Abstract
Seven esters of α-truxillic acid have been synthesized: bis-3-piperidylpropyl ester and its quaternary bis-N-ethyl derivative, bis-N-diethylaminopropyl ester and its quaternary bis-N-methyl derivative, and bis-4-piperidylbutyl ester and its quaternary bis-N-methyl and bis-N-ethyl derivatives.
All esters inhibited the specific binding of muscarinic receptor antagonist [methyl-3H]-N-methylscopolamine ([3H]-NMS) to muscarinic receptors in membranes of CHO cell lines stably expressing the human gene for the M1, M2, M3 or M4 subtype of muscarinic receptors. All esters displayed the highest potency at the M2 and the lowest potency at the M3 receptor subtype.
In experiments performed on the M2 muscarinic receptor subtype, the affinity between the receptors and the esters was greatly increased when the concentration of ions was diminished. The highest affinities were found for the tertiary bis-3-piperidylpropyl and bis-4-piperidylbutyl aminoesters (equilibrium dissociation constants of 52 and 179 pM, respectively, in the low ionic strength medium).
All investigated esters slowed down the dissociation of [3H]-NMS from the M2 muscarinic receptor subtype. [3H]-NMS dissociation from the M1, M3 and M4 muscarinic receptor subtypes was investigated in experiments with the bis-4-piperidylbutyl aminoester and also found to be decelerated.
It is concluded that the esters of α-truxillic acid act as M2-selective allosteric modulators of muscarinic receptors and that, by their potency, the tertiary bis-3-piperidylpropyl and bis-4-piperidylbutyl aminoesters surpass the other known allosteric modulators of these receptors.
Keywords: Muscarinic receptors, α-truxillic acid esters, allosteric modulation, piperidylpropyl esters of α-truxillic acid, piperidylbutyl esters of α-truxillic acid, CHO cells
Introduction
It has been shown that several esters of α-truxillic acid are potent neuromuscular blockers, and some of them were introduced into clinical use as myorelaxants in the former Soviet Union (Kharkevich & Skoldinov, 1986). These neuromuscular blockers (i.e., nicotinic antagonists) also display anti-muscarinic effects in pharmacological experiments, which are particularly prominent on the heart and occur at several fold lower doses than the neuromuscular blockade (Kharkevich & Shorr, 1986). Cardio-selective anti-muscarinic effects of several other neuromuscular blockers had been discovered in earlier studies, and had been shown to be allosteric in nature (Clark & Mitchelson, 1976; Stockton et al., 1983; Dunlap & Brown, 1983; reviews Lee & El-Fakahany, 1991; Holzgrabe & Mohr, 1998; Christopoulos et al., 1998).
In the present work, we synthesized three esters of α-truxillic acid (=(1α, 2α, 3β, 4β)-2,4-diphenyl-1,3-cyclobutane-1,3-dicarboxylic acid, CA No 490-20-0) containing two tertiary nitrogen atoms each, and four bis-N-methylated or bis-N-ethylated quaternary derivatives of these esters, with the intention to investigate their anti-muscarinic properties in radioligand binding experiments on membranes containing defined muscarinic receptor subtypes. The purpose of our experiments was to establish the subtype specificity of the anticipated anti-muscarinic action of the esters, to determine the competitive or allosteric nature of such action, and to find (if possible) a compound with a high affinity for the receptors, which might serve as the starting material for the development of a specific radioligand.
Methods
Preparation of α-truxillic acid and of aminoalcohols
α-Truxillic acid (Figure 1) was obtained by photodimerization of E-cinnamonic acid (Arendaruk et al., 1967), using one of three ways (exposure to sunshine for 3 months, exposure to u.v. light in solid state for 175–200 h, or exposure of a water suspension in a photoreactor for 70 h), all with a yield of 35–40%. Its melting point was 281–284°C and its structure and purity were checked by 1H and 13C nuclear magnetic resonance. Its dichloride was prepared by heating it in excess of thionyl chloride with the addition of pyridine.
Figure 1.
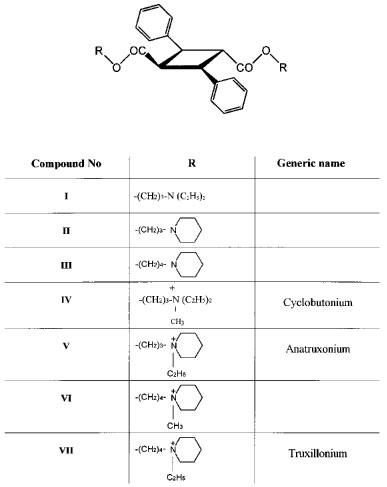
Esters of α-truxillic acid.
4-Piperidylbutan-1-ol was prepared using the sequence of reactions in which tetrahydrofuran plus acetylbromide yield 4-bromobutylacetate, which reacts with piperidine to form 4-piperidylbutylacetate, which is deacetylated by boiling with 10% KOH to form 4-piperidylbutan-1-ol (Smorgonskii & Goldfarb, 1940; Solovev et al., 1959).
3-Piperidylpropan-1-ol was prepared by heating piperidine with sodium dissolved in allylalcohol (Hromatka, 1942).
3-Diethylaminopropan-1-ol was obtained from Aldrich-Sigma.
Preparation of truxillic acid esters
α-Truxillic acid esters (described as compounds I, II and III in Figure 1) were prepared from α-truxillic acid dichloride and corresponding aminoalcohols, based on the procedure described by Arendaruk et al. (1967; 1973). The purity of the products was checked by thin layer chromatography on silica gel (Merck) in chloroform : methanol : ammonia (6 : 4 : 0.2) (I,III) or ethyl acetate : ammonia (100 : 2) (II) and on aluminium oxide (Merck) in toluene : ethanol (8 : 2) (I, III). The structure of II was confirmed by 1H and 13C nuclear magnetic resonance. Although not determined directly, the pKa values of compounds I, II and III were expected to be 10.5 or higher (CRC Handbook, 1983). Consequently, the three amino esters were probably fully protonized during incubations at pH of 7.4.
Quaternary derivatives of the esters (compounds IV–VII in Figure 1) were prepared by heating the parent compounds with excess methyl iodide or ethyl iodide in methanol or ethanol and recrystallization. Their purities were checked by elementary analysis (VI, VII) and by comparing the IR spectra with a standard donated by D. Kharkevich (IV). Their melting points were 211–212°C (IV), 210–215°C (V), 227–228°C (VI) and 166–167°C (VII).
Cell membranes
Interactions between the esters of α-truxillic acid and muscarinic receptors were investigated on membranes of CHO (Chinese hamster ovary) cell lines stably transfected with human genes for the M1–M4 muscarinic receptor subtypes (Buckley et al., 1989). Cells were grown in Dulbecco's modified Eagle's medium complemented with 10% foetal calf serum and 0.005% geneticin as described by Jakubík et al. (1995). After 7 days in culture, they were collected using mild trypsinization and centrifugation, and suspended in medium A (see below) at a concentration of 20×106 cells ml−1.
Suspended cells were homogenized with an Ultra-Turrax homogenizer (Janke and Kunkel, Staufen, Germany). The homogenate was centrifuged for 3 min at 310×g, the sediment was recentrifuged at the same acceleration, and the membranes contained in the combined supernatants were sedimented by 30 min centrifugation at 60,000×g. The sediment was resuspended in medium A or, for incubations at low ionic strength, in medium B. In the latter case, the membranes were washed by an additional centrifugation (60 min at 60,000×g) in medium B. Membranes were kept frozen at −40°C for up to 6 weeks.
Media used for homogenization and incubation
Most experiments with cell membranes were performed with medium A, consisting of (mM) NaCl 136, KCl 5, MgSO4 1, Na-phosphate 1; pH 7.4 and Na-HEPES 10; pH 7.4 in water. Where indicated, low ionic strength medium B was applied, consisting of Na-HEPES (10 mM, pH 7.4) in water.
Radioligand binding experiments
[Methyl-3H]-N-methylscopolamine ([3H]-NMS) was used as a specific muscarinic radioligand with low subtype selectivity. In saturation binding experiments performed under the conditions described below, Kd values for [3H]-NMS binding to the M1, M2, M3 and M4 receptor subtypes in medium A were 180, 215, 110 and 120 pM, respectively.
Experiments with radioligand binding and dissociation were performed at 25°C using methods described previously (Jakubík et al., 1995; 1997). Membranes corresponding to 106 cells were incubated in 0.8 ml of medium A or B supplemented with [3H]-NMS and the investigated compounds. The incubations were arrested by dilution and filtration through Whatman GF/B glass fibre filters (presoaked in 0.3% polyethyleneimine) in a Brandel cell harvester. The radioactivity retained on filters was measured by scintillation counting in Bray's solution. Atropine sulphate (5 μmol l−1) was included in the incubation medium to determine the nonspecific binding of the radioligand.
Three types of experiments were performed: (a) Saturation (Scatchard type) experiments, yielding values of Bmax (maximum binding capacity) and Kd for [3H]-NMS binding. Membranes were incubated for 120 min with 18–600 pM [3H]-NMS; (b) Displacement (competition-type) experiments. In these experiments, membranes were preincubated with a fixed concentration of [3H]-NMS. After 1 h, α-truxillic acid esters were added at increasing concentrations and the incubations were continued for 12 h in experiments with medium A and for 15 h in experiments with medium B. The concentrations of [3H]-NMS used were 260, 278, 176 and 176 pM for the M1, M2, M3 and M4 receptor subtype, respectively, and (c) Experiments with [3H]-NMS dissociation. Membranes were preincubated with [3H]-NMS for 60 or 120 min, after which time atropine (5 μM) was added either alone or together with α-truxillic acid esters. The concentrations of [3H]-NMS and of the esters varied for different subtypes and are indicated in Results. Incubations were arrested by dilution and filtration at various time intervals after the addition of atropine.
Data analysis
Values of Kd (equilibrium dissociation constant of the radioligand), Bmax (maximum number of binding sites for the radioligand), KA (equilibrium dissociation constant for the binding of an allosteric ligand to the allosteric binding site on a receptor with unoccupied orthosteric binding site), α (cooperativity factor, see below) and koff were computed by nonlinear regression as described in previous work (Jakubík & Tuček, 1994b; Jakubík et al., 1997) with the use of Lotus or GraphPad Prism programs. The cooperativity factor α (Ehlert, 1988) indicates the fold change of the Kd of a receptor for its orthosteric ligand ([3H]-NMS in our work) induced by the binding of an allosteric ligand to the allosteric binding site. Values of α and KA were computed by fitting Ehlert's (1988) equation No. 6 to data on the binding of [3H]-NMS to receptors in the presence of varying concentrations of the allosteric ligand, i.e.:
where A stands for the allosteric ligand and B and Bo describe the binding of [3H]-NMS in the presence and the absence of the allosteric ligand, respectively.
Materials
E-cinnamonic acid and silica gel and alumina TLC plates were from Merck (Darmstadt, Germany). [3H]-NMS (83 Ci mmol−1) was from Amersham Life Sci. Ltd. (Little Chalfont, U.K.) CHO cell lines stably transfected with genes for muscarinic receptors were kindly provided by Drs Tom Bonner and Mark Brann.
Results
Inhibition of [3H]-NMS binding by α-truxillic acid esters
All α-truxillic acid esters inhibited the binding of [3H]-NMS to the M1–M4 receptor subtypes, but the inhibition did not reach 100% even at the highest ester concentrations used. Figure 2 shows the inhibitory curves obtained with the bis-4-piperidylbutyl ester (III) and its bis-N-methyl (VI) and bis-N-ethyl (VII) derivatives. The inhibitory curves obtained with the bis-3-piperidylpropylester (II) and its bis-N-ethyl derivative (V) and with the bis-diethylaminopropyl ester (I) and its bis-N-methyl derivative (IV) were similar but are not being shown. Values of KA and of the cooperativity factors α computed from data on the inhibition of [3H]-NMS binding by all tested esters have been listed in Table 1.
Figure 2.
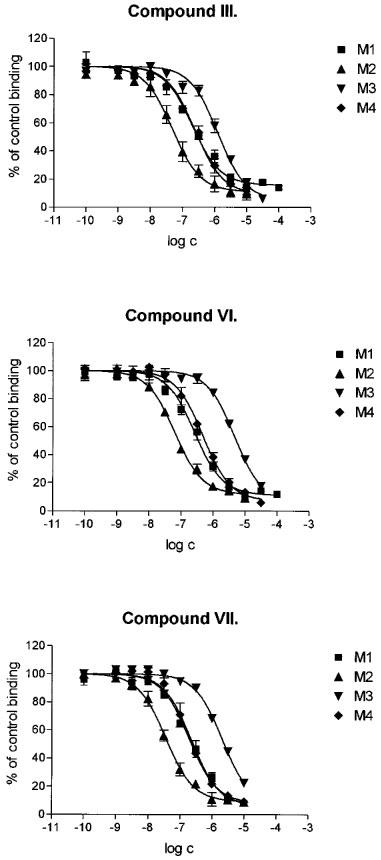
Inhibition of the binding of [3H]-NMS to the M1–M4 subtypes of muscarinic receptors by α-truxillic acid bis-4-piperidylbutyl ester (compound III, top panel), its quaternary bis-N-methyl derivative (compound VI, middle panel) and bis-N-ethyl derivative (compound VII, bottom panel). Abscissa: log of the concentration of the ester. Ordinate: [3H]-NMS binding in the presence of the ester, expressed as per cent of the binding in the absence of the ester. Data are means±s.e.mean of at least two experiments with incubations performed in triplicates.
Table 1.
Negative logarithms (pKA) of equilibrium dissociation constants (M) for the binding of α-truxillic acid esters to the allosteric sites on muscarinic receptors and cooperativity factors α for their interaction with the binding of [3H]-NMS
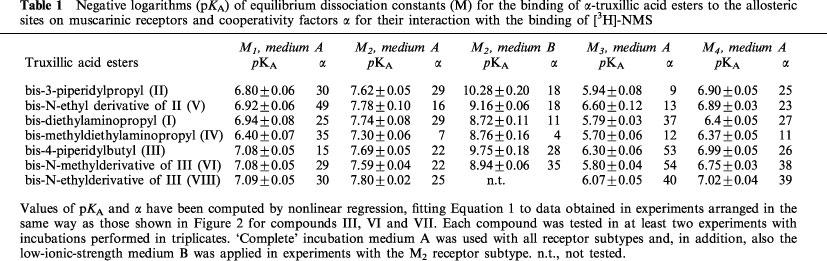
As indicated in Table 1, the inhibition of [3H]-NMS binding by truxillic acid esters was investigated with the use of the ‘complete' medium A on membranes of cells expressing the M1–M4 receptor subtypes. It is apparent from Table 1 that the affinity of α-truxillic acid esters was highest for the M2 muscarinic receptor subtype, intermediate for the M1 and M4 receptor subtypes, and lowest for the M3 muscarinic receptor subtype. No systematic differences were found in experiments with medium A between the affinities for receptors displayed by the tertiary aminoesters as compared to their quaternary derivatives.
The effect of diminishing the concentration of ions in the incubation medium on the inhibition of [3H]-NMS binding by the esters has been investigated on membranes of cells expressing the M2 muscarinic receptor subtype. Figure 3 shows data obtained with the bis-4-piperidylbutyl ester (III) and its N-methyl derivative (VI). The results obtained with these two and four other esters have been summarized in separate columns in Table 1. The affinity between the M2 receptors and the esters was considerably higher in 10 mM Na-HEPES (medium B) than in the ‘complete' medium A. The highest shifts in the affinities were observed for the tertiary bis-3-piperidylpropyl ester (II; 457 fold) and bis-4-piperidylbutyl ester (III; 115 fold). As a result, receptor affinities for these tertiary aminoesters were 6–13 fold higher than the affinities for their quaternary derivatives in the low ionic-strength medium. Surprisingly, no such difference was found between the affinities for the bis-N-diethylaminopropyl ester (I) and its quaternary derivative (IV).
Figure 3.
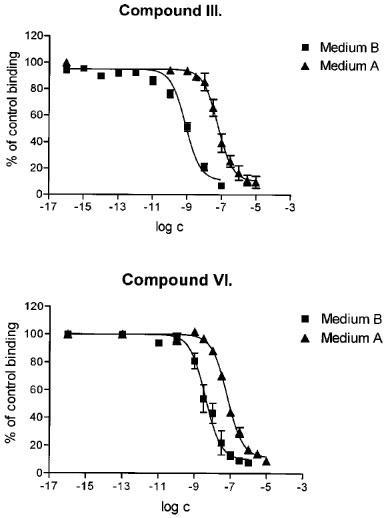
Effect of lowering the concentration of ions in the medium on the inhibition of [3H]-NMS binding to the M2 subtype of muscarinic receptors by α-truxillic acid bis-4-piperidylbutyl ester (compound III, top panel) and its bis-N-methyl derivative (compound VI, bottom panel). Experiments were performed using either the ‘complete' medium A, or the low-ionic-strength medium B. Abscissa: log of the concentration of the ester. Ordinate: [3H]-NMS binding in the presence of the ester, expressed as per cent of the binding in the absence of the ester. Data are means±s.e.mean of at least two experiments with incubations performed in triplicates.
α-Truxillic acid esters and [3H]-NMS dissociation
Experiments were performed to investigate whether the esters of α-truxillic acid have an effect on the rate of [3H]-NMS dissociation from the receptors, observed after the addition of a high concentration of atropine. As shown in Figure 4, all esters slowed down the dissociation of [3H]-NMS from the M2 receptors. In the presence of individual esters applied at 10 μM concentration, the overall rate of dissociation was slowed down 2–9 times. While the time course of the dissociation revealed after the addition of atropine alone or of atropine plus four of the esters was monophasic, data for the combination of atropine with three compounds (II, V and VI) could better be fitted to a biphasic exponential curve. Detailed investigation of the kinetics of [3H]-NMS dissociation was hindered by a shortage of supply of the esters, which had to be applied at comparatively high concentrations.
Figure 4.
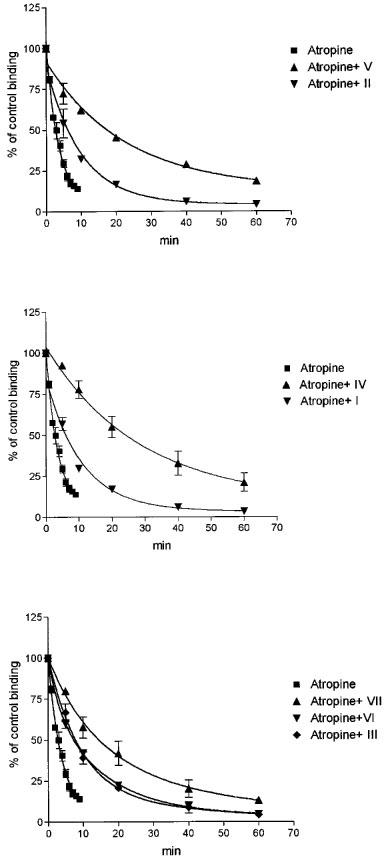
Effects of α-truxillic acid esters applied at a single concentration of 10 μM on the rates of atropine-induced dissociation of [3H]-NMS from muscarinic receptors of the M2 subtype. Membranes were preincubated in medium A for 60 min with 250 pM [3H]-NMS, after which 5 μM atropine was added either alone or simultaneously with 10 μM ester. Abscissa: Time (min) after the addition of atropine. Ordinate: [3H]-NMS binding, expressed as per cent of the binding immediately before the addition of atropine. Top panel: effects of the bis-3-piperidylpropyl ester (compound II) and of its N-ethyl derivative (compound V). Middle panel: effects of the bis-diethylaminopropyl ester (compound I) and its bis-N-methyl derivative (compound IV). Bottom panel: effects of the bis-4-piperidylbutyl ester (compound III), its bis-N-methyl derivative (compound VI) and bis-N-ethyl derivative (compound VII). Data are means±s.e.mean of two experiments with incubations performed in duplicates. The koff value computed for the rate of dissociation observed after the addition of atropine was 0.239 min−1 and the dissociation was decelerated 2.8, 2.2, 8.9, 2.2, 5.4, 2.8 and 2.8 fold when compounds V, II, IV, I, VII, VI and III, respectively, were added together with atropine.
To obtain information about the concentration-effect relationship with regard to the deceleration of [3H]-NMS dissociation, we performed single-time-point determinations of the koff values observed after the addition of atropine and different concentrations of bis-4-piperidylbutyl truxillate (compound III). As may be seen from Figure 5, the inhibitory effect of increasing concentrations of the ester on the rate of atropine-induced [3H]-NMS dissociation could be described by a sigmoidal curve with a slope factor (nH) of 0.88±0.04. The maximum deceleration achieved was close to 90% and the concentration ensuring the half-maximum effect (EC50) was 2.29×10−6 M.
Figure 5.
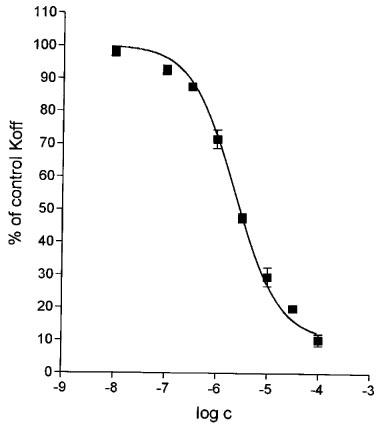
Effect of various concentrations of bis-4-piperidylbutyl α-truxillate (compound III) on the rate of [3H]-NMS dissociation from the M2 receptors observed after the addition of 5 μM atropine. Abscissa: log concentration (M) of the ester. Ordinate: koff determined in the presence of atropine plus the ester, expressed as per cent of the koff determined in the presence of atropine alone. Incubations were performed in the ‘complete' medium A and the koff values were computed from single-time-point determinations of the specifically bound radioactivity measured 3–25 min after the addition of atropine and the ester. To prepare the stock solution of the ester, ethanol had to be used. To avoid irregularities which might arise because of the presence of different concentrations of ethanol in tubes with different concentrations of the ester, the same concentration of ethanol (0.1% at final dilution) was included in all tubes, independently of the concentration of truxillate. After the addition of atropine alone, koff was 0.375 min−1. Data are means±s.e.mean of three experiments (seven filtrations for each point).
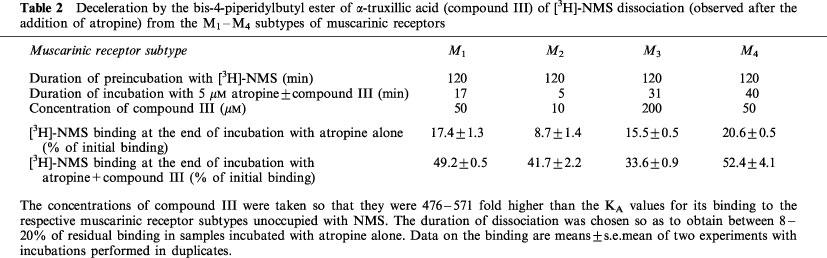
To find out whether the dissociation of [3H]-NMS from the M1, M3 and M4 muscarinic receptor subtypes is also susceptible to deceleration by an α-truxillic acid ester, additional single-time-point experiments were performed with [3H]-NMS dissociation in the presence of atropine and the bis-4-piperidylbutyl ester (III). The results have been summarized in Table 2. It is evident that the bis-4-piperidylbutyl α-truxillate (III) slowed down the dissociation of [3H]-NMS from all four muscarinic receptor subtypes studied.
Table 2.
Deceleration by the bis-4-piperidylbutyl ester of α-truxillic acid (compound III) of [3H]-NMS dissociation (observed after the addition of atropine) from the M1–M4 subtypes of muscarinic receptors
Discussion
The effects of the investigated esters were remarkably similar in that they all inhibited the binding of [3H]-NMS to muscarinic receptors with the highest affinity for the M2 and with the lowest affinity for the M3 receptor subtype. The nature of the inhibition was allosteric. This conclusion is based on the following observations: (a) The inhibition of [3H]-NMS binding did not reach 100%. Since the allosteric inhibitors diminish the binding of orthosteric ligands by decreasing the affinity of receptors for the orthosteric ligand to a maximal limit determined by the magnitude of the cooperativity factor (rather than by physical displacement), apparent 100% inhibition of the binding is only observed when the Kd for the binding of the orthosteric ligand becomes substantially elevated, and the lack of 100% inhibition is a frequent feature of allosteric interactions: (b) The esters slowed down the dissociation of [3H]-NMS from the receptors. This is a feature typical of the action of most allosteric ligands on muscarinic receptors (e.g., Nedoma et al., 1986; Waelbroeck, 1994; Lazareno & Birdsall, 1995). When associated with the allosteric binding site, allosteric modulators probably interfere sterically with the access of substances to and departure from the orthosteric binding sites (Proška & Tuček, 1994; Jakubík & Tuček, 1994a; 1995; Matsui et al., 1995), and (c) The affinities for the esters became strongly enhanced in the low ionic strength medium B, in harmony with observations made for gallamine, another allosteric modulator of muscarinic receptors (Pedder et al., 1991; Tränkle et al., 1996).
The conclusion on the allosteric nature of the action of truxillic acid esters on muscarinic receptors agrees with data on the action of other M2-selective myorelaxants on these receptors (Lee & El-Fakahany, 1991; Tuček & Proška, 1995), and also with a recent poster by Urbanský & Proška (1997), demonstrating that the binding of [3H]-NMS to cardiac muscarinic receptors became enhanced by an ester of truxillic acid.
When determined in the ‘complete' medium A, differences between the affinities for the seven investigated truxillic acid esters on one and the same muscarinic receptor subtype were small. Substantial differences between the affinities for individual esters became apparent during incubations in the low ionic strength medium B. The affinities of the M2 receptors for the tertiary bis-3-piperidylpropyl (II) and bis-4-piperidylbutyl (III) aminoesters were clearly higher than those for the quaternary derivatives of these esters. We cannot offer an unequivocal explanation for the finding that the observed affinity for the tertiary compounds was more enhanced in the low ionic strength medium (when compared to the ‘complete' medium) than that for the quaternary compounds. It seems likely that, in the absence of Na+ ions, the affinity for the tertiary compounds II and III is higher than that for their quaternary derivatives because of a better fit of of the tertiary compounds to the binding domain. There is little doubt that the positive charge on the protonated tertiary nitrogen or on the quaternary nitrogen of the allosteric modulators is important for the interaction with a negative charge (two negative charges? – see Jakubík & Tuček, (1995)) on the receptor. In the presence of Na+ ions, the modulators have to compete with the Na+ ions for the negative charge(s) on the receptor. It is not clear why the protonated amino compounds are less successful in the competition with Na+ than their quaternary ammonium counterparts. It is also not clear why we could not observe a similar difference between the affinities for the tertiary bis-N-diethylaminopropyl and the quaternary bis-N-methyldiethylaminopropyl esters (I and IV, respectively).
It is interesting to compare the affinities of muscarinic receptors for the esters of α-truxillic acid with their affinities for the other known allosteric modulators. Most data on the affinities of muscarinic receptors for their allosteric modulators originate from experiments in which the ionic composition of the incubation medium was not far from that of physiological saline solution, particularly as far as the concentration of Na+ ions is concerned. Such data can be compared with the present data obtained with medium A. These are examples of the affinities (expressed as KA values) of the M2 muscarinic receptors described in the literature: 769 nM for gallamine (Stockton et al., 1983), 667 nM for alcuronium (Proška & Tuček, 1994), 11 μM for strychnine (Lazareno et al., 1998), 180 nM for the compound C7/3′-phth (Lanzafame et al., 1996), and 160 nM for the compound W84 (Tränkle et al., 1998). Compared to these values, the affinities of the M2 muscarinic receptors for α-truxillic esters discovered in experiments with medium A (16 and 27 nM for the bis-3-piperidylpropyl and bis-4-piperidylbutyl truxillate, respectively) are very high.
The affinity of muscarinic receptors for the allosteric modulators is known to increase in low ionic strength media. In the work of Pedder et al. (1991), the KA of cardiac muscarinic receptors for gallamine was found to correspond to 794 and 40 nM in a high and low ionic strength medium, respectively. In comparison to the latter value, the affinities of the M2 receptors for α-truxillic acid esters which we discovered in experiments with the low ionic strength medium are remarkably high (KA of 0.087 nM for the bis-3-piperidylpropyl truxillate and of 0.147 nM for the bis-4-piperidylbutyl truxillate).
As already mentioned, most allosteric modulators of muscarinic receptors decelerate the dissociation of classical ligands from the orthosteric binding sites and some block it completely. An exception to this rule of deceleration has been observed in experiments with [3H]-quinuclidinyl benzilate and low concentrations of gallamine (Ellis & Seidenberg, 1989). The relation between the effects which an allosteric modulator has on the rate of the dissociation of a classical ligand and on the affinity of the receptor for this ligand is not straightforward. It has been found repeatedly that the effects of allosteric modulators on the rates of the dissociation of orthosteric ligands are independent of the cooperativity with which they modulate the affinity for orthosteric ligands measured at equilibrium (e.g., Kostenis et al., 1994; Tränkle et al., 1997). Data in Figure 5 indicate that bis-4-piperidylbutyl truxillate (III) does not completely inhibit [3H]-NMS dissociation because the dissociation proceeded at 10% of control rate even in the presence of 100 μM ester. At this concentration, the receptor-[3H]-NMS complex is expected to be fully saturated with the modulator since the Kd value for the binding of compound III to the [3H]-NMS-occupied receptor is much (44–227 fold) lower. According to the equilibrium binding experiments, the Kd for the binding of compound III to the [3H]-NMS-receptor complex is expected to be 0.44 μM (KA×α, see Table 1). According to the experiments with [3H]-NMS dissociation, the Kd for the binding of compound III to the [3H]-NMS-receptor complex is expected to correspond to its EC50 value determined for the deceleration of [3H]-NMS dissociation (Lazareno & Birdsall, 1995), i.e. to 2.29 μM (see Figure 5).
Because of their high affinity and subtype selectivity, α-truxillic acid esters are likely to become useful tools for studies involving the allosteric modulation of muscarinic receptors, particularly of their M2 subtype, and perhaps may serve as the starting material for the development of drugs acting via the allosteric binding sites on these receptors.
This work was supported by grants from the Grant Agency of the Czech Republic (309/96/1287 and 309/99/0214) and from NIH (FIRC Award No. 2-RO3-TW00171 to E.E. El-Fakahany). We thank Professor D. Kharkevich for the donation of small samples of several esters, Dr A.P. Skoldinov for advice on synthetic procedures, Professor E.E. El-Fakahany for discussions and generous help, and Mrs D. Ungerová for technical assistance. We acknowledge the participation of Dr J. Proška in preliminary experiments on cardiac membranes. M. Lysíková completed this work as a part of her postgraduate study of neuroscience at the First Medical Faculty of Charles University in Prague, Czech Republic.
Abbreviations
- CHO
Chinese hamster ovary
- [3H]-NMS
[methyl-3H]-N-methylscopolamine
References
- ARENDARUK A.P., SKOLDINOV A.P., KHARKEVICH D.A. Investigations of the series of cyclobutanedicarbonyl acids. IV. Synthesis of bis-quaternary salts of alkamine esters of alpha-truxillic acid. (In Russian) Khim. Farm. Zh. 1967;4:3–8. [Google Scholar]
- ARENDARUK A.P., SKOLDINOV A.P., KHARKEVICH D.A.Method of production diiodomethylate of di-(1,3-diethylaminopropanol ester) of alpha-truxillic acid (cyclobutonium) Chem. Abstr. 197379(In Russian). USSR patent 360849, Byull. No. 22P66036d [Google Scholar]
- BUCKLEY N.J., BONNER T.I., BUCKLEY C.M., BRANN M.R. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol. Pharmacol. 1989;35:469–476. [PubMed] [Google Scholar]
- CHRISTOPOULOS A., LANZAFAME A., MITCHELSON F. Allosteric interactions at muscarinic cholinoceptors. Clin. Exp. Pharmacol. Physiol. 1998;25:185–194. doi: 10.1111/j.1440-1681.1998.t01-4-.x. [DOI] [PubMed] [Google Scholar]
- CLARK A.L., MITCHELSON F. The inhibitory effect of gallamine on muscarinic receptors. Br. J. Pharmacol. 1976;58:323–331. doi: 10.1111/j.1476-5381.1976.tb07708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRC HAND BOOK OF CHEMISTRY AND PHYSICS 1983CRC Press Inc., Boca Raton; D164–D166.64th Edition [Google Scholar]
- DUNLAP J., BROWN J.H. Heterogeneity of binding sites on cardiac muscarinic receptors induced by the neuromuscular blocking agents gallamine and pancuronium. Mol. Pharmacol. 1983;24:15–22. [PubMed] [Google Scholar]
- EHLERT F.J. Estimation of the affinities of allosteric ligands using radioligand binding and pharmacological null methods. Mol. Pharmacol. 1988;83:187–194. [PubMed] [Google Scholar]
- ELLIS J., SEIDENBERG M. Gallamine exerts biphasic allosteric effects at muscarinic receptors. Mol. Pharmacol. 1989;35:173–176. [PubMed] [Google Scholar]
- HOLZGRABE U., MOHR K. Allosteric modulation of ligand binding to muscarinic acetylcholine receptors. Drug Dev. Today. 1998;3:214–222. [Google Scholar]
- HROMATKA O. Über die Synthese von Aminopropanolen. I. Mitteilung. Ber. Dtsch. Chem. Ges., B. 1942;75:131–138. [Google Scholar]
- JAKUBÍK J., BAčÁKOVÁ L., EL-FAKAHANY E.E., TUčEK S. Subtype selectivity of the positive allosteric action of alcuronium at cloned M1–M5 muscarinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 1995;274:1077–1083. [PubMed] [Google Scholar]
- JAKUBÍK J., BAčÁKOVÁ L., EL-FAKAHANY E.E., TUčEK S. Positive cooperativity of acetylcholine and other agonists with allosteric ligands on muscarinic acetylcholine receptors. Mol. Pharmacol. 1997;52:172–179. doi: 10.1124/mol.52.1.172. [DOI] [PubMed] [Google Scholar]
- JAKUBÍK J., TUčEK S. Protection by alcuronium of muscarinic receptors against chemical inactivation and location of the allosteric binding site for alcuronium. J. Neurochem. 1994a;63:1932–1940. doi: 10.1046/j.1471-4159.1994.63051932.x. [DOI] [PubMed] [Google Scholar]
- JAKUBÍK J., TUčEK S. Two populations of muscarinic binding sites in the chick heart distinguished by affinities for ligands and selective inactivation. Br. J. Pharmacol. 1994b;113:1529–1537. doi: 10.1111/j.1476-5381.1994.tb17170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAKUBÍK J., TUčEK S. Positive allosteric interactions on cardiac muscarinic receptors: effects of chemical modifications of disulphide and carboxyl groups. Eur. J. Pharmacol. 1995;289:311–319. doi: 10.1016/0922-4106(95)90109-4. [DOI] [PubMed] [Google Scholar]
- KHARKEVICH D.A., SHORR V.A.Antimuscarinic and ganglion-blocking activity of neuromuscular blocking agents. New Neuromuscular Blocking Agents 1986Springer Verlag, Berlin; 191–224.In: Kharkevich D.A. ed. Handbook of Experimental Pharmacology, Vol. 79, pp [Google Scholar]
- KHARKEVICH D.A., SKOLDINOV A.P.The derivatives of carboxylic acids Handbook of Experimental Pharmacology 198679Springer Verlag, Berlin; 323–369.New Neuromuscular Blocking Agents. ed. Kharkevich D.A. [Google Scholar]
- KOSTENIS E., HOLZGRABE U., MOHR K. Allosteric effect on muscarinic M2-receptors of derivatives of the alkane-bis-ammonium compound W84. Comparison with bispyridinium-type allosteric modulators. Eur. J. Med. Chem. 1994;29:947–953. [Google Scholar]
- LANZAFAME A., CHRISTOPOULOS A., MITCHELSON F. Interactions of agonists with an allosteric antagonist at muscarinic acetylcholine M2 receptors. Eur. J. Pharmacol. 1996;316:27–32. doi: 10.1016/s0014-2999(96)00639-5. [DOI] [PubMed] [Google Scholar]
- LAZARENO S., BIRDSALL N.J.M. Detection, quantitation, and verification of allosteric interactions of agents with labeled and unlabeled ligands at G protein-coupled receptors: interactions of strychnine and acetylcholine at muscarinic receptors. Mol. Pharmacol. 1995;48:362–378. [PubMed] [Google Scholar]
- LAZARENO S., GHARAGOZLOO P., KUONEN D., POPHAM A., BIRDSALL N.J.M. Sutype-selective positive cooperative interactions between brucine analogues and acetylcholine at muscarinic receptors: radioligand binding sites. Mol. Pharmacol. 1998;53:573–589. doi: 10.1124/mol.53.3.573. [DOI] [PubMed] [Google Scholar]
- LEE N.H., EL-FAKAHANY E.E. Allosteric antagonists of the muscarinic acetylcholine receptor. Biochem. Pharmacol. 1991;42:199–205. doi: 10.1016/0006-2952(91)90703-8. [DOI] [PubMed] [Google Scholar]
- MATSUI H., LAZARENO S., BIRDSALL N.J.M. Probing of the location of the allosteric site on m1 muscarinic receptors by site-directed mutagenesis. Mol. Pharmacol. 1995;47:88–98. [PubMed] [Google Scholar]
- NEDOMA J., TUčEK S., DANILOV A.F., SHELKOVNIKOV S.A. Stabilization of antagonist binding to cardiac muscarinic acetylcholine receptors by gallamine and other neuromuscular blocking drugs. J. Pharmacol. Exp. Ther. 1986;236:219–223. [PubMed] [Google Scholar]
- PEDDER E.K., EVELEIGH P., POYNER D., HULME E.C., BIRDSALL N.J.M. Modulation of the structure-binding relationships of antagonists for muscarinic acetylcholine receptor subtypes. Br. J. Pharmacol. 1991;103:1561–1567. doi: 10.1111/j.1476-5381.1991.tb09827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROšKA J., TUčEK S. Mechanisms of steric and cooperative actions of alcuronium on cardiac muscarinic acetylcholine receptors. Mol. Pharmacol. 1994;45:709–717. [PubMed] [Google Scholar]
- SMORGONSKII L.M., GOLDFARB YA.L. On the effect of acid haloanhydrides on tetrahydrofurane and on some derivatives of 4-diethylaminobutanol-1. (In Russian) Zh. Obsh. Khim. 1940;10:1113–1119. [Google Scholar]
- SOLOVEV V.M., ARENDARUK A.P., SKOLDINOV A.P. Dialkylaminoalkyl esters of 3,4,5-trimethoxybenzoic acid. (In Russian) Zh. Obsh. Khim. 1959;29:631–635. [Google Scholar]
- STOCKTON J.M., BIRDSALL N.J.M., BURGEN A.S.V., HULME E.C. Modification of the binding properties of muscarinic receptors by gallamine. Mol. Pharmacol. 1983;23:551–557. [PubMed] [Google Scholar]
- TRÄNKLE C., ANDRESEN I., LAMBRECHT G., MOHR K. M2 receptor binding of the selective antagonist AF-DX 384: possible involvement of the common allosteric site. Mol. Pharmacol. 1998;53:304–312. doi: 10.1124/mol.53.2.304. [DOI] [PubMed] [Google Scholar]
- TRÄNKLE C., ELIS K., WIESE M., MOHR K. Molecular rigidity and potency of bispyridinium type allosteric modulators at muscarinic M2-receptors. Life Sci. 1997;22:1995–2003. doi: 10.1016/s0024-3205(97)00164-1. [DOI] [PubMed] [Google Scholar]
- TRÄNKLE C., KOSTENIS E., BURGMER U., MOHR K. Search for lead structures to develop new allosteric modulators of muscarinic receptors. J. Pharmacol. Exp. Ther. 1996;279:926–933. [PubMed] [Google Scholar]
- TUčEK S., PROšKA J. Allosteric modulation of muscarinic acetylcholine receptors. Trends Pharmacol. Sci. 1995;16:205–212. doi: 10.1016/s0165-6147(00)89023-9. [DOI] [PubMed] [Google Scholar]
- URBANSKÝ M., PROšKA J. Truxillic acid derivatives: high affinity, M2 selective allosteric modulators. Probes for mapping the muscarinic receptors. Life Sci. 1997;60:1170. [Google Scholar]
- WAELBROECK M. Identification of drugs competing with d-tubocurarine for an allosteric site on cardiac muscarinic receptors. Mol. Pharmacol. 1994;46:685–692. [PubMed] [Google Scholar]


