Abstract
5-HT1-like and 5-HT2 receptors have both been described to mediate contractions to 5-HT in the human umbilical artery (HUA). However, the nature of the 5-HT receptor subtypes is unknown.
In isometric force studies with ring preparations of HUA α-methyl-5-hydroxytryptamine (α-Me-5-HT) and 5-hydroxytryptamine (5-HT) contracted HUA with pED50 values of 8.04 and 7.74, respectively. In the presence of a subthreshold concentration of another vasoconstrictor sumatriptan and 5-nonyloxytryptamine elicited concentration-dependent contractions with pEC50 values of 7.21 and 7.67, respectively.
In the presence of the selective 5-HT1B/D receptor antagonist GR127935, contractile responses elicited by sumatriptan and 5-nonyloxytryptamine were competitively antagonized (pKB 9.01 and 9.02, respectively). In the experiments with 5-HT, GR127935 appeared to be non-competitive with shallow Schild plot slopes. The data were fitted with two linear regression lines and the calculated pKB of the high affinity component (8.90) was comparable to that expected for GR127935 at the 5-HT1B/1D receptor.
Several 5-HT2 selective receptor antagonists (spiperone, cyproheptadine, pirenperone) competitively inhibited responses to 5-HT. The selective 5-HT2A antagonist ketanserin against sumatriptan and 5-nonyloxytryptamine behaved as a weak antagonist while against 5-HT demonstrated a competitive antagonism (pKB 8.56).
Using specific primers for human 5-HT1B, 5-HT1D and 5-HT2A receptor genes, the reverse transcriptase-polymerase chain reaction revealed mRNA expression of 5-HT1B and 5-HT2A receptors in the HUA.
The results suggest that the HUA has a functional population of 5-HT1B and 5-HT2A receptor subtypes which are involved in the contractile response to 5-HT. Contractions mediated by 5-HT1B receptors can be ‘uncovered' by exposure to other vasoactive agents.
Keywords: 5-HT1B and 5-HT2A receptors, human umbilical artery, vasoconstriction, RT–PCR
Introduction
It is well established that 5-hyroxytryptamine (5-HT) can elicit both contraction and relaxation of blood vessels (Feniuk & Humphrey, 1989; Martin, 1994; Mylecharane, 1990). Extensive efforts have been made to unravel the molecular identity of 5-HT receptors involved in the regulation of blood vessel tone and the cellular mechanism whereby they exert their actions. Analysis of 5-HT receptor mRNA expression in different vascular tissues has shown that only five of the 13 known G-protein coupled 5-HT receptor mRNAs are expressed in blood vessels: 5-HT1B/1D (formerly 5-HT1Dα/β), 5-HT2A, 5-HT2B, 5-HT4 and 5-HT7 (Ullmer et al., 1995).
A number of studies have reported that a mixed population of 5-HT1-like and 5-HT2 receptors mediate 5-HT-induced contraction in human blood vessels including umbilical, coronary, basilar, pulmonary and pial arteries (Connor et al., 1989; Cortijo et al., 1997; Hamel et al., 1993; Kaumann et al., 1994; MacLennan et al., 1989; Parsons et al., 1989). Responses mediated by 5-HT1-like receptors in rabbit (Choppin & O'Connor, 1993; De La Lande, 1992; MacLennan & Martin, 1992; Movahedi & Purdy, 1997; Yildiz & Tuncer 1995a,1995b) and bovine pulmonary arteries (Sweeny et al., 1995) can be ‘uncovered' or enhanced following concomitant exposure to other vasoactive agents such as thromboxane or prostaglandin F2α. This unmasking of contractile 5-HT1 receptors was first reported in guinea-pig iliac artery (Sahin-Erdemli et al., 1991). Furthermore, it has been shown that in human coronary (Cocks et al., 1993) and mammary arteries (Yildiz et al., 1996), the 5-HT1B/1D selective agonist, sumatriptan, elicits poor contractions or failed to show any contractile responses when applied alone, whereas 5-HT contracts these vessels. However, when active force is induced in those vessels with other vasoactive agents, sumatriptan-induced contractions are significantly enhanced. This phenomenon has been related to an increase in the mobilization of intracellular calcium (Young et al., 1986; Cocks et al., 1993), as a result of the first vasoconstrictor agent priming the tissue such that a response to the 5-HT1-like agonist can be initiated.
5-HT is a potent vasoconstrictor agent of human umbilical blood vessels (HUA) (Altura et al., 1972; Reiffenstein & Triggle, 1974), and a preliminary characterization of the receptors mediating the 5-HT-induced contraction of the HUA suggests an involvement of both 5-HT1-like and 5-HT2 receptors (McLennan et al., 1989). However, the particular subtypes of the receptors present in this artery are unknown.
Our studies were performed to characterize the 5-HT1- and 5-HT2- receptor subtypes mediating 5-HT- induced contractions in the HUA. We have studied the effects of α-methyl-5-hydroxytryptamine (α-Me-5-HT, non-selective 5-HT2 receptor agonist), sumatriptan and 5-nonyloxytryptamine (5-HT1B/1D receptor agonists) and 5-HT itself. In our pharmacological studies to characterize the 5-HT receptor populations in HUA, the 5-HT1B/1D receptor antagonist, GR127935, was utilized, as well as the 5-HT2 antagonists ketanserin, spiperone, pirenperone, cyproheptadine, rauwolscine and also 1-(m-chlorphenyl) piperazine (m-CPP), the latter possessing both agonist and antagonist activity (Hoyer et al., 1994; Baxter et al., 1995). Our results suggest that the 5-HT-induced contraction in the HUA is mediated by 5-HT1B and 5-HT2A receptor subtypes, and this conclusion is supported by our reverse transcriptase-polymerase chain reaction (RT–PCR) data utilizing primers designed to amplify human cDNA sequences for 5-HT1B, 5-HT1D and 5-HT2A receptors.
In the present study, the new 5-HT receptor nomenclature (Hartig et al., 1996) is used. The rat 5-HT1B and human 5-HT1Dβ receptor subtypes are classified together as 5-HT1B and the human 5-HT1Dα receptor subtype as 5-HT1D. Based on pharmacological and molecular biological analysis, there is increasing evidence that the 5-HT1B (formerly 5-HT1Dβ) receptor subtype is the most common 5-HT1 receptor subtype found in blood vessels (Skingle et al., 1996). Thus, the term 5-HT1B is used in the present study to refer to the 5-HT1 receptor subtype in the HUA since it has been characterized by both pharmacological and molecular biological analysis.
Methods
Human umbilical artery tissue
Umbilical cords were cut from the placenta as soon as possible after delivery but normally within 1 h. The cord was placed in oxygenated (95% oxygen/ 5% carbon dioxide) physiological salt solution, PSS (composition, mM: NaCl 118; KCl 4.7; CaCl2 2.5; KH2PO4 1.2; MgSO4 1.2; NaHCO3 12.5; glucose 11.1) at 4°C. Arteries were dissected free of surrounding Wharton's jelly and tested the same day or stored at 4°C and studied the next day. Previous studies (Xie & Triggle, 1994a) have shown that overnight storage at 4°C does not affect the contractile responsiveness of the HUA. Six to ten rings, 3–5 mm in length, obtained from the same cord were carefully suspended in 25 ml organ baths containing PSS with or without 3 μM indomethacin at 37°C bubbled with 95% oxygen/5% carbon dioxide under a preload of 2 g. In all experiments tissues were equilibrated for 3–4 h prior to any experimental protocols with bath solution being changed every 30 min. Isometric force was monitored using Grass FT-03 transducers and a Grass model 7D polygraph. In a previous study (Xie & Triggle, 1994b) it was reported that, utilizing 95% O2/5% CO2, the endothelium did not modulate the responsiveness of the HUA to 5-HT. Thus, in the current study the endothelium was not removed.
Concentration-response curves to 5-HT receptor agonists and antagonists
After the equilibration period, the rings were contracted with a high concentration of KCl (50 mM). After a further 1 h period of recovery, with repeated washing every 15 min, tissues were challenged with 5-HT, α-Me-5-HT or m-CPP. Cumulative concentration-response curves to agonists were constructed by increasing the bathing solution concentration by 0.5 log10 increments at intervals when the preceding response had reached a plateau, approximately 3 min for all agonists. In the experiments with sumatriptan and 5-nonyloxytryptamine, responses were obtained after the tissue had been treated with a subthreshold concentration of KCl (15–25 mM). The subthreshold concentration had been assessed for each ring preparations by prior testing. In order to study 5-HT2 receptor-mediated contraction in the absence of 5-HT1 receptor activation, indomethacin was added to the PSS where indicated in the text.
Each ring preparation was exposed to only one agonist and antagonist. In any given protocol, a cumulative concentration-response curve to a single agonist was obtained and then repeated in the presence of an antagonist. In all experiments, one HUA tissue served as a time control and was only exposed to one agonist. The antagonists were examined at a minimum of three concentrations (one concentration per tissue) and were allowed to equilibrate for 1 h with the tissue before repeating the concentration-response curve to the same agonist.
RT–PCR
Human umbilical arteries were dissected The endothelial cells were removed by gently scraping the vessel lumen with cotton swab, and frozen with liquid nitrogen. Total RNA from the arteries was isolated using the GITC-CsCl centrifugation method (Sambrook et al., 1989). Two μg of total RNA was reverse transcribed by Superscript II reverse transcriptase (Life Technologies) using an oligo-(dT) primer, and the cDNAs were amplified using specific pairs of primers. The amplification reactions were also performed with reverse transcriptase reactions where Superscript II reverse transcriptase was omitted. Primer pairs were based on known human 5-HT cDNA sequences. C1 (TCCATGCCAAT ACCAGTCTTTG, sense, 617–640) and C1′ (GACCCTGGCTCCCTATGGAT, antisense, 895–915) were designed for 5-HT2A cDNA (Saltzman et al., 1991), C2 (CCTGGAAAGTACTGCTGGTTAT, sense, 139–161) and C2′ (CGGTCCTGTTGGGCGTCTGT, antisense, 723–742) for 5-HT1B cDNA, and C3 (CCATCACCCACACCTGGAAC, sense, 339–358) and C3′ (GCTTCCCATAGAGTGAGGGT, antisense, 736–755) for 5-HT1D cDNA (Weinshank et al., 1992; Levy et al., 1992). As a positive control for the 5-HT1D subtype, the C3/C3′ primer pair was used to amplify human brain cDNA (Clontech). The amplified products were gel-purified and sequenced with the Amplitaq FS kit from Perkin Elmer. Fluorescently labelled sequencing reactions were analysed at the core DNA facility of the University of Calgary. Nucleic acid sequence analysis was performed with the MacVector software package (Oxford Molecular Group) and by connection to the National Centre for Biotechnology information at the NIH.
Data analysis
All data is reported as the mean±s.e.mean. Throughout, n values refer to the number of individual umbilical cords from which arteries were obtained. Contractile data is reported in absolute values (g). Concentration-response curves were analysed by using a logistic non-linear curve fitting program (MicroCal Origin, version 3.0, Northampton, MA, U.S.A.) from the following equation (1):
The EC50 values (agonist concentration necessary to produce a half-maximal response) are converted to the pEC50 (the negative logarithm of the mean of individual EC50) for statistical analysis.
Antagonist affinity, expressed as pA2 value, was obtained from the x-intercept of the plot of log (r−1) against log molar antagonist concentration where slope was not significantly different from unity (Arunlakshana & Schild, 1959). The ratio of EC50 estimates for each pair of curves (control and in the presence of antagonist) represents r.
pKB values were obtained for all antagonists after imposing the unity constraint on the Schild plot.
If the slope was significantly less than unity, affinity was calculated by using the lowest concentration of the antagonist. For results using GR127935 in experiments with 5-HT as the agonist, two linear regressions were fitted to the data as this was found to be a better fit than a single linear regression. The sets of points at the point of inflexion are joined (Figure 3b) to illustrate the plateau.
Figure 3.
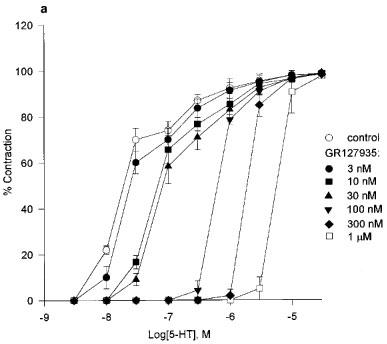
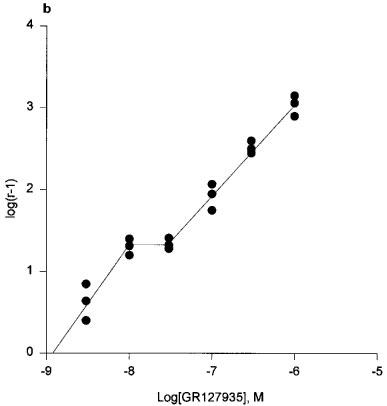
(a) Cumulative concentration-response curves to 5-HT in the presence of GR127935 (3 nM–1 μM). Each point represents the arithmetic mean±s.e.mean. of n=5 experimental determinations. (b) Schild plot constructed with the concentration-ratios from individual experiments (n=3). The sets of points at the point of inflection are joined to illustrate the ‘plateau'.
pA2 and pKB values are expressed with 95% confidence limits and slopes are expressed with standard errors. All those statistical analyses were performed using a computer program based on procedures outlined by Tallarida & Murray (1986).
Statistical differences were assessed by the use of Student's t-test and considered significant at the level of P<0.05.
Materials
The following compounds were used: 5-hydroxytryptamine creatine sulphate complex (5-HT) and 1-(m-chlorphenyl)piperazine (mCPP) from Sigma Chemicals Co. (St. Louis, MO, U.S.A.); α-methyl-5-hydroxytryptamine (α-Me-5-HT), 5-nonyloxytryptamine and ketanserin tartarate from RBI (Natick, MA, U.S.A.); sumatriptan and GR127935 were a generous gift from Glaxo Wellcome (Ware, U.K.); spiperone hydrochloride and pirenperone were gifts from Janssen (Belgium); cyproheptadine hydrochloride from DuPont Pharmaceuticals (Wilmington, DE, U.S.A.) and rauwolscine hydrochloride from Carl Roth Pharmaceuticals (Karlsruhe, Germany). 5-nonyloxytryptamine, spiperone and pirenperone solutions were made in ethanol. GR127935 was initially dissolved in a few drops of concentrated acetic acid, and then diluted in PSS. All other solutions were made in double distilled water.
Results
Effects of 5-HT, α-Me-5-HT and m-CPP in HUA
In the presence of indomethacin, 5-HT and α-Me-5-HT were both potent contractile agents in the HUA, producing a concentration-dependent increase in tone (Figure 1). 5-HT (3 nM–10 μM) produced concentration-response curves with pEC50 7.74±0.12 and slope 1.21±0.05 (n=15). Concentration-response curves to α-Me-5-HT (3 nM–10 μM) gave pEC50 8.04±0.06 and slope 0.67±0.19 (n=9). In the HUA m-CPP (0.1–10 μM) failed to show any agonist activity (n=7).
Figure 1.
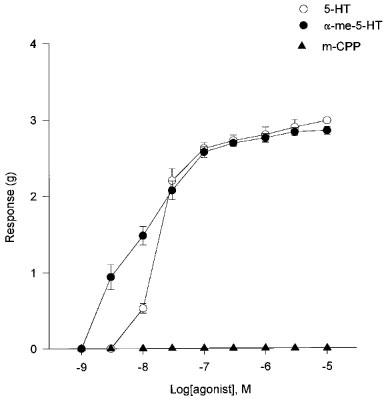
Concentration-response curves for 5-HT, α-Me-5-HT and m-CPP in the presence of indomethacin in the HUA. Each point of the graph represents the mean±s.e.mean. (n=7–15).
Effects of sumatriptan and 5-nonyloxytryptamine in HUA
This set of experiments was carried out in the absence of indomethacin. In the absence of another vasoconstrictor, sumatriptan and 5-nonyloxytryptamine initiated tone in only three of ten and two of ten vessels, respectively. Preliminary studies showed that if the tissues were treated with a contractile subthreshold concentration of KCl, prostaglandin F2α, U44069, histamine or 5-HT then the 5-HT1B receptor agonists sumatriptan and 5-nonyloxytryptamine contracted all vessels. Under these conditions (absence of indomethacin), the 5-HT concentration response curves became biphasic (see Figure 3a). In the present study the sumatriptan and 5-nonyloxytryptamine (Figure 2) elicited concentration-dependent contractions are presented for tissues pretreated with a subthreshold concentration of KCl (15–25 mM). Concentration-response curves to sumatriptan (3 nM–10 μM) were steeper than those to 5-HT with midpoint slope 2.6±0.27 and pEC50 7.21±0.16 (n=13). 5-nonyloxytryptamine gave pEC50 7.67±0.19 and slope 1.26±0.09 (n=12).
Figure 2.

Concentration-response curves for sumatriptan and 5-nonyloxytryptamine without the presence of indomethacin in the HUA. Each point of the graph represents the mean±s.e.mean. (n=12–13).
Effects of GR127935 on agonist-induced contractions
In the present study, we have evaluated the effect of the selective 5-HT1B/1D receptor antagonist, GR127935, on 5-HT induced contractions, as well as sumatriptan- and 5-nonyloxytryptamine-induced contractions of the HUA in the absence of indomethacin.
Application of GR127935 (3 nM–1 μM) evoked a rightward displacement of the second response curve to 5-HT with no attenuation of the maximum response to 5-HT (n=7), consistent with competitive antagonism (Figure 3a). GR127935 displaced the concentration response curves of 5-HT in a non-parallel manner. Schild analysis of the entire data yielded a pA2 estimate of 9.58 [8.91–10.25] and a Schild slope parameter of 0.78±0.13. In Figure 3b, we have presented Schild plots based on six different concentrations of GR127935 vs 5-HT with data points from tissues obtained from three different umbilical cords (each point represents the mean value of one cord), and these data suggest a two receptor site model rather than one site. Therefore, Schild regression was applied separately to the two lower, and to the four higher concentrations of GR127935. This yielded two slopes, the first one was not different from unity (slope 1.01) but the second component had a slope that was less than unity (slope 0.85). Therefore, a high affinity estimate for GR127935 was obtained with the lowest concentration, pKB 8.90 [8.18–9.63]. However, in contrast to the Schild plot seen for GR127935 against 5-HT, in the studies of GR127935 against sumatriptan (n=5) and 5-nonyloxytryptamine (n=3) the inhibition curves were monophasic. GR127935 (1–100 nM) caused a rightward displacement of the sumatriptan response curve with pA2 value 9.15[8.43–9.88] and Schild slope 0.87±0.18 (Figure 4). GR127935 also produced displacement of the 5-nonyloxytryptamine concentration-response curve with pA2 of 9.69[7.91–11.47] and slope 0.80±0.27 (Figure 5). When Schild slopes for sumatriptan and 5-nonyloxytryptamine were constrained to unity, estimated pKB values were 9.01[8.63–9.39] and 9.02[7.98–10.06], respectively.
Figure 4.

Cumulative concentration-response curves to sumatriptan in the 1, 10 and 100 nM of GR127935. Each point represents the arithmetic mean±s.e.mean. of n=5 experimental determinations.
Figure 5.

Cumulative concentration-response curves to 5-nonyloxytryptamine in the presence of 1, 10 and 100 nM of GR127935. Each point represents the arithmetic mean±s.e.mean. of n=3 experimental determinations.
Effects of ketanserin on 5-HT1B/1D and 5-HT2A agonist-induced contractions
Ketanserin (1 nM–1 μM) in the presence of indomethacin produced a concentration related shift to the right of the 5-HT concentration-response curve with a pA2 of 8.67[7.95–9.40] and slope 0.86±0.14. When slope was constrained to unity, pKB was 8.56[8.00–9.12] (n=10).
Antagonism by ketanserin against the contractile response to sumatriptan (n=5) and 5-nonyloxytryptamine (n=5) without the presence of indomethacin was assessed. The effect of ketanserin was very weak against either sumatriptan or 5-nonyloxytryptamine, and only at 1 μM or more did ketanserin produce a rightward shift of the agonist response curve. The calculated pKB values for ketanserin in the presence of sumatriptan and 5-nonyloxytryptamine were 6.39±0.41 and 5.93±0.45, respectively.
Effects of 5-HT2 receptor antagonists on 5-HT2 agonist-induced contractions
5-HT-induced responses in the HUA were examined in the presence of a variety of 5-HT receptor antagonists with addition of the indomethacin. Table 1 lists the pA2, slope and pKB values (including 95% confidence limits) for the antagonists against 5-HT-induced contractions. Several compounds which are known to act as 5-HT2A receptor antagonists including spiperone, cyproheptadine and pirenperone (Hoyer et al., 1994) were highly potent antagonists in the HUA. Spiperone in the concentrations 1 nM–1 μM had effects similar to those of ketanserin on 5-HT-induced contractions. Cyproheptadine and pirenperone induced a significant rightward shift of the concentration-response curve to 5-HT. Rauwolscine in concentrations up to 1 μM did not influence the contraction to 5-HT, however, at concentrations of rauwolscine ⩾1 μM, a competitive antagonism was noted and a pKB of 6.67 obtained. In the HUA, m-CPP (0.1–10 μM) acted in a non-competitive manner, with pA2 value of 7.10[6.64–7.56] and slope 0.53±0.19.
Table 1.
Summary of pA2, pKB and slope values for 5-HT2 receptor antagonists in HUA in the presence of indomethacin

RT–PCR
The C1 and C1′ primer pair, the C2 and C2′ primer pair, and the C3 and C3′ primer pair were expected to amplify a 298 bp fragment product of 5-HT2A, a 603 bp fragment product of 5-HT1B and a 416 bp fragment product of 5-HT1D, respectively. Following the RT–PCR protocol as shown in Figure 6, a ∼300 bp band was observed for 5-HT2A, and a ∼600 bp band for 5-HT1B, only in the presence, but not in the absence of Superscript II reverse transcriptase. As for 5-HT1D, no product band was visible in either the presence or absence of Superscript II reverse transcriptase, but a band of ∼400 bp was observed with human brain cDNA as a positive control. Sequencing confirmed the identity of the above-described amplified fragments with 5-HT2A, 5-HT1B and 5-HT1D (data not shown).
Figure 6.
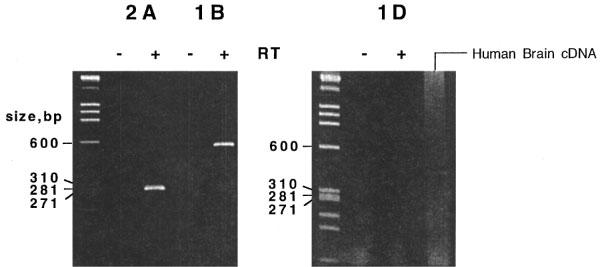
RT–PCR results. Two μg of total RNA from human umbilical artery was reverse transcribed using oligo-(dT) either with (+) or without (−) reverse transcriptase, and the cDNAs were amplified by PCR using pairs of primers C1 C1′ (5-HT2A); C2 C2′ (5-HT1B) and C3 C3′ (5-HT1D). Human brain cDNA was amplified with the primer pair C3, C3′. Products were separated by polyacrylamide (5%) gel electrophoresis and visualized by ethidium bromide staining. HaeIII-digested ΦX-174 RF DNA molecular size markers of indicated size (Pharmacia) were run in lanes on the left of each gel.
Discussion
The aim of this study was to pharmacologically characterize the 5-HT receptors that are involved in mediating the vasoconstrictor response to 5-HT in the HUA. It has been previously suggested that a mixed population of 5-HT1-like and 5-HT2 receptors mediates 5-HT-induced contraction in the HUA (MacLennan et al., 1989). Using 5-HT1 and 5-HT2 receptor subtype selective ligands we have provided pharmacological evidence that 5-HT1B/1D and 5-HT2A receptor subtypes mediate 5-HT induced contractions in the HUA. In addition, the RT–PCR analysis of 5-HT receptor mRNA expression in the HUA revealed that 5-HT1B (formerly 5-HT1Bβ) and 5-HT2A receptor subtypes mRNAs are expressed in the HUA, but not 5-HT1D. Furthermore, although no priming of the tissue is required to elicit a contractile response to 5-HT, precontraction of the HUA with another vasoconstrictors unmasks a constictor response to sumatriptan in HUA tissue which are otherwise unresponsive to this 5-HT1-like receptor agonist.
Functional characterization of 5-HT1B/1D receptors involved in 5-HT-induced contraction
MacLennan et al. (1989) reported that at physiological pO2 5-HT2 receptors almost exclusively mediate contractions induced by 5-HT and high pO2 unmasks a previously quiescent population of 5-HT1-like receptors in the HUA. It has also been shown in the HUA that the thromboxane A2 receptor antagonism, or blockade of its synthesis, selectively attenuates oxygen-induced contractions, suggesting that the elevation of tone induced by high oxygen tension is mediated by the release of endogenous thromboxane A2 (Templeton et al., 1991). In some of our experimental protocols, indomethacin was included in order to study the responsiveness to 5-HT in the absence of thromboxane-induced tone. However, indomethacin was excluded in the protocols designed to study the contractions of 5-HT1 receptors, as it has been reported that a functional cyclo-oxygenase is required in order to demonstrate a response following 5-HT1 receptor activation (MacLennan et al., 1989).
In our experiments, the HUA contracted to selective 5-HT1B/1D receptor subtype agonists (sumatriptan and 5-nonyloxytryptamine) only after exposure (priming) of the tissue to a contractile subthreshold concentration of another vasoactive agent. A number of recent studies have demonstrated the ‘unmasking' of 5-HT1-like receptors mediated responses in the rabbit renal, ear and iliac arteries as well as in the guinea-pig iliac artery (Choppin & O'Connor, 1993; De La Lande 1992; Movahedi & Purdy, 1997; Sahin-Erdemli et al., 1991; Yildiz & Tuncer, 1995a). Studies with human large coronary arteries and human internal mammary arteries have shown that threshold activation of thromboxane A2 receptors greatly amplified 5-HT1B/1D receptor mediated contraction initiated by the addition of the 5-HT1B/1D selective agonist, sumatriptan (Cocks et al., 1993; Yildiz et al., 1996). Therefore, the presence of a contractile subthreshold concentrations of prostaglandin F2α, histamine, U 44069 or elevated extracellular potassium is obligatory for contraction induced by activation of 5-HT1B/1D receptor subtype with agonists such as sumatriptan and 5-nonyloxytryptamine in the HUA. The cellular basis of action of this ‘priming' effect of subthreshold concentrations of one vasoconstrictor agonist on the effects of another is unknown and requires further investigation, although it has been suggested that it involves the increased mobilization of intracellular calcium (Young et al., 1986) and also involves PKC activation (Li et al., 1994). The pEC50s for sumatriptan and 5-nonyloxytryptamine could not be determined in non-pretreated vessels because of the complete lack of contractions generated under such conditions.
In vivo, however, it is conceivable that low concentrations of circulating 5-HT interacting with other vasoconstricting stimuli, such as prostaglandin or histamine, could initiate contraction of the HUA. Sumatriptan is a 5-HT1B/1D agonist (Humphrey et al., 1988) showing a degree of selectivity for the 5-HT1B/1D receptors mediating smooth muscle contraction. In rabbit, cow and dog, sumatriptan is typically 4–10 fold less potent than 5-HT as a vasoconstrictor. This has been interpreted as indicating that the vasoconstrictor effect of 5-HT is mediated mainly through 5-HT2A receptors (Frenken & Kaumann, 1984; Humphrey et al., 1988; MacLean et al., 1994; Parsons & Whalley, 1989). Similarly in human basilar and coronary arteries, sumatriptan is less potent than 5-HT (Boulanger et al., 1995; Connor et al., 1989; Parsons et al., 1989). However, a very high potency, pEC50 8.2±0.2, even higher than that of 5-HT, for sumatriptan is seen in human umbilical vein endothelial cells (Schoeffter et al., 1995). 5-nonyloxytryptamine binds at human 5-HT1B/1D receptors with about five times higher affinity than sumatriptan, Ki=1.2 and 5.5 nM, respectively (Glennon et al., 1994). The pEC50 values that we have obtained in the HUA for 5-HT, sumatriptan and 5-nonyloxytryptamine (7.74, 7.21 and 7.67, respectively) are thus comparable with the published literature and consistent with their action at 5-HT1B/1D receptors. Of interest also is that we did not observe an endothelium-dependent component of the effects of 5-HT on the HUA indicating a difference between the HUA and the human umbilical vein.
Antagonism of the vasoconstrictor response to 5-HT by GR127935
A series of piperazinylbenzanilide derivatives with high affinity for and antagonist activity at 5-HT1B/1D receptors has been described (Clitherow et al., 1994). One such derivative is GR127935. In human umbilical vein endothelial cells at 1 nM, GR127935 produced a dramatic rightward shift of the 5-HT curve with reduction of the maximal effect, and an apparent pKB of 10.8 (Schoeffter et al., 1995). A similar non-competitive antagonism by GR127935 has been noted in the dog basilar artery against sumatriptan (Skingle et al., 1996). In the canine coronary artery, GR127935 competitively inhibited sumatriptan-induced contractions (pA2 10.03) while 5-HT induced contractions were non-competitively inhibited (Terrón, 1996). In the rabbit coronary artery, the apparent pA2 value with GR127935 was 8.92 (Ellwood & Curtis, 1997). A pKi of 8.5 (Skingle et al., 1996) has been reported for GR127935 in guinea-pig striatum.
The receptor antagonist, GR127935, was the only 5-HT1B/1D antagonist used in this study and demonstrated competitive antagonism. An antagonist inhibition curve was used to see if the nature of inhibition of 5-HT-induced contraction by GR127935 was consistent with responses being mediated via a single or a heterogenous receptor population. These experiments were conducted without indomethacin so as to allow activation of all 5-HT receptor subtypes in the HUA (MacLennan et al., 1989). The Schild analysis of the GR127935 data revealed a pronounced flattening of the Schild plot resulting in a shallow Schild slope (Figure 3b), consistent with a heterogenous receptor population (Kenakin, 1982). The calculated pKB of the high affinity component (8.90) was comparable to that expected for GR127935 at the 5-HT1B/1D receptor subtype. Moreover, high affinity estimates were obtained for GR127935 against sumatriptan and 5-nonyloxytryptamine contractions in the HUA (pKB 9.01 and 9.02, respectively). These results are in accordance with responses mediated via the activation of the 5-HT1B/1D receptor subtype. In addition, ketanserin, against selective 5-HT1B/1D agonists sumatriptan and 5-nonyloxytryptamine behaved as a weak antagonist, yielding pKB values 6.39 and 5.93, respectively. These low affinity estimates for ketanserin are in agreement with its low affinity for human cloned 5-HT1B/1D receptors (Bard et al., 1996). Furthermore, vascular 5-HT1B/1D receptor mediated contraction has been detected in some blood vessels under unusual experimental circumstances, namely in vessels precontracted with one of several agonists such as histamine, angiotensin II or prostaglandin F2α (Choppin & O'Conner, 1993; Yildiz & Tuncer, 1995b). Such data are in an agreement with our detection of 5-HT1B/1D receptors only after pretreatment with subthreshold concentrations of a vasopressor but we again emphasize that in the in vivo setting activation of 5-HT1B/1D receptors may contribute to the vasoconstrictor response of the HUA to 5-HT. Our results are comparable to previous published data wherein it has been demonstrated in a variety of vascular preparations, for instance, rabbit blood vessels of the following origin: renal (Choppin & O'Conner, 1993), femoral (MacLennan & Martin, 1992), iliac (Yildiz & Tuncer, 1993) and ear (De la Lande, 1992) and also human coronary (Cocks et al., 1993). Collectively, these results indicate that, under appropriate conditions, activation of the 5-HT1-like receptor in vascular tissue, including the HUA, can result in a significant vasoconstriction and, thus, reduction in blood flow.
Role of 5-HT2A receptors involved in 5-HT-induced contraction
α-Me-5-HT possesses a high affinity for 5-HT2 receptors (Baxter et al., 1995). In our experiments, α-Me-5-HT showed greater potency than 5-HT (pEC50 8.04) thus indicating the likely involvement of 5-HT2 receptors in mediating vasoconstriction in the HUA. The slope value for α-Me-5-HT was 0.67, whereas that for 5-HT was 1.21 and this was surprising given the stated selectivity of α-Me-5-HT for 5-HT2 receptors (Baxter et al., 1995).
The effects of a number of 5-HT2 receptor antagonists on 5-HT-induced contraction in the presence of indomethacin were also investigated. Ketanserin, originally developed as a 5-HT2 receptor antagonist, is 1000 fold more selective for the 5-HT2A receptor compared to the 5-HT2B receptor and is, therefore, a useful pharmacological probe to discriminate between these two receptor subtypes (Leyson et al., 1982). Ketanserin showed a competitive antagonism against 5-HT with a pKB value 8.56 indicating the presence of 5-HT2A receptor subtypes in addition to the 5-HT1B/1D subtypes. Pirenperone and cyproheptadine were also used in our experiments and are relatively selective for the 5-HT2A receptor with affinities for 5-HT2C binding sites that have been reported to be 4–10 fold lower than for 5-HT2A (Hoyer et al., 1994). The two antagonists shifted the response curves to 5-HT in the HUA in a rightward and parallel fashion with pKB values of 9.69 and 9.30 respectively. Spiperone is also reasonably selective for the 5-HT2A receptor with an affinity for 5-HT1 approximately 80 fold less than for the 5-HT2 (Leyson et al., 1982; Wainscott et al., 1993). Spiperone also competitively inhibited the concentration response curves to 5-HT in the HUA with a pKB of 9.06.
The 5-HT2B receptor has a low affinity for compounds like spiperone, cinanserine and ketanserin, whereas 5-HT2B receptors show comparatively high affinity for yohimbine and rauwolscine (Hoyer et al., 1994). Rauwolscine has a high affinity for 5-HT2B receptor subtype (pKB 8.5) with only low affinity for 5-HT2A or 5-HT2C receptors (Clineschmidt et al., 1985; Hoyer, 1989). Rauwolscine in concentrations up to 1 μM did not influence the response to 5-HT in the HUA, indicating that the 5-HT2B receptor subtype is unlikely to be involved in mediating contractions, and only with rauwolscine concentrations of 1 μM or greater was inhibition of 5-HT mediated contractions observed (pKB of 6.67).
Among the many mixed non-tryptamine agonists that act at 5-HT2B and 5-HT2C receptors m-chlorphenylpiperazine is particularly interesting. It is now evident that mCPP acts as a partial agonist at both 5-HT2B and 5-HT2C receptors, and furthermore possesses much lower efficacy at 5-HT2A receptors, usually displaying only antagonist activity (Baxter et al., 1995). We found that mCPP had no direct agonist activity in the HUA but non-competitively inhibited 5-HT-induced contraction in HUA, thus supporting our other data that suggests the presence of the 5-HT2A receptor subtype, together with the 5-HT1B/1D, in the HUA, and that these receptor subtypes are responsible for mediating the vasoconstrictor response to 5-HT.
mRNA expression for 5-HT1B, 5-HT1D and 5-HT2A receptors in HUA
As previously noted, only five G-protein coupled 5-HT receptor mRNAs have been shown to be expressed in blood vessels (5-HT1B, 5-HT2A, 5-HT2B, 5-HT4 and 5-HT7) (Ullmer et al., 1995). The human 5-HT2A receptor gene has been cloned (Saltzman et al., 1991). Two distinct genes coding for 5-HT1D receptors have been cloned and the corresponding gene products named 5-HT1D (previously designated 5-HT1Dα) and 5-HT1B (previously designated 5-HT1Dβ) exhibit a very similar pharmacology (Hartig et al., 1996). Although the mRNAs for both human 5-HT1B and 5-HT1D receptors have been found in the brain, only 5-HT1B receptor mRNA was detected in cerebral and temporal arteries (Hamel et al., 1993; Verheggen et al., 1998). Using specific primers for human 5-HT1B and 5-HT1D receptor genes (Weinshank et al., 1992), as well as 5-HT2A (Saltzman et al., 1991), signals for 5-HT1B and 5-HT2A receptor mRNAs were clearly present in the HUA and that for the 5-HT1D receptor, mRNA was not detectable. Similar observations have been made in other blood vessels (Hamel et al., 1993; Schoeffter et al., 1995; Ullmer et al., 1995). It is recognized in studies of both human and other mammalian species that the presence of mRNA does not necessarily infer expression of the functional protein.
Conclusion
Collectively these data indicate that the contractile response of the HUA to 5-HT is mediated by a heterogenous populations of 5-HT1B and 5-HT2A receptor subtypes. Since the 5-HT1B/D ligands used in this study are non-selective in their ability to distinguish 5HT1B and 5-HT1D receptors, the RT–PCR analysis was performed and 5-HT1B receptor mRNA was identified. Furthermore, contractions mediated by 5-HT1B receptors can be ‘uncovered' by exposure to other vasoactive agents at low concentrations which activate 5-HT2A receptors and result in the ‘unmasking' of a contractile response mediated via 5-HT1B receptors, suggesting that the 5-HT1B receptor may significantly contribute in the in vivo setting to the vasoconstrictor response to 5-HT.
Acknowledgments
The support of the Heart & Stroke Foundation of Alberta (to C.R. Triggle) is gratefully acknowledged. We would like to thank Glaxo Wellcome Inc., Janssen Du Pont Pharmaceutical Co. and Carl Roth for their generous donation of drugs, the staff of the delivery room at the Foothills Hospital, Calgary for their cooperation, and Ms Nadine Magoski for the technical assistance with some of the experiments.
Abbreviations
- m-CPP
1-(m-chlorphentl)piperazine
- HUA
human umbilical artery
- 5-HT
5-hydroxytryptamine
References
- ALTURA B.M., MALAVIYA D., REICH C.F., OARKIN L.R. Effects of vasoactive agents on isolated human umbilical arteries and veins. Am. J. Physiol. 1972;222:345–355. doi: 10.1152/ajplegacy.1972.222.2.345. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonism. Br. J. Pharmacol. Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARD J.A., KUCHAREWICH S.A., ZGOMBICK J.M., WEINSHANK R.L., BRANCHEK T.A., COHEN M.L. Difference in ligand binding profiles between cloned rabbit and human 5-HT1Dα and 5-HT1Dβ receptors: ketanserin and methiotepin distinguish between rabbit 5-HT1D receptor subtypes. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;354:237–244. doi: 10.1007/BF00171053. [DOI] [PubMed] [Google Scholar]
- BAXTER G., KENNETT G., BLANEY F., BLACKBURN T. 5-HT2 receptor subtypes: a family re-united. TiPS. 1995;16:105–110. doi: 10.1016/s0165-6147(00)88991-9. [DOI] [PubMed] [Google Scholar]
- BOULANGER C.M., LONGMORE J., DESTA B., SCHOFIELD W., HILL R.G., TAYLOR A.A. Further studies on the response of human coronary arteries to the 5-HT1D receptor agonists sumatriptan and MK-462. Br. J. Pharmacol. 1995;116:38P. [Google Scholar]
- CHOPPIN A., O'CONNER S.E. Pre-contraction with histamine and U46619 unmasks a 5-HT1 like receptor in rabbit renal artery. Eur. J. Pharmacol. 1993;231:469–472. doi: 10.1016/0014-2999(93)90126-3. [DOI] [PubMed] [Google Scholar]
- CLINESCHMIDT B.V., REISS D.R., PETTIBONE D.J., ROBINSON J.L. Characterization of 5-hydroxytryptamine receptors in rat stomach fundus. J. Pharmacol. Exp. Ther. 1985;235:696–708. [PubMed] [Google Scholar]
- CLITHEROW J.W., SCOPES D.I., SKINGLE M., JORDAN C.C., FENIUK W., CAMPBELL I.B., CARTE M.C., COLLINGTON E.W., CONNOR H.E., HIGGINS G.A., BEATTIE D., KELLY H.A., MITCHELL W.L., OXFORD A.W., WADSWORTH A.H., TYERS M.B. Evolution of a novel series of [(N, N-dimethylamino)propyl] and piperazinylbenzanilides as the first selective 5-HT1D antagonists. J. Med. Chem. 1994;37:2253–2257. doi: 10.1021/jm00041a001. [DOI] [PubMed] [Google Scholar]
- COCKS T.M., KEMP B.K., PRUNEU D., ANGUS J.A. Comparation of contractile responses to 5-hydroxyrtyptamine and sumatriptan in human isolated coronary artery: synergy with thromboxane A2-receptor agonist U46619. Br. J. Pharmacol. 1993;110:360–369. doi: 10.1111/j.1476-5381.1993.tb13818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR H.E., FENIUK W., HUMPHREY P.P.A. 5-hydroxytryptamine contracts human coronary arteries predominantly via 5-HT2 receptor activation. Eur. J. Pharmacol. 1989;161:91–94. doi: 10.1016/0014-2999(89)90184-2. [DOI] [PubMed] [Google Scholar]
- CORTIJO J., CABRERA M.M., BARNABEU E., DOMENECH T., BOU J., FERNANDEZ A.G., BELETA J., PALACIOS J.M., MORICELLO E. Characterization of 5-HT receptors on human pulmonary artery and vein: functional and binding studies. Br. J. Pharmacol. 1997;122:1455–1463. doi: 10.1038/sj.bjp.0701509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LA LANDE I.S. Evidence for a 5-HT1 like receptor mediating the amplifying action of 5-HT in the rabbit ear artery. Br. J. Pharmacol. 1992;106:550–555. [PMC free article] [PubMed] [Google Scholar]
- ELLWOOD A., CURTIS M.J. Involvement of 5-HT1B/1D and 5-HT2A receptors in 5-HT-induced contraction of endothelium-denued rabbit epicardial coronary arteries. Br. J. Pharmacol. 1997;122:875–884. doi: 10.1038/sj.bjp.0701470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENIUK W., HUMPHREY P.P.A.In {it}Serotonin: Actions, Receptors, Patophysiology 1989London, U.K.: Macmillan Press; 100–122.Eds. Mylecharane, E.J., Angus, J.A., De La Laude, I.S. & Humphrey, P.P.A. pp [Google Scholar]
- FRENKEN M., KAUMANN A.J. Interaction of ketanserin and its metabolite ketanserinol with 5-HT2 receptors in the pulmonary and coronary arteries of calf. Naunyn-Schmideberg's Arch. Pharmacol. 1984;326:334–339. doi: 10.1007/BF00501438. [DOI] [PubMed] [Google Scholar]
- GLENNON R.A., HONG S., DUKAT M., TIETLER M., DAVIS K. 5-(Nonyloxy)tryptamine: A novel high-affinity 5-HT1Dβ serotonin receptor agonist. J. Med. Chem. 1994;37:2828–2830. doi: 10.1021/jm00044a001. [DOI] [PubMed] [Google Scholar]
- HAMEL E., LINVILLE D., YING V., VILLEMURE J.G., CHIA L.S. Exspression of mRNA for the serotonin 5-hydroxytryptamine 1Dβ receptor subtype in human and bovine cerebral arteries. Mol. Pharmacol. 1993;44:242–246. [PubMed] [Google Scholar]
- HARTIG P.R., HOYER D., HUMPHREY P.P.A., MARTIN G.R. Alignment of receptor nomenclature with the human genome: classification of the 5-HT1B and 5-HT1D receptor subtypes. Trends Pharmacol. Sci. 1996;17:103–105. doi: 10.1016/0165-6147(96)30002-3. [DOI] [PubMed] [Google Scholar]
- HOYER D.5-Hydroxytryptamine receptors and effector coupling mechanisms in peripheral tissues The peripheral actions of 5-hydroxytryptamine 1989Oxford: University Press; 72–99.ed. Fozard, J. pp [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P.A. VII. International Union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- HUMPHREY P.P.A., FENIUK W., PERREN M.J., CONNOR H.E., OXFORD A.W., COATES J.H., BUTINA D. GR 43175 a selective agonist for the 5-HT1 like receptor on dog saphenous vein. Br. J. Pharmacol. 1988;94:1123–1132. doi: 10.1111/j.1476-5381.1988.tb11630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J., FRENKEN M., POSIVAL H., BROWN A. Variable participation of 5-HT1 like receptors and 5-HT2 receptors in serotonin-induced contraction of human isolated coronary arteries. Circulation. 1994;90:1141–1153. doi: 10.1161/01.cir.90.3.1141. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.P. The Schild regression in the process of receptor classification. Can. J. Physiol. Pharmacol. 1982;60:249–265. doi: 10.1139/y82-036. [DOI] [PubMed] [Google Scholar]
- LEVY F.O., GUDERMAN T., PEREZ-REYES E., BIRNBAUMER M., KAUMANN A.J., BIRNBAUMER L. Molecular cloning of a human serotonin receptor (S12) with a pharmacological profile resembling that of the 5-HT1D subtype. J. Biol. Chem. 1992;267:7553–7562. [PubMed] [Google Scholar]
- LEYSON J.E., NIEMAGEERS C.J., VAN NUETEN J.M., LAUDRON P.M. [3H]ketanserin ( R41468) a selective [3H] ligand for serotonin 2 receptor binding sites. Mol. Pharmacol. 1982;21:301–314. [PubMed] [Google Scholar]
- LI X-F., ALLEN B.G., WALSH M.P., TRIGGLE C.R.Tension development, myosin light chain phosphorylation and change in intracellular free [Ca2+]i induced by α1 and α2-adrenoreceptor agonists The resistance arteries 1994N. Jersey: Human Press; 31–41.eds. Halpern, W., Bevan, J., Brayden, J., Dustan, H., Nelson, M. & Osol G. pp [Google Scholar]
- MACLEAN M.R., CLAYTON R.A., HILLIS S.W., MCINTYRE P.D., PEACOCK A.J., TEMPLETON A.G.B. 5-HT1-receptor mediated vasoconstriction in bovine isolated pulmonary arteries: influence of vascular endothelium and tone. Pulm. Pharmacol. 1994;7:65–72. doi: 10.1006/pulp.1994.1007. [DOI] [PubMed] [Google Scholar]
- MACLENNAN S.J., MARTIN G.R. Effect of thromboxane A2- mimetic U 46619 on 5-HT1 like and 5-HT2 receptor-mediated contraction of the rabbit isolated femoral artery. Br. J. Pharmacol. 1992;107:418–421. doi: 10.1111/j.1476-5381.1992.tb12761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLENNAN S.J., WHITTLE M.J., MCGRATH J.C. 5-HT1-like receptors requiring functional cyclo-oxygenase and 5-HT2 receptors independent of cyclo-oxygenase mediate contraction of the human umbilical artery. Br. J. Pharmacol. 1989;97:921–933. doi: 10.1111/j.1476-5381.1989.tb12033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN G.R. Vascular receptors for 5-hydroxytryptamine: distribution, function and classification. Pharmacol. Ther. 1994;62:283–324. doi: 10.1016/0163-7258(94)90048-5. [DOI] [PubMed] [Google Scholar]
- MOVAHEDI H., PURDY R.E. Pharmacological characterization of the silent 5-hydroxytryptamine1B-like receptors of rabbit ear artery. J. Pharmacol. Exp. Ther. 1997;288:653–660. [PubMed] [Google Scholar]
- MYLECHARANE E.J. Mechanisms involved in serotonin-induced vasodilatation. Blood Vessels. 1990;27:116–126. doi: 10.1159/000158802. [DOI] [PubMed] [Google Scholar]
- PARSONS A.A., WHALLEY E.T. Evidence for the presence of 5-HT1-like receptors in rabbit isolated basilar arteries. Eur. J. Pharmacol. 1989;174:189–196. doi: 10.1016/0014-2999(89)90311-7. [DOI] [PubMed] [Google Scholar]
- PARSONS A.A., WHALLEY E.T., FENIUK W., CONNOR H.E., HUMPHREY P.P.A. 5-HT1-like receptors mediate 5-hydroxytryptamine-induced contraction of human isolated basilar artery. Br. J. Pharmacol. 1989;96:434–440. doi: 10.1111/j.1476-5381.1989.tb11835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REIFFENSTEIN R.J., TRIGGLE C.R. Cocaine-induced supersensitivity in the human umbilical artery. Can. J. Physiol. Pharmacol. 1974;52:687–698. doi: 10.1139/y74-088. [DOI] [PubMed] [Google Scholar]
- SAHIN-ERDEMLI I., HOYER D., STOLL A., SEILER M.D., SCHOEFFTER D. 5-HT1-like receptors mediate 5-hydroxytryptamine-induced contraction of guinea pig iliac artery. Br. J. Pharmacol. 1991;102:386–390. doi: 10.1111/j.1476-5381.1991.tb12183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTZMAN A.G., MORSE B., WHITMAN M.M., IVANSHCHENKO Y., JAYE M., FELDER S. Cloning of the human serotonin 5-HT2 and 5-HT1C receptor subtypes. Biochem. Biophys. Res. Comm. 1991;181:1469–1478. doi: 10.1016/0006-291x(91)92105-s. [DOI] [PubMed] [Google Scholar]
- SAMBROOK J., FRITSCH E.F., MANIATIS T.Extraction, purification, and analysis of messenger RNA from Eucaryotic cells Molecular Cloning 1989New York: Cold Spring Harbor Laboratory Press; 7.1–7.3.2nd Edition. pp [Google Scholar]
- SCHOEFFTER P., ULLMER C., GUTIERREZ M., WEITZ-SCHMIDT G., LÜBBERT H. Functional serotonin 5-HT1D receptors and 5-HT1Dβ receptor mRNA expression in human umbilical vein endothelial cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:580–582. doi: 10.1007/BF00169394. [DOI] [PubMed] [Google Scholar]
- SKINGLE M., BEATTIE D.T., SCOPES D.I., STARKEY S.J., CONNOR H.E., FENIUK W., TYERS M.B. GR127935: a potent and selective 5-HT1D receptor antagonist [Review] Behav. Brain Res. 1996;73:157–161. doi: 10.1016/0166-4328(96)00089-7. [DOI] [PubMed] [Google Scholar]
- SWEENY G., TEMPLETON A., CLAYTON R.A., BAIRD M., SHERIDEN S., JOGNSTON E.D., MACLEAN M.R. Contractile responses to sumatriptan in isolated bovine pulmonary artery rings: relationship to tone and cyclic nucleotide levels. J. Cardiovasc. Pharmacol. 1995;26:751–760. doi: 10.1097/00005344-199511000-00012. [DOI] [PubMed] [Google Scholar]
- TALLARIDA R.J., MURRAY R.B. Manual of Pharmacologic Calculations with Computer Programs. New York: Springer-Verlag; 1986. [Google Scholar]
- TEMPLETON A.G.B., MCGRATH J.E., WHITTLE M.J. The role of endogenous thromboxane in contractions to U46619, oxygen, 5-HT and 5-CT in the human umbilical artery. Br. J. Pharmacol. 1991;103:1079–1084. doi: 10.1111/j.1476-5381.1991.tb12303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERRÓN J.A. GR127935 is a potent antagonist of the 5-HT1-like receptor mediating contraction in the canine coronary artery. Eur. J. Pharmacol. 1996;300:109–112. doi: 10.1016/0014-2999(96)00041-6. [DOI] [PubMed] [Google Scholar]
- ULLMER C., SCHMUCK K., KALKMAN H.O., LÜBBERT H. Expression of serotonin receptor mRNAs in blood vessels. FEBS Letters. 1995;370:215–221. doi: 10.1016/0014-5793(95)00828-w. [DOI] [PubMed] [Google Scholar]
- VERHEGGEN R., HUNDESHAGEN A.G., BROWN A.M., SCHINDLER M., KAUMANN A.J. 5-HT1B receptor-mediated contractions in human temporal artery: evidence from selective antagonists and 5-HT receptor mRNA expression. Br. J. Pharmacol. 1998;124:1345–1354. doi: 10.1038/sj.bjp.0701929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAINSCOTT D.B., CHOEN M.L., SCHENCK K.W., AUDIA J.E., NISSEN J.S., BAEZ M., KURSAR J.D., LUCATIES V.L., NELSON D.L. Pharmacological characteristics of the newly cloned rat 5-hydroxytryptamine2F receptor. Mol. Pharmacol. 1993;43:419–426. [PubMed] [Google Scholar]
- WEINSHANK R.L., ZGOMBICK J.M., MACCHI M.J., BRANCHEK T.A., HARTIG P.R. Human serotonin 1D receptor is encoded by a subfamily of two distinct genes 5-HT 1Dα and 5-HT 1Dβ. Proc. Natl. Acad. Sci. U.S.A. 1992;89:3630–3634. doi: 10.1073/pnas.89.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIE H., TRIGGLE C.R. The endothelium contributes to the contractile responses of the human umbilical artery to 5-hydroxytryptamine and endothelin-1 under low but not high pO2 conditions. Can. J. Physiol. Pharmacol. 1994a;72:1171–1179. doi: 10.1139/y94-166. [DOI] [PubMed] [Google Scholar]
- XIE H., TRIGGLE C.R. Endothelium-independent relaxations to acetylcholine and A23187 in the human umbilical artery. J. Vasc. Res. 1994b;31:92–105. doi: 10.1159/000159035. [DOI] [PubMed] [Google Scholar]
- YILDIZ O., TUNCER M. Characterization of 5-HT receptors in rabbit isolated iliac artery. Arch. Int. Pharmacodyn. 1993;326:72–83. [PubMed] [Google Scholar]
- YILDIZ O., CICEK S., AY I., TATAR H., TUNCER M. 5-HT1-like receptor-mediated contraction in the human internal mammary artery. J. Cardiovasc. Pharmacol. 1996;2:6–10. doi: 10.1097/00005344-199607000-00002. [DOI] [PubMed] [Google Scholar]
- YILDIZ O., TUNCER M. The amplification of responses to sumatriptan by various agonists in rabbit isolated iliac artery. J. Cardiovasc. Pharmacol. 1995a;25:508–510. doi: 10.1097/00005344-199503000-00025. [DOI] [PubMed] [Google Scholar]
- YILDIZ O., TUNCER M. 5-HT1-like and 5-HT2A receptors mediate 5-hydroxytryptamine-induced contraction of rabbit isolated mesenteric artery. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995b;352:127–131. doi: 10.1007/BF00176765. [DOI] [PubMed] [Google Scholar]
- YOUNG M.S., IWANOV V., MOULDS R.F.W. Interaction between platelet-released serotonin and thromboxane A2 on human digital arteries. Clin. Exp. Pharmacol. Physiol. 1986;13:143–152. doi: 10.1111/j.1440-1681.1986.tb00328.x. [DOI] [PubMed] [Google Scholar]


