Abstract
Nitric oxide (NO) donors prevent experimentally-induced gastric mucosal damage, but their clinical utility is limited by short duration of action or unsuitability of the pharmaceutical form employed. This study analyses the gastroprotection elicited by a clinically used mode of continuous administration of an NO donor, namely the nitroglycerin patch.
Application to rats of a transdermal patch that releases doses of nitroglycerin comparable to those used in man (40, 80, 160 and 400 ng min−1 rat−1) reduced gastric damage induced by indomethacin (25 mg kg−1, p.o. or s.c.). The nitroglycerin patch (160 ng min−1 rat−1) also diminished damage by oral administration (1 ml) of acidified bile salts (100 mg kg−1 taurocholic acid in 150 mM HCl) or 50% ethanol.
Transdermal nitroglycerin (160 ng min−1 rat−1) did not influence basal gastric blood flow, as measured by lasser-doppler flowmetry, but prevented its reduction by indomethacin.
Transdermal nitroglycerin (160 ng min−1 rat−1) prevented in vivo leukocyte rolling and adherence in the rat mesentery microvessels superfused with indomethacin, as evaluated by intravital microscopy.
The transdermal nitroglycerin patch protects the gastric mucosa from damage by mechanisms that involve maintenance of mucosal blood flow and reduction of leukocyte-endothelial cell interaction.
Keywords: Nitric oxide, nitroglycerin patch, gastric mucosal damage, gastric blood flow, leukocyte adhesion, indomethacin, NSAIDs
Introduction
There is substantial evidence that intragastric topical application or local intravascular administration of NO-releasing substances protect the gastric mucosa from damage induced by a variety of injurious agents such as ethanol (MacNaughton et al., 1989), acid (Kitagawa et al., 1990) or endothelin (López-Belmonte et al., 1993). Furthermore, intravenously administered NO-donors prevented PAF (Boughton-Smith et al., 1992) or ischaemia-reperfusion (Andrews et al., 1994) induced gastric injury. However, the clinical applicability of these experimental studies is limited by the pharmaceutical nature of the NO donors employed, in particular the short duration of action of the oral preparations or the unsuitability of the parenteral forms. These circumstances led us to test whether a clinically used, safe and effective mode of continuous administration of an NO donor, namely the transdermal nitroglycerin patch, would be useful to provide gastric mucosal protection against injury. First synthesized in 1846, nitroglycerin (glyceryl trinitrate) was shown to relieve angina in 1879 and has been used ever since for treating acute angina pectoris attacks. The sublingual route has been the classical method of administration of this drug. However, the short duration of action of the nitroglycerin thus dispensed has led to the development of alternative methods of drug delivery, of which the transdermal patch has become one of the most popular (Todd et al., 1990). The primary aim of the patch is to obtain a constant release of the drug across the skin into the systemic circulation which achieves a uniform steady-state plasma concentration of nitroglycerin and, consequently, a longer duration of action. In preliminary results we have shown that the nitroglycerin patch may reduce gastric damage induced by parenteral administration of indomethacin (Barrachina et al., 1995), and subsequently it has been reported that patients who are non steroidal anti-inflammatory drug (NSAID) users and are treated on a chronic regular basis with nitrates, either orally or transdermally, exhibit a significantly lower risk of gastrointestinal bleeding (Lanas et al., 1998). On this basis, the main objective of this study was to characterize experimentally the effects of the nitroglycerin patch on the mucosal damage induced by NSAIDs in the rat. Furthermore, in order to investigate the mechanisms responsible for such a protective action, we have also studied whether application of the nitroglycerin patch could prevent other forms of gastric injury. Likewise, the haemodynamic effects of this transdermally-administered nitrovasodilator have also been evaluated, both systemically and on the blood flow of the gastric mucosa. Finally, since leukocyte adherence to the vascular endothelium is one of the early events in the process of NSAIDs-induced mucosal injury, we have also characterized by intravital microscopy whether pretreatment with the patch could prevent indomethacin-induced leukocyte adherence to the endothelium in vivo. A preliminary account of this work has been presented in abstract form (Bello et al., 1996; Calatayud et al., 1997).
Methods
General
Male Sprague-Dawley rats (250–300 g) were deprived of food, but not water for 18–20 h before the application of a nitroglycerin patch to the dorso-cervical area of the body which had been shaved under light ether anaesthesia the previous day. Patches were cut to release doses of nitroglycerin of 40, 80, 160, 400 and 3200 ng min−1 rat−1. Control animals were treated with placebo patches (PP) without nitroglycerin, but of a similar composition and a size equal to that of the patches used for administering the highest dose of nitroglycerin employed in our experiments. All patches were maintained in place till the end of the different experimental periods. When necessary, rats were anaesthetized with sodium pentobarbitone (50 mg kg−1 i.p., 1 ml kg−1) and the trachea, right carotid artery and jugular vein were cannulated with polyethylene tubing of the appropriate size to facilitate spontaneous breathing, to measure systemic arterial blood pressure through a pressure transducer (Spectramed Stathan P-23XL) connected to a channel recorder (GRASS RPS7C8B, Quincy, MA, U.S.A.) and for additional anaesthetic administration, respectively. All experimental protocols were performed following the guidelines approved by the Ethical Committee for Experimental Research of the Faculty of Medicine of the University of Valencia.
Assessment of mucosal integrity
Nitroglycerin patches or PP were applied 30 min before the administration of the various mucosal damaging agents. The first group of rats was treated with indomethacin (25 mg kg−1; 2.5 ml kg−1 s.c. or 4 ml kg−1, p.o.) and sacrificed 3 h later. In a second set of experiments, acidified bile salts (100 mg kg−1 taurocholic acid in 150 mM HCl, 4 ml kg−1) or 50% ethanol (1 ml rat−1) were administered p.o. and animals sacrificed 10 min or 3 h later. Following cervical dislocation, the stomachs were removed, opened along the greater curvature, pinned to a wax block, coded to avoid observer bias and photographed on color transparency film. When damage was induced by indomethacin or acidified bile salts, the lengths of all macroscopic individual lesions were measured from the photographs and added to provide a total lesion length (mm) for each rat. When evaluating ethanol-induced injury the area of mucosal damage was calculated via computerized planimetry and expressed as a percentage (%) of the total area of gastric mucosa showing macroscopically-visible lesions.
Haemodynamic studies
A midline incision was performed and the anaesthetized rats had their pylorus ligated with 4–0 silk. Thereafter the stomach was opened along the greater curvature, pinned over a plexiglas platform and clamped with a plexiglas cylinder to form an ex vivo gastric chamber (MacNaughton et al., 1989; Beck et al., 1992). The mucosa was then continuously bathed (0.5 ml min−1) with 5 ml of an isotonic solution of mannitol (200 mM+50 mM HCl). Changes in gastric and dermal blood flow (GBF and DBF respectively) were determined by laser Doppler flowmetry (Oxford Array, Oxford Optronix, Oxford) as described before (Casadevall et al., 1992). In brief, a pencil probe (Array 1 OOA 036, Oxford Optronix, Oxford) was placed perpendicularly 5 mm above the glandular mucosa surface for measuring GBF and another probe was applied on a shaved abdominal area for determining DBF. Thirty minutes after application of the nitroglycerin patch (160 ng min−1 rat−1) animals received 25 mg kg−1 of indomethacin (s.c.) and mean arterial blood pressure (MABP), GBF and DBF were recorded for the following 60 min and expressed as a percentage of basal values. In other experiments, the effects of nitroglycerin (160 or 3200 ng min−1 rat−1) on these haemodynamic parameters were evaluated during 60 min in rats without indomethacin treatment.
Intravital microscopy
The experimental preparation used in this study was the same as that described previously (Wallace et al., 1993). In brief, a midline abdominal incision was made in these rats and a segment of the mid-jejunal mesentery was gently exteriorized and carefully placed over an optically clear viewing pedestal that permitted transillumination of a 2 cm2 segment of the tissue. The temperature of the pedestal was maintained at 37°C. The exposed mesentery was superfused continuously at a rate of 1 ml min−1 with a warmed bicarbonate-buffered saline (pH 7.4). An orthostatic microscope (Nikon Optiphot-2, SMZ1, Badhoevedorp, The Netherlands) equipped with a 20× objective lens (Nikon SLDW, Badhoevedorp, The Netherlands) and a 10× eyepiece permitted the visualization of the tissue. A video camera (Sony SSC-C350P, Koeln, Germany) mounted on the microscope projected the image onto a colour monitor (Sony Trinitron PVM-14N2E, Koeln, Germany), and the images were recorded for playback analysis using a video recorder (Sony SVT-S3000P, Koeln, Germany). The final magnification of the video screen was ×1300. Animal temperature was monitored using a rectal electrothermometer and kept at 37°C with an infrared heat lamp. All exposed tissues were moistened with saline-soaked gauze to minimize evaporation. Single unbranched mesenteric venules (20–40 μm in diameter) were selected for study and the diameter was measured on-line with a video caliper (Microcirculation Research Institute, Texas A&M University, College Station, TX, U.S.A.). The number of rolling, adherent and emigrated leukocytes was determined off-line during playback of videotaped images. A leukocyte was defined as adherent to venular endothelium if it remained stationary for a period equal to or greater than 30 s. Adherent cells were expressed as the number per 100 μm length of venule. Rolling leukocytes were defined as those white blood cells that move at a velocity less than that of erythrocytes in the same vessel. Leukocyte rolling velocity (Vwbc) was determined by the time required for a leukocyte to transverse a given distance along the length of the venule and is expressed as μm s−1. Flux of rolling leukocytes was measured as those cells that could be seen moving past a defined reference point in the vessel. The same reference point was used throughout the experiment because leukocytes may roll for only a section of the vessel before rejoining the flow of the blood or firmly adhering. The number of rolling leukocytes per 100 μm venule length was calculated as the leukocyte flux. Vwbc−1. Centreline red blood cell velocity (Vrbc) was also measured on-line by using an optical Doppler velocimeter (Microcirculation Research Institute, Texas A&M University, College Station, TX, U.S.A.). Venular blood flow was calculated from the product of mean red blood cell velocity (Vmean=centreline velocity. 1.6−1) and cross sectional area, assuming cylindrical geometry. Venular wall shear rate (γ) was calculated based on the Newtonian definition: γ=8 (Vmean. Dv−1), in which Dv is venular diameter (House & Lipowsky, 1987).
The experimental protocol was performed as follows: nitroglycerin patch or PP were applied before starting surgical procedures for intravital microscopy, the mesentery was superfused for 30 min with bicarbonate-buffer saline until stabilization and basal parameters recorded for 5 min (time 0). Thereafter, the tissue was superfused for a further 60 min period with bicarbonate-buffer saline containing indomethacin (25 μg ml−1) and the afore-mentioned parameters measured for 5 min at times 15, 30 and 60 min. The dose of indomethacin used was selected based on results of previous studies (Wallace et al., 1993).
Statistical analysis
Comparisons between groups were made by ANOVA, followed by a Tukey test. The paired or unpaired Student t-test was used when appropriate in the haemodynamic and intravital microscopic experiments. Values are expressed as mean ±s.e.mean and statistical significance was set at P<0.05.
Drugs
Placebo and nitroglycerin patches (Nitro-Dur® 15) were a gift from Schering-Plough Laboratories. Indomethacin (Sigma) was dissolved in 5% sodium bicarbonate (10 mg ml−1 s.c., 6.25 mg ml−1 p.o.). Sodium taurocholate (Sigma) was dissolved in 150 mM HCl (25 mg ml−1) and absolute ethanol (Panreac) was diluted with saline (50% v v−1). Mannitol was used as the clinically available preparation (Apiroserum Manitol 20%®, Pharmacia).
Results
Gastric mucosal integrity studies
As shown in Figure 1, control animals receiving subcutaneous indomethacin (25 mg kg−1) presented gastric lesions with a total length of 30±5 mm. Pretreatment with the nitroglycerin patch (40, 80 and 160 ng min−1 rat−1) induced a dose-dependent reduction of gastric damage (respectively 30±14, 63±14 and 67±12% reduction). Higher doses of transdermal nitroglycerin (400 ng min−1 rat−1) did not induce any further protection against indomethacin induced gastric damage (60±9% reduction). Furthermore, when rats were treated with a nitroglycerin patch releasing 3200 ng min−1 rat−1 no significant protection has been observed (38±24% reduction, n=12). However, this high dose of nitroglycerin did not cause any damage by itself when administered to rats without indomethacin treatment (n=4). The level of damage induced by 25 mg kg−1 of indomethacin was significantly (P<0.05) increased when this NSAID was administered orally, and again it was significantly reduced by pretreatment with the transdermal patch, with doses of 160 and 400 ng min−1 rat−1 of nitroglycerin decreasing the length of gastric injury by 28±4 and 30±9% respectively. As shown in Figure 2, the administration of 1 ml acidified bile salts (100 mg kg−1 taurocholic acid in 150 mM HCl) induced significant macroscopic lesions to the gastric mucosa as determined 10 min or 3 h later. Pretreatment with the nitroglycerin patch (160 ng min−1 rat−1) significantly reduced this level of damage by 54±7% (10 min) and 83±3% (3 h). Likewise pretreatment with transdermal nitroglycerin (160 ng min−1 rat−1) significantly diminished by 56±10% the area of injury induced by the acute (10 min) oral administration of 1 ml ethanol 50% (Figure 3).
Figure 1.
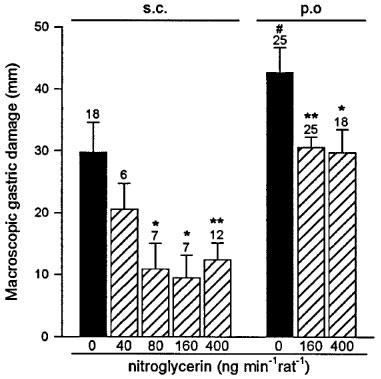
Nitroglycerin administered by the transdermal patch reduces gastric mucosal injury induced by subcutaneous or orally administered indomethacin (25 mg kg−1). Each column and bar represent the mean±s.e.mean, number above is the number of rats used. Significant difference from respective control group is shown as *P<0.05 and **P<0.01 and between the two indomethacin treated groups as #P<0.05.
Figure 2.
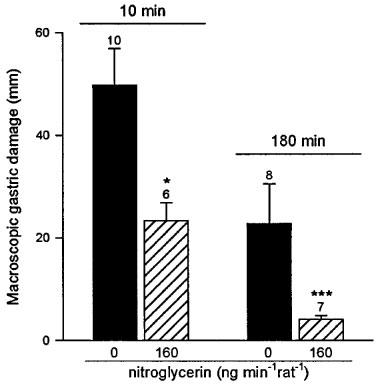
Nitroglycerin (160 ng min−1 rat−1) administered via the transdermal patch reduces gastric mucosal injury observed 10 min or 3 h after acidified bile salts administration (100 mg kg−1 taurocholic acid in 150 mM HCl). Each column and bar represent the mean±s.e.mean and the number above is the number of rats used. Significant difference from the respective control group is shown as *P<0.05 and ***P<0.001.
Figure 3.
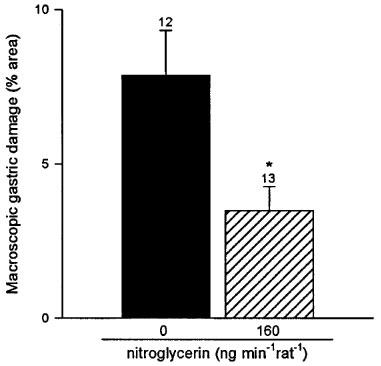
Nitroglycerin (160 ng min−1rat−1) administered via a transdermal patch reduces gastric mucosal injury induced by 1 ml ethanol 50% p.o. Each column and bar represent the mean±s.e.mean and the number above is the number of rats used. Significant difference from the control group is shown as *P<0.05.
Haemodynamic studies
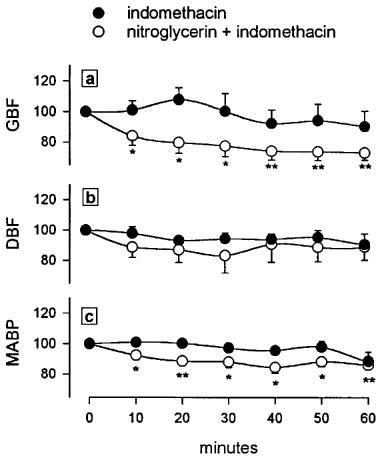
Treatment with indomethacin (25 mg kg−1, s.c.) induced a progressive and significant reduction of GBF which appeared 20 min later and lasted during the remaining of the 60 min experimental period (Figure 4a). This dose of indomethacin did not influence DBF (Figure 4b) but significantly decreased MABP at 60 min (Figure 4c). Pretreatment with the nitroglycerin patch (160 ng min−1 rat−1) prevented the indomethacin-induced fall in GBF (Figure 4a) and in MABP (Figure 4c). In control, non indomethacin-treated rats, administration of the nitroglycerin patch (160 ng min−1 rat−1, n=4) did not generate any change in GBF, DBF or MABP during the 60 min experimental period. Treatment of control, non indomethacin-treated, animals with a patch releasing higher amounts of nitroglycerin (3200 ng min−1 rat−1) produced an immediate and significant fall in GBF (Figure 5a) and MABP (Figure 5c), without modifying DBF (Figure 5b).
Figure 4.
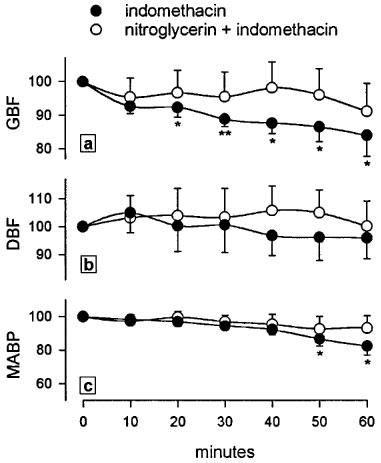
Effects of nitroglycerin (160 ng min−1 rat−1) administered via a transdermal patch on gastric blood flow (a), dermal blood flow (b) and mean arterial blood pressure (c) measured by lasser-doppler flowmetry in anaesthetized rats treated with indomethacin (25 mg kg−1, s.c.). Results are expressed as percentage from basal values. Each point and bar represent the mean±s.e.mean of a minimum of six animals and significant difference from the respective basal value is shown as *P<0.05 and **P<0.01.
Figure 5.
Effects of nitroglycerin (3200 ng min−1 rat−1) administered via a transdermal patch on gastric blood flow (a), dermal blood flow (b) and mean arterial blood pressure (c) measured in anaesthetized rats. Results are expressed as percentage from basal values. Each point and bar represent the mean±s.e.mean of a minimum of six animals and significant difference from the respective basal value is shown as *P<0.05 and **P<0.01.
Intravital microscopy
Pretreatment with the transdermal nitroglycerin patch (160 ng min−1 rat−1) produced no changes on the basal flux of rolling leukocytes when compared with the values present in the placebo-treated group, 13.0±2.4 cells min−1 vs 16.3±6.8 cells min−1 respectively. Indomethacin superfusion produced a significant increase in the number (Figure 6a) and flux (Figure 6b) of rolling leukocytes after 30 min. Treatment with the nitroglycerin patch induced a significant reduction in both parameters, with a return to levels similar to those present in basal conditions (Figure 6a and b). As shown in Figure 6c, leukocyte rolling velocity throughout the 60 min experimental period was not influenced either by superfusion with indomethacin or by treatment with the nitroglycerin patch.
Figure 6.
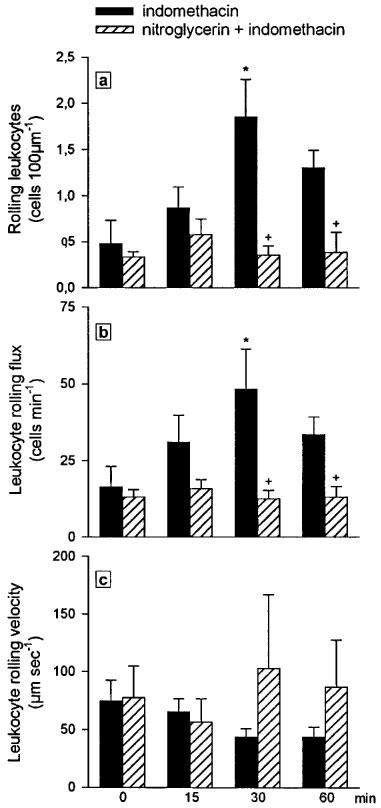
Effect of nitroglycerin (160 ng min−1 rat−1) administered via a transdermal patch on number of rolling leukocytes per 100 μm length of venule (a), leukocyte rolling flux (b) and leukocyte rolling velocity (c) induced by the 60 min superfusion with indomethacin (25 μg ml−1). Results are expressed as mean±s.e.mean of four animals per group. Significant difference from the respective basal value is shown as *P<0.05 and from the placebo group as +P<0.05.
Superfusion with indomethacin also resulted in a significant increase in the number of adherent leukocytes at the three different time periods measured (Figure 7a). Treatment with the nitroglycerin patch (160 ng min−1 rat−1) induced a significant diminution in leukocyte adherence to levels that were no different from those present in basal conditions (Figure 7a). As seen in Figure 7b, leukocyte emigration was neither affected by indomethacin superfusion nor by pretreatment with the nitroglycerin patch. Likewise, recruitment of rolling and adherent leukocytes was not accompanied by changes in venular shear rate and venular haemodynamics were also unaffected by the nitroglycerin patch (data not shown).
Figure 7.
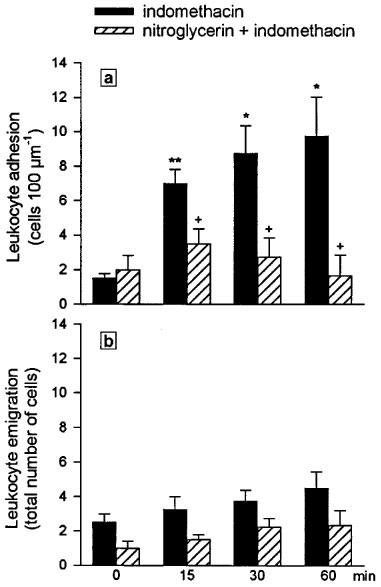
Effect of nitroglycerin (160 ng min−1 rat−1) administered via a transdermal patch on number of adherent leukocytes per 100 μm length of venule (a) and leukocyte emigration (b) elicited during 60 min of indomethacin superfusion (25 μg ml−1). Results are expressed as mean±s.e.mean of four animals per group. Significant difference from the respective basal value is shown as *P<0.05 and **P<0.01, and from the placebo group as +P<0.05.
Discussion
The present study demonstrates that the continuous delivery of NO elicited by the application of a transdermal patch of nitroglycerin protected the rat gastric mucosa from the deleterious actions of orally and subcutaneously administered indomethacin. Furthermore, it is also important to emphasize that this protection was obtained with doses of nitroglycerin that in principle and, on a weight basis, are analogous to those clinically used in man (Todd et al., 1990). Pretreatment with the nitroglycerin patch also prevented damage induced by a more acute challenge with acidified bile salts and ethanol. This indicates that these protective actions are not limited to the defined type of injury represented by NSAIDs, which needs several hours to become visible, but equally influence more acute challenges with topically acting agents. When used in humans, patches similar to those employed here achieve an effective concentration of nitroglycerin within 30 min of application (Chien, 1984) and, in animal studies, the nitroglycerin released from the transdermal patch penetrates the hairless mouse skin at a constant rate (Chien, 1984). Since a rapid initial increase in the levels of circulating NO is thus rather unlikely, the prevention of the damage induced by ethanol or acidified bile salts suggest that even low doses of circulating NO are capable of preventing some critical events in the chain leading to the appearance of macroscopic damage generated by these rapidly acting agents. The critical role of endogenous NO in the protection of the gastric mucosa has been described in recent years, and it is now well established that this mediator modulates important functions involved in the pathogenesis of gastric mucosal injury like mucosal blood flow (Piqué et al., 1989; 1992), leukocyte-adherence (Kubes et al., 1991), acid secretion (Esplugues et al., 1996) and mucous gel secretion (Brown et al., 1992). Furthermore, administration of NO donors has already been proven to be highly effective in preventing injury induced by a wide variety of deleterious agents (Whittle, 1994). Indeed, there are novel NSAIDs-derivatives that contain a moiety similar to that found in many organic nitrates and exhibit less ulcerogenic effects on the stomach (Wallace et al., 1994). However, in addition to pharmacokinetical considerations such as short duration of action or unsuitability of the parenteral form employed, several studies have shown that some of these NO-donors exhibit a dual NO effect in tissue integrity which may limit their use as gastroprotective drugs. For instance, while low doses of agents such as S-nitroso-N-acetyl-penicillamine (SNAP) or sodium nitroprusside prevent the ulcerogenic effects of endothelin, extensive haemorrhagic mucosal damage appeared following the administration of doses 2–4 times higher than those that protected the mucosa (López-Belmonte et al., 1993; Lamarque & Whittle, 1995a,1995b). This dual behaviour was not observed in our experiments with the nitroglycerin patch, and administration of a dose 20 times greater than that used to obtain protection had no direct harmful effect on the gastric mucosa. This may reflect the specific requirement of nitroglycerin to be enzymatically activated to release NO, a process regulated and limited by the metabolic capacity of the microvascular tissue (Ignarro et al., 1981; Feelisch & Noack, 1987) and, in addition, is in keeping with the well established safety record of nitroglycerin after decades of extensive clinical use (Todd et al., 1990; Ahlner et al., 1991).
It has been recognized for several years that the reduction of gastric mucosal blood flow by NSAIDs is a leading factor in the induction of NSAIDs-induced gastropathy (Wallace, 1997). NO generated in the mucosa has been implicated in the maintenance of mucosal circulation during gastroprotection (Casadevall et al., 1994) and nitroglycerin has been shown to induce vasodilatation within gastric microvessels (Walder et al., 1990; Chen et al., 1993). Our experiments show that a dose of transdermal nitroglycerin that elicited protection prevented the reduction in GBF induced by indomethacin, thus suggesting that maintenance of an appropriate blood perfusion may account for the prevention of mucosal injury afforded by the patch. Support for this hypothesis is given by the absence of such a protective effect when the patch was releasing much higher doses of nitroglycerin which have a direct effect on GBF. Changes in blood flow could also be responsible for the protection by the patch against damage by ethanol or acidified bile salts, although in both these cases they would have to be very acute to prevent injury by these topically acting agents. With regard to DBF we have observed that it remained constant in all experimental groups, even when the dose of nitroglycerin was great enough to reduce both MABP and GBF. With the present results we can only speculate as to whether this difference between the effects of this nitrovasodilator on gastric and dermal blood perfusion are due to a selective action of the drug or, alternately, is consequence of a physiological circulatory redistribution in response to the hypotension.
It is relevant to point out that these protective doses of nitroglycerin had no effect on any of the vascular parameters evaluated when administered in control animals, in particular neither gastric blood flow nor systemic arterial blood pressure were affected. This suggests that we are not dealing with a direct effect on the vascular wall but with the prevention of some action of indomethacin which results in a reduction of blood flow and is also highly sensitive to the effects of NO. It has been shown by various investigators that both endogenous NO and NO donors inhibit leukocyte adherence to the microvascular endothelium (Kubes et al., 1991; Gaboury et al., 1993). This effect has been related to a diminished expression of adhesion molecules or, more recently, to the inhibition of mast cell activation, which indirectly influences the outcome of the leukocyte extravasation process (Henricks & Nijkamp, 1988; Gaboury et al., 1993). Such an interaction of neutrophils with the vascular endothelium is a very early event after NSAIDs administration, and it has been suggested that it contributes to the pathogenesis of gastric mucosal injury in two related ways that could lead to reduction of blood flow (Wallace & Granger, 1992). Firstly, the factors that trigger the adherence of the neutrophils also provoke activation of these cells and the release of oxygen derived free radicals capable of damaging the vascular endothelium (Vaananen et al., 1991). Second, neutrophil adherence to the endothelium could lead to obstruction of capillaries and the subsequent reduction of blood blow, with induction of local ischaemia and a diminution in the defensive capabilities of the mucosa. Indeed the reduction in gastric mucosal blood that appears with NSAIDs administration has been related to the appearance of white thrombi in the gastric microcirculation (Kitahora & Guth, 1987). It is thus relevant that in our in vivo experiments with intravital microscopy, gastroprotective doses of nitroglycerin induced an almost complete inhibition on the flux of rolling and adhered leukocytes that follow superfusion with indomethacin. This effect was obtained with doses of nitroglycerin that neither modify mucosal blood flow nor mean arterial blood pressure, thus clearly indicating a potent anti-adhesive activity of this form of NO administration. It is also possible that nitroglycerin acted by inhibiting the release and action of endothelin since both have been implicated in the gastrolesive effects of NSAIDs (Kitajima et al., 1993) and nitric oxide inhibit the synthesis of this peptide and its vasoconstrictor and injurious action in the gastric mucosa (Saijonmaa et al., 1990; Boulanger & Luscher, 1991; López-Belmonte et al., 1993; Wood et al., 1996). Although gastric hypermotility also seems to be involved in the microcirculatory disturbances appearing after indomethacin administration (Takeuchi et al., 1989; 1991) and NO is known to reduce gastrointestinal contractility (Shikano et al., 1987; Buga et al., 1989; Lefebvre et al., 1992; Jin et al., 1993; Martinez Cuesta et al., 1996), the implication of this element in our experimental design with the gastric chamber looks unlikely.
In conclusion, the results of this study demonstrate that nitroglycerin increases gastric mucosal resistance against indomethacin and other exogenous damaging agents. This protection was achieved by a clinically used method of systemic administration, namely the nitroglycerin patch, and at doses that are clinically relevant. Maintenance of an adequate gastric blood perfusion seems to be implicated in the prevention of indomethacin-induced damage and this effect might be due to a reduction on leukocyte-endothelial cell interaction. The present study thus suggests that the nitroglycerin patch, with its well established therapeutic and safety record, may represent a rational clinical alternative for the prevention of gastric damage and, in particular, of NSAIDs-induced gastropathy.
Acknowledgments
The present study has been supported by grants PM 95 0145, SAF 95 0472 and SAF 98 0118. S.Calatayud is the recipient of a fellowship sponsored by Consellería de Educación y Ciencia (Generalitat Valenciana).
Abbreviations
- DBF
dermal blood flow
- GBF
gastric blood flow
- MABP
mean arterial blood pressure
- NO
nitric oxide
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PP
placebo patches
- SNAP
S-nitroso-N-acetyl-penicillamine
- Vrbc
red blood cell velocity
- Vwbc
leukocyte rolling velocity
References
- AHLNER J., ANDERSSON R.G.G., TORFGARD K., AXELSSON K.L. Organic nitrate esters: Clinical use and mechanisms of action. Pharmacol. Rev. 1991;43:351–423. [PubMed] [Google Scholar]
- ANDREWS F.J., MALCONTENTI WILSON C., O'BRIEN P.E. Protection against gastric ischemia-reperfusion injury by nitric oxide generators. Dig. Dis. Sci. 1994;39:366–373. doi: 10.1007/BF02090210. [DOI] [PubMed] [Google Scholar]
- BARRACHINA M.D., CALATAYUD S., CANET A., BELLO R., DIAZ DE ROJAS F., GUTH P.H., ESPLUGUES J.V. Transdermal nitroglycerin prevents nonsteroidal anti-inflammatory drug gastropathy. Eur. J. Pharmacol. 1995;281:R3–R4. doi: 10.1016/0014-2999(95)00399-6. [DOI] [PubMed] [Google Scholar]
- BECK P.L., LEE S.S., MCKNIGHT W., WALLACE J.L. Characterization of spontaneous and ethanol-induced gastric damage in cirrhotic rats. Gastroenterology. 1992;103:1048–1055. doi: 10.1016/0016-5085(92)90042-w. [DOI] [PubMed] [Google Scholar]
- BELLO R., CANET A., CALATAYUD S., DÍAZ DE ROJAS F., ESPLUGUES J.V. Transdermal nitroglycerin prevents gastric damage induced by nonsteroidal-antiinflammatory drugs and bile salts. Gastroenterology. 1996;110:A64. [Google Scholar]
- BOUGHTON-SMITH N.K., DEAKIN A.M., WHITTLE B.J. Actions of nitric oxide on the acute gastrointestinal damage induced by PAF in the rat. Agents Actions. 1992. pp. C3–C9. [PubMed]
- BOULANGER C.M., LUSCHER T.F. Hirudin and nitrates inhibit the thrombin-induced release of endothelin from the intact porcine aorta. Circ. Res. 1991;68:1768–1772. doi: 10.1161/01.res.68.6.1768. [DOI] [PubMed] [Google Scholar]
- BROWN J.F., HANSON P.J., WHITTLE B.J. Nitric oxide donors increase mucus gel thickness in rat stomach. Eur. J. Pharmacol. 1992;223:103–104. doi: 10.1016/0014-2999(92)90824-n. [DOI] [PubMed] [Google Scholar]
- BUGA G.M., GOLD M.E., WOOD K.S., CHAUDHURI G., IGNARRO L.J. Endothelium-derived nitric oxide relaxes nonvascular smooth muscle. Eur. J. Pharmacol. 1989;161:61–72. doi: 10.1016/0014-2999(89)90180-5. [DOI] [PubMed] [Google Scholar]
- CALATAYUD S., DÍAZ DE ROJAS F., BELLO R., CANET A., ESPLUGUES J.V. Transdermal nitroglycerin prevents the decrease in gastric mucosal blood flow induced by NSAIDs. Gastroenterology. 1997;112:A81. [Google Scholar]
- CASADEVALL M., PANES J., PIQUE J.M., BOSCH J., TERES J., RODES J. Limitations of laser-Doppler velocimetry and reflectance spectrophotometry in estimating gastric mucosal blood flow. Am. J. Physiol. 1992;263:G810–G815. doi: 10.1152/ajpgi.1992.263.5.G810. [DOI] [PubMed] [Google Scholar]
- CASADEVALL M., PIQUE J.M., CIRERA I., BARRACHINA M.D., TERES J. Acute normovolaemic anaemia prevents ethanol-induced gastric damage in rats through a blood flow related mechanism. Naunyn. Schmiedebergs. Arch. Pharmacol. 1994;350:569–574. doi: 10.1007/BF00173028. [DOI] [PubMed] [Google Scholar]
- CHEN R.Y., ROSS G., CHYU K.Y., GUTH P.H. Role of L-arginine-derived nitric oxide in cholinergic dilation of gastric arterioles. Am. J. Physiol. 1993;265:H2110–H2116. doi: 10.1152/ajpheart.1993.265.6.H2110. [DOI] [PubMed] [Google Scholar]
- CHIEN Y.W. Pharmaceutical considerations of transdermal nitroglycerin delivery: the various approaches. Am. Heart. J. 1984;108:207–215. doi: 10.1016/0002-8703(84)90577-5. [DOI] [PubMed] [Google Scholar]
- ESPLUGUES J.V., BARRACHINA M.D., BELTRAN B., CALATAYUD S., WHITTLE B.J., MONCADA S. Inhibition of gastric acid secretion by stress: a protective reflex mediated by cerebral nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14839–14844. doi: 10.1073/pnas.93.25.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEELISCH M., NOACK E.A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur. J. Pharmacol. 1987;139:19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- GABOURY J., WOODMAN R.C., GRANGER D.N., REINHARDT P., KUBES P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am. J. Physiol. 1993;265:H862–H867. doi: 10.1152/ajpheart.1993.265.3.H862. [DOI] [PubMed] [Google Scholar]
- HENRICKS P.A.J., NIJKAMP F.P. Pharmacological modulation of cell adhesion molecules. Eur. J. Pharmacol. 1988;344:1–13. doi: 10.1016/s0014-2999(98)00036-3. [DOI] [PubMed] [Google Scholar]
- HOUSE S.D., LIPOWSKY H.H. Leukocyte-endothelium adhesion: microhemodynamics in mesentery of the cat. Microvasc. Res. 1987. pp. 363–379. [DOI] [PubMed]
- IGNARRO L.J., LIPPTON H., EDWARDS J.C., BARICOS W.H., HYMAN A.L., KADOWITZ P.J., GRUETTER C.A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J. Pharmacol. Exp. Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- JIN J.G., MURTHY K.S., GRIDER J.R., MAKHLOUF G.M. Activation of distinct cAMP- and cGMP-dependent pathways by relaxant agents in isolated gastric muscle cells. Am. J. Physiol. 1993;264:G470–G477. doi: 10.1152/ajpgi.1993.264.3.G470. [DOI] [PubMed] [Google Scholar]
- KITAGAWA H., TAKEDA F., KOHEI H. Effect of endothelium-derived relaxing factor on the gastric lesion induced by HCl in rats. J. Pharmacol. Exp. Ther. 1990;253:1133–1137. [PubMed] [Google Scholar]
- KITAHORA T., GUTH P.H. Effect of aspirin plus hydrochloric acid on the gastric mucosal microcirculation. Gastroenterology. 1987;93:810–817. doi: 10.1016/0016-5085(87)90444-6. [DOI] [PubMed] [Google Scholar]
- KITAJIMA T., YAMAGUCHI T., TANI K., KUBOTA Y., OKUHIRA M., INOUE K., YAMADA H. Role of endothelin and platelet-activating factor in indomethacin-induced gastric mucosal injury in rats. Digestion. 1993;54:156–159. doi: 10.1159/000201030. [DOI] [PubMed] [Google Scholar]
- KUBES P., SUZUKI M., GRANGER D.N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMARQUE D., WHITTLE B.J. Involvement of superoxide and xanthine oxidase in neutrophil-independent rat gastric damage induced by NO donors. Br. J. Pharmacol. 1995a;116:1843–1848. doi: 10.1111/j.1476-5381.1995.tb16672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMARQUE D., WHITTLE B.J. Role of oxygen-derived metabolites in the rat gastric mucosal injury induced by nitric oxide donors. Eur. J. Pharmacol. 1995b;277:187–194. doi: 10.1016/0014-2999(95)00075-v. [DOI] [PubMed] [Google Scholar]
- LANAS A., BAJADOR A., ARROYO M., FUENTES J., SERRANO P., SANTOLARIA S. Effects of nitrate and prophylactic aspirin on upper gastrointestinal bleeding: a retrospective case-control study. J. Int. Med. Res. 1998;26:120–128. doi: 10.1177/030006059802600302. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A., HASRAT J., GOBERT A. Influence of NG-nitro-L-arginine methyl ester on vagally induced gastric relaxation in the anaesthetized rat. Br. J. Pharmacol. 1992;105:315–320. doi: 10.1111/j.1476-5381.1992.tb14252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPEZ-BELMONTE J., WHITTLE B.J., MONCADA S. The actions of nitric oxide donors in the prevention or induction of injury to the rat gastric mucosa. Br. J. Pharmacol. 1993;108:73–78. doi: 10.1111/j.1476-5381.1993.tb13442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACNAUGHTON W.K., CIRINO G., WALLACE J.L. Endothelium-derived relaxing factor (nitric oxide) has protective actions in the stomach. Life Sci. 1989;45:1869–1876. doi: 10.1016/0024-3205(89)90540-7. [DOI] [PubMed] [Google Scholar]
- MARTINEZ CUESTA M.A., ESPLUGUES J.V., WHITTLE B.J. Modulation by nitric oxide of spontaneous motility of the rat isolated duodenum: role of tachykinins. Br. J. Pharmacol. 1996;118:1335–1340. doi: 10.1111/j.1476-5381.1996.tb15542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIQUE J.M., WHITTLE B.J., ESPLUGUES J.V. The vasodilator role of endogenous nitric oxide in the rat gastric microcirculation. Eur. J. Pharmacol. 1989;174:293–296. doi: 10.1016/0014-2999(89)90324-5. [DOI] [PubMed] [Google Scholar]
- PIQUE J.M., ESPLUGUES J.V., WHITTLE B.J. Endogenous nitric oxide as a mediator of gastric mucosal vasodilatation during acid secretion. Gastroenterology. 1992;102:168–174. doi: 10.1016/0016-5085(92)91797-8. [DOI] [PubMed] [Google Scholar]
- SAIJONMAA O., RISTIMAKI A., FYHRQUIST F. Atrial natriuretic peptide, nitroglycerine, and nitroprusside reduce basal and stimulated endothelin production from cultured endothelial cells. Biochem. Biophys. Res. Commun. 1990;173:514–520. doi: 10.1016/s0006-291x(05)80064-6. [DOI] [PubMed] [Google Scholar]
- SHIKANO K., OHLSTEIN E.H., BERKOWITZ B.A. Differential selectivity of endothelium-derived relaxing factor and nitric oxide in smooth muscle. Br. J. Pharmacol. 1987;92:483–485. doi: 10.1111/j.1476-5381.1987.tb11347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI K., NISHIWAKI H., OKADA M., NIIDA H., OKABE S. Bilateral adrenalectomy worsens gastric mucosal lesions induced by indomethacin in the rat. Role of enhanced gastric motility. Gastroenterology. 1989;97:284–293. doi: 10.1016/0016-5085(89)90063-2. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI K., UESHIMA K., HIRONAKA Y., FUJIOKA Y., MATSUMOTO J., OKABE S. Oxygen free radicals and lipid peroxidation in the pathogenesis of gastric mucosal lesions induced by indomethacin in rats. Relation to gastric hypermotility. Digestion. 1991;49:175–184. doi: 10.1159/000200718. [DOI] [PubMed] [Google Scholar]
- TODD P.A., GOA K.L., LANGTRY H.D. Transdermal nitroglycerin (glyceryl trinitrate). A review of its pharmacology and therapeutic use. Drugs. 1990;40:880–902. doi: 10.2165/00003495-199040060-00009. [DOI] [PubMed] [Google Scholar]
- VAANANEN P.M., MEDDINGS J.B., WALLACE J.L. Role of oxygen-derived free radicals in indomethacin-induced gastric injury. Am. J. Physiol. 1991;261:G470–G475. doi: 10.1152/ajpgi.1991.261.3.G470. [DOI] [PubMed] [Google Scholar]
- WALDER C.E., THIEMERMANN C., VANE J.R. Endothelium-derived relaxing factor participates in the increased blood flow in response to pentagastrin in the rat stomach mucosa. Proc. R. Soc. Lond. B. Biol. Sci. 1990;241:195–200. doi: 10.1098/rspb.1990.0085. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., GRANGER D.N. The pathogenesis of NSAID-gastropathy–are neutrophils the culprits. Trends. Pharmacol. Sci. 1992;13:129–131. doi: 10.1016/0165-6147(92)90046-9. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., MIYASAKA M., TAMATANI T., PAULSON J., ANDERSON D.C., GRANGER D.N., KUBES P. Role of endothelial adhesion molecules in NSAID-induced gastric mucosal injury. Am. J. Physiol. 1993;265:G993–G998. doi: 10.1152/ajpgi.1993.265.5.G993. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., REUTER B., CICALA C., MCKNIGHT W., GRISHAM M.T., CIRINO G. Novel nonsteroidal anti-inflammatory drugs derivatives with markedly reduced ulcerogenic properties in the rat. Gastroenterology. 1994;107:173–179. doi: 10.1016/0016-5085(94)90074-4. [DOI] [PubMed] [Google Scholar]
- WHITTLE B.J.Nitric oxide in gastrointestinal physiology and pathology Physiology of the gastrointestinal tract 1994New York: Raven Press; 267–294.ed.; Johnson, L.R. pp [Google Scholar]
- WOOD J.G., ZHANG Q., YAN Z.Y., CHEUNG L.Y. Nitric oxide attenuates endothelin-1-induced vasoconstriction in canine stomach. Am. J. Physiol. 1996;271:G27–G35. doi: 10.1152/ajpgi.1996.271.1.G27. [DOI] [PubMed] [Google Scholar]


