Abstract
Microthrombi produced have a potential to form larger thrombi, leading to vascular occlusions. Recently, a new device to easily detect microaggregates using laser-light scattering (LS) has been developed. We adopted this device to comparatively evaluate the inhibitory effects of aspirin (1, 3 or 10 mg kg−1), vapiprost (0.3, 1 or 3 mg kg−1) or GR144053 (0.1, 0.3 or 1 mg kg−1) on ex vivo aggregation of hamster platelets in relation to their in vivo antithrombotic effects.
A transluminal thrombus was produced in the hamster femoral artery by the photochemical reaction. Each compound was injected i.v. as a bolus 10 min prior to the reaction, showing a dose-dependent antithrombotic effect, i.e. they prolonged the time before the artery occluded. At that time cyclic flow reductions occurred more marked when aspirin or vapiprost was given.
At the end of experiments, blood was collected to evaluate the platelet aggregation using both the new LS device and the conventional optical density (OD) method. Many more small aggregates were still formed when the highest dose of aspirin or vapiprost was used as compared with that of GR144053, although suppression of the platelet aggregation using the OD method, prolongation of the occlusion time and the bleeding time were quite similar.
In conclusion, a GPIIb/IIIa antagonist markedly suppressed the microthrombi and reduced the cyclic flow reduction. This further indicates the importance of small aggregates as triggers of thrombosis and shows that prevention of their formation may result in improved vascular patency after thrombotic insult.
Keywords: Microaggregation, GPIIb/IIIa antagonist, platelets
Introduction
Platelets play an important role in the development of thrombi and platelet microaggregates produced in the early phase of platelet activation are now considered to have a potential to aggravate thrombus formation, finally leading to vascular occlusions (Ruggeri, 1997). Platelet aggregation usually is measured using either the optical density (OD) method (Born & Hume, 1967) or the impedance method (Cardinal & Flower, 1979), both of which are indispensable for clinical evaluation of platelet function. However, these methods provide us with no information about subtle but significant changes in the number of platelet microaggregates in response to some small increment in aggregating stimuli or in the pro-aggregatory status of the platelets. The light scattering (LS) method, which has primarily been used for experimental research, provides a tool with a greater sensitivity for detecting platelet microaggregates than the conventional light transmittance method (Latimer et al., 1977, Latimer 1979). Recently, a particle-counting method which employs LS has been developed (Ozaki et al., 1994), allowing the identification of particle size in terms of light intensity and minimizing the interference with the neighbouring platelets, which may attenuate high-intensity light scattered by larger particles (Tohgi et al., 1996).
Currently available antiplatelet agents such as aspirin show clinical efficacy in the treatment of arterial thrombotic disorders by inhibiting platelet aggregation (Topol, 1995; Schafer, 1996). However, since microthrombi produced in the early phase of platelet activation, may be a trigger for further development of larger thrombi (Menys et al., 1994) the inhibitory effect of these agents on the formation of microthrombi should be evaluated. Therefore, in the present study, we compared the effects of three antiplatelet agents with different mechanisms of action, i.e., aspirin, a cyclooxygenase inhibitor, vapiprost, a thromboxane A2 (TXA2) receptor antagonist and GR144053, a platelet glycoprotein (GP) IIb/IIIa (a final common pathway to the platelet aggregation) antagonist, on the in vivo formation of a photochemically-induced thrombus in the hamster femoral artery with special reference to platelet microaggregates formed ex vivo in response to collagen.
Methods
Animals
Male hamsters (Gold, SLC, Japan) weighing 100–120 g were selected and fed a standard chow (RC4, Oriental Yeast Co., Ltd, Japan). Hamsters were anaesthetized by intraperitoneal injection of 50 mg kg−1 sodium pentobarbital. All experiments were performed in accordance with institutional guidelines.
Reagents
Vapiprost and GR144053, 4-[4-{4-(Aminoiminomethyl)phenyl}-1-piperazinyl]-1-piperidineacetic acid hydrochloride trihydrate, were synthesized in Glaxo Research & Development Limited. Both compounds were kind gifts from Glaxo Wellcome U.K. L-lysine aspirin and the other chemical substances were obtained from Yoshitomi Co. Ltd. (Tokyo, Japan) and Sigma (St. Louis, MO, U.S.A.), respectively.
In vivo thrombus induction
The experimental procedure to induce a thrombus by endothelial injury was applied as described previously (Matsuno et al., 1992; 1993). In brief, the left femoral artery and the region of bifurcation were exposed under sodium pentobarbital (50 mg kg−1, i.p.) anaesthesia. A 5 mm-long portion of the left femoral artery distal to the inguninal ligament was isolated by rubbing it against the blade of a forceps. A doppler flow probe (Model PDV-20, Crystal Biotech Co. Ltd. U.S.A.) was positioned to monitor blood flow during the experiments. The left jugular vein was canulated for a bolus injection of rose bengal or drug delivery by intravenous injection as a bolus. The dye after a bolus injection was irradiated by an optic fibre mounted on a micromanipulator and positioned such that the head would be close to the flow probe: about 5 mm away from the left femoral artery, proximal to the flow probe. The right carotid artery was also canulated for monitoring blood pressure and pulse rate. Ten minutes after monitoring baseline blood flow, the irradiation with green light was started and then rose bengal (20 mg kg−1) was injected intravenously as a bolus through the left jugular vein via a cannula (polyethylene sp3, Natsume Co. Ltd. Japan). The arterial blood flow, blood pressure and pulse rate were continuously monitored during the experiments. A thrombus formation was judged to be occlusive when blood flow was zero. After the end of experiments, all animals were killed by an overdose of pentobarbital.
Bolus injection of antiplatelet agents
Aspirin, vapiprost and GR144053 were injected as a bolus via the left jugular vein. Each drug was injected 10 min before the endothelium was injured. Hamsters were divided (n=8 each) into a control group, groups treated with aspirin at doses of 1.0, 3.0 or 10.0 mg kg−1, groups treated with vapiprost at doses of 0.3, 1.0 or 3.0 mg kg−1 or groups treated with GR144053 at doses of 0.1, 0.3 and 1.0 mg kg−1. Blood flow was continuously monitored during each experiment.
Platelet aggregation studies ex vivo
Blood (4.0 ml each) was collected by heart puncture in sodium citrate (3.15%) at the end of the experiments. The inhibitory effect of each drug on the ex vivo platelet aggregation was investigated in platelet rich plasma (PRP). To this end blood samples from each hamster were centrifuged for 10 min at 155×g. Platelet count was adjusted to 4×108 cells ml−1. Aggregation in PRP was simultaneously determined by evaluating the maximum per cent decrease in the optical density (OD) and by assessing the light scattering (LS) intensity using an AG 10 apparatus (Kowa Co. Ltd., Tokyo, Japan). The concentration of collagen (5.0 μg ml−1) was chosen such that about 60% aggregation was induced in control hamster PRP. The principles of the LS methods have been described previously (Tohgi et al., 1996). Briefly, a laser beam measuring 40 μm in diameter was generated using a 20 mW diode laser (675 nm, Toshiba, Japan), and was passed through PRP (300 μl) stirred in a cylindrical glass cuvette with a 5 mm internal diameter. The light scattered from the observation volume (48×140×20 μm) was detected by a photocell array. Light intensity corresponds to particle size. The signal frequency was recorded at 10 s intervals. Data were expressed as the change over time in the number of aggregates (counts per s) of individual sizes (determined by light intensity, expressed in volts). Data were recorded as a two-dimensional graph showing the change over time of total light intensity expressed as a cumulative summation at 10 s intervals of scattered light intensity (li) and the number of particles corresponding to that intensity (Ni) in terms of particle size (intensity) (ΣliNi)(volt×count per s). The total light intensities of small, medium and large aggregates were determined. Particles with an intensity of 25–400 mV represented small aggregates (9–25 μm), those with an intensity of 400–1000 mV represented medium aggregates (25–50 μm), and those with an intensity of 1000–2048 mV represented large aggregates (50–70 μm).
Template bleeding time
Bleeding time was measured at 30 min after the injection of each drug. A template bleeding time device (Simplate; Organon Teknika Co., Durham NC, U.S.A.) was placed on the abdominal region (Lethagen & Kling 1993; Matsuno et al., 1997). The region of incision was carefully shaved before performance of the first bleeding time. Blood flowing from the incision was wiped away with filter paper every 15 s.
Statistics
All data are expressed as means±s.e.mean. The significance of drug effects (*P<0.1) versus the control was determined by ANOVA followed by the Student Newman-Keuls test or Willcoxon's test for the time to occlusion. χ2 test was also performed for the judgment of the vascular patency after endothelial injury in each drug treated group.
Results
Antithrombotic effects in vivo
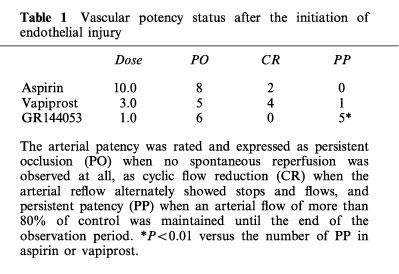
Thrombotic occlusion of the femoral artery in control experiments (infusion of saline) occurred 9.1±0.7 min after the induction of vascular injury. Each drug prolonged the time required to occlude the femoral artery in a dose-dependent manner (Figure 1). However a lot of cyclic flow reductions were observed when animals were treated with either aspirin or vapiprost even when the occlusion time was significantly prolonged. In contrast, the number of cyclic flow reductions was markedly reduced at the highest dose (1.0 mg kg−1) of GR144053. Changes in blood flow of hamsters treated with the highest dose of each drug is illustrated in Figure 2. The arterial patency was rated and expressed as persistent occlusion (PO) when no spontaneous reperfusion was observed at all, as cyclic flow reduction (CR) when the arterial reflow alternately showed stops and flows, and persistent patency (PP) when an arterial flow of more than 80% of control was maintained until the end of the observation period. The vascular patency is given in Table 1.
Figure 1.
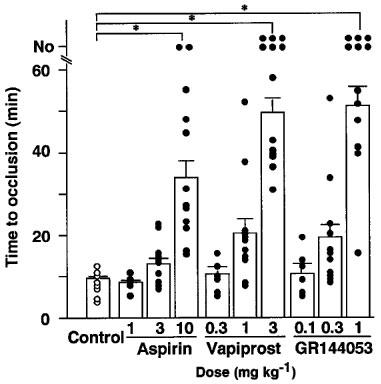
Inhibitory effects of aspirin, vapiprost or GR 144053 on thrombus formation in the hamster femoral artery. Arterial blood flow was continuously monitored for 60 min. When no occlusion was observed within the observation period, time was expressed as No. Open or closed circles represented the time required to occlusion, open bars show the mean time to occlusion in each group. Times longer than 60 min are used as 60 min for the calculation of means±s.e.mean *P<0.01 versus control.
Figure 2.
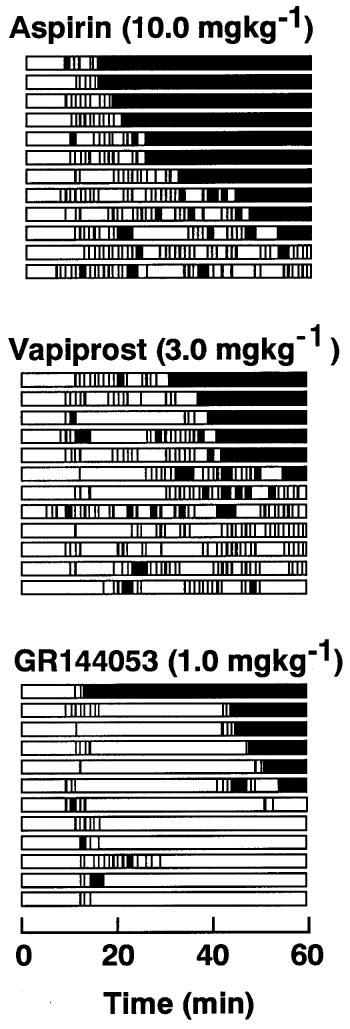
Effect of aspirin, vapiprost or GR 144053 on femoral artery patency after endothelial injury. The time-profile of vascular patency is schematically illustrated. The black columns indicate vascular occlusion. Infusion of each drug was started 30 min before the initiation of endothelial injury and continued for 60 min thereafter. 0 min indicates the start of endothelial injury by the photochemical reaction.
Table 1.
Vascular potency status after the initiation of endothelial injury
Antiplatelet activity ex vivo
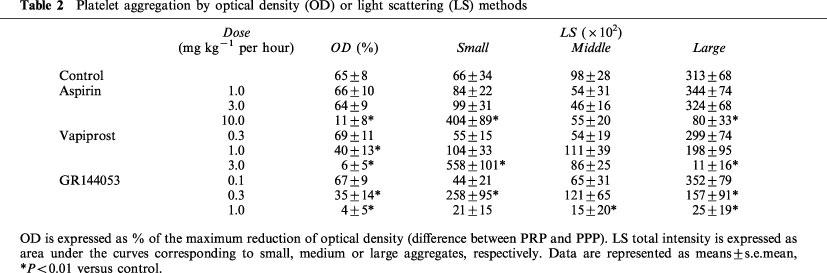
Blood samples were collected from each hamster at the end of the infusion with each drug, and platelet aggregation induced by collagen was examined ex vivo using the OD or LS detection system. When studied by OD detection, platelet aggregation was inhibited dose dependently by each drug and the groups treated with the highest dose showed a complete inhibition of platelet aggregation (Figure 3b, Figure 3c and Figure 3d, a thick line). Estimated EC50 values for aspirin, vapiprost or GR144053 were 5.3, 1.1 and 0.6 mg kg−1, respectively. At the same time, we determined the presence of small, medium or large aggregates after stimulation with collagen by LS detection. In the control group (Figure 3a), the occurrence of small aggregates increased rapidly and gradually decreased with the formation of middle or large aggregates. The appearance of medium or large aggregates paralleled the changes seen with OD detection. In treated groups, the occurrence of medium and large aggregates was decreased dose dependently, whereas the change in number of small aggregates showed a different pattern. When the highest dose of either aspirin or vapiprost was injected in hamsters, the amount of small aggregates was markedly increased even when no platelet aggregation could be measured by the OD method (Figure 3b and c). On the contrary, no aggregates could be detected in the hamsters treated with GR144053 at a dose of 1.0 mg kg−1 h− (Figure 3d). Table 2 shows the quantification of the individual aggregate size in each group.
Figure 3.
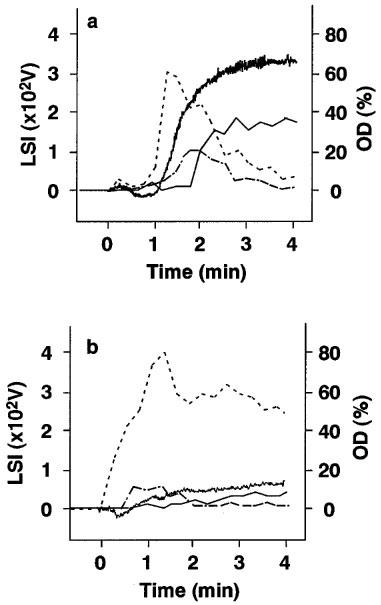
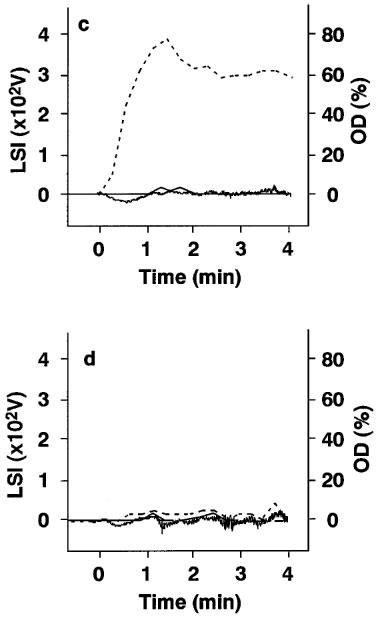
Platelet aggregation induced by 5.0 μg m−1 collagen and assessed by changes in optical density (OD) or in light scattering intensity (LSI) on ex vivo samples obtained from (a) control animals, (b) animals treated with 10.0 mg kg−1 aspirin, (c) with 3.0 mg kg−1 vapiprost or (d) with 1.0 mg kg−1 GR144053. Bold line: optical density; thin line: large aggregates; broken line: middle aggregates; dashed line: small aggregates. OD is scaled on the right side, LSI on left side.
Table 2.
Platelet aggregation by optical density (OD) or light scattering (LS) methods
Template bleeding time
Bleeding times were measured at the end of the infusion. The template bleeding time in the control group was 38.3±8.8 s (n=12). In animals treated with aspirin, vapiprost or GR144053, this parameter was prolonged in a dose-dependent manner (Figure 4).
Figure 4.
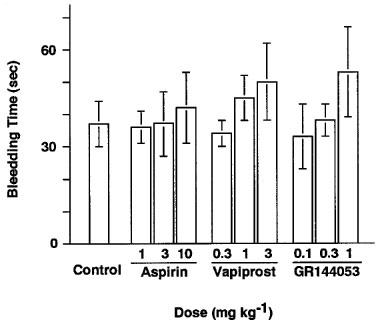
Dose-dependent changes in bleeding time in hamsters treated with saline (control), aspirin, vapiprost or GR144053, injected intravenously 10 min before the initiation of thrombus formation. Bleeding time was measured 30 min after the injection of each drug.
Discussion
In the present study, we investigated the presence of platelet microaggregates in relation to thrombus formation after endothelial injury and evaluated the effects of the antiplatelet drugs aspirin, vapiprost, a TXA2 receptor antagonist and GR144053, a GPIIb/IIIa antagonist, on this. At sites of vascular endothelial damage, platelet activation will be initiated by interactions between von Willebrand factor (vWF), collagen and their specific receptors on platelets. Platelet aggregation further requires activation of the fibrinogen receptor, GPIIb/IIIa or integrin αIIb/β3, leading to binding of fibrinogen and von Willebrand Factor, which both can cross link adjacent platelets. These results in platelet aggregation and associated intravascular microthrombus formation (Fressinaud & Meyer, 1991). Therefore microaggregation of platelets plays an initiating and hence key role in the in vivo development of a thrombus.
Aspirin is an irreversible, non-competitive inhibitor of cyclooxygenase and acts as an antiplatelet agent through the prevention of TXA2 generation in platelets (Smith & Willis, 1971). Vapiprost, a TXA2 receptor antagonist (Armstrong et al., 1993), and GR114053, a GPIIb/IIIa antagonist (Kawasaki et al., 1996), have been reported to inhibit platelet activation and thrombus formation in vitro and in vivo (White et al., 1994; Matsuno et al., 1992; 1997; 1998). In our thrombus model in hamsters, we could now show that these drugs indeed have a significant effect against vascular occlusion due to thrombus formation, especially at the highest dose of each drug used. However the vascular patency after the initiation of endothelial injury in each group was different: indeed cyclic flow reductions were much more present when either aspirin or vapiprost was used even when the time to occlusion was significantly prolonged. In contrast, when GR144053 at a dose of 1.0 mg kg−1 was administered, few cyclic flow reductions were observed and blood flow was clearly maintained. All this is in line with our previous histological findings that indicated that microthrombi remained on injured endothelial surfaces even under treatment with antiplatelet agents (Matsuno et al., 1991; 1992). Cyclic flow reduction (CR) is an indication of a thrombogenic condition in vivo (Adrie et al., 1996; Nicolini et al., 1992). Therefore the condition of CR after endothelial injury in the highest dose treated groups with either aspirin or vapiprost indicated a risk which the vascular patency will be changed to the persistent occlusion (PO).
In order to further define a role of microaggregate formation and cyclic flow alteration on vascular stenosis, we performed platelet aggregation studies using the OD or the LS method at the end of the observation period. Collagen was chosen as an agonist on the basis that platelets adhere to collagen in the damaged vessel wall in vivo, and subsequent TXA2 formation and release of ADP and 5-hydroxytryptamine (5-HT) effect platelet aggregation and haemostasis (Menys et al., 1994). In hamsters, platelet aggregation induced by collagen was clearly detected by the OD method as well as microaggregation as detected by the LS method. When looking at these results, it is clear that the small aggregated forms of platelets are a trigger for the development of larger ones ultimately leading to thrombus formation since small aggregates were rapidly detected upon platelet stimulation with collagen, followed by the appearance of middle and large forms concomitant with a gradual decrement of small aggregated forms. Platelet aggregation measured by the OD method paralleled the formation of the large aggregates detected by the LS method. Platelet aggregation ex vivo, measured by the OD method, was completely inhibited following treatment of the hamsters with each drug. However, when platelet aggregation was detected using the LS method, small aggregate formation was not prevented by treatment with either aspirin or vapiprost, in line with TXA2-formation playing an amplifying role in platelet activation, which however can be overruled by strong stimuli. In contrast, GR144053 prevented all aggregate formation, indicating that antagonism of GPIIb/IIIa, the ultimate receptor needed to have platelet aggregation, can completely inhibit platelet aggregation.
We furthermore could observe that the number of small aggregates normally decreased when middle or large aggregates started to form, which probably are responsible for the formation of an occlusive thrombus (Tohgi et al., 1996). Cyclic flow changes were seen in vivo after the treatment with either aspirin or vapiprost, under these conditions the number of small aggregates that formed was much increased, however no occlusive thrombus developed. These data indicate that microaggregation of platelets has important implications in the induction of cyclic flow reductions after endothelial injury, a known thrombogenic condition.
On the other hand, it should be noted that antithrombotic interventions may easily be complicated by haemorrhagic events. In this experiment, the highest dose of each drug elicited a prolongation of bleeding time which however never reached a statistically significant difference. We therefore believe that it should be possible to use antiplatelet drugs powerful enough to prevent the development of a thrombus without inducing a marked bleeding tendency. Furthermore, the inhibition of the microaggregation of platelets by a GPIIb/IIIa antagonist also does not seem to influence the bleeding risk significantly.
In conclusion, the presence of small platelet aggregates, as can be detected by the LS method, seem to play a key role in arterial thrombus formation, and seem to be especially related to the occurrence of cyclic flow reductions. A GPIIb/IIIa antagonist prevents the formation of all kind of platelet aggregates, in contrast to aspirin or an TXA2 receptor antagonist, that do not affect microaggregate formation.
Acknowledgments
The authors are grateful to Prof H. Deckmyn (Laboratory for Thrombosis Research, KU Leuven, Belgium) for help with this study and the English language.
Abbreviations
- CR
cyclic flow reduction
- GP
glycoprotein
- LS
light scattering
- OD
optical density
- PO
persistent occlusion
- PP
persistent patency
- TXA2
thromboxane A2
References
- ADRIE C., BLOCH K.D., MORENO P.R., HURFORD W.E., GUERRERO J.L., HOLT R., ZAPOL W.M., GOLD H.K., SEMIGRAN M.J. Inhaled nitric oxide increases coronary artery patency after thrombolysis. Circulation. 1996;94:1919–1926. doi: 10.1161/01.cir.94.8.1919. [DOI] [PubMed] [Google Scholar]
- ARMSTRONG R.A., HUMPHREY P.P., LUMLEY P. Characteristics of the binding of [3H]-GR32191 to the thromboxane (TP-) receptor of human platelets. Br. J. Pharmacol. 1993;110:539–547. doi: 10.1111/j.1476-5381.1993.tb13844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORN G.V., HUME M. Effects of the numbers and sizes of platelet aggregates on the optical density of plasma. Nature. 1967;215:1027–1029. doi: 10.1038/2151027a0. [DOI] [PubMed] [Google Scholar]
- CARDINAL D.C., FLOWER R.J. The study of platelet aggregation in whole blood. Br. J. Pharmacol. 1979;66:94P–95P. [PMC free article] [PubMed] [Google Scholar]
- FRESSINAUD E., MEYER D. von Willebrand factor and platelet interactions with the vessel wall. Blood Coagulation & Fibrinolysis. 1991;2:333–340. doi: 10.1097/00001721-199104000-00017. [DOI] [PubMed] [Google Scholar]
- KAWASAKI T., SAKAI Y., TANIUCHI Y., SATO K., MARUYAMA K., SHIMIZU M., KAKU S., YANO S., INAGAKI O., TOMIOKA K., YANAGISAWA I., TAKENAKA T. Biochemical characterization of a new disintegrin, flavostatin, isolated from Trimeresurus flavoviridis venom. Biochimie. 1996;78:245–252. doi: 10.1016/0300-9084(96)82187-0. [DOI] [PubMed] [Google Scholar]
- LATIMER P. Light scattering vs. microscopy for measuring average cell size and shape. Biophys. J. 1979;27:117–126. doi: 10.1016/S0006-3495(79)85206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATIMER P., BORN G.V., MICHAL F. Application of light-scattering theory to the optical effects associated with the morphology of blood platelets. Arch. Biochem. Biophys. 1977;180:151–159. doi: 10.1016/0003-9861(77)90019-4. [DOI] [PubMed] [Google Scholar]
- LETHAGEN S., KLING S. New bleeding time devices with retractable blades evaluated in children, healthy volunteers and patients with prolonged bleeding time. Thromb. Haemost. 1993;70:595–597. [PubMed] [Google Scholar]
- MATSUNO H., KOZAWA O., NIWA M., ITO T., TANABE K., NISHIDA M., HAYASHI H., UEMATSU T. Effect of GR144053, a fibrinogen-receptor antagonist, on thrombus formation and vascular patency after thrombolysis by tPA in the injured carotid artery of the hamster. J. Cardiovasc. Pharmacol. 1998;32:191–197. doi: 10.1097/00005344-199808000-00004. [DOI] [PubMed] [Google Scholar]
- MATSUNO H., KOZAWA O., NIWA M., UEMATSU T. Inhibition of von Willebrand factor binding to platelet GP lb by a fractionated aurintricarboxylic acid prevents restenosis after vascular injury in hamster carotid artery. Circulation. 1997;96:1299–1304. doi: 10.1161/01.cir.96.4.1299. [DOI] [PubMed] [Google Scholar]
- MATSUNO H., UEMATSU T., NAKASHIMA M. Thrombolytic effect of a plasminogen-plasminogen activator chimera in a photochemically induced thrombosis (PIT) model. Br. J. Pharmacol. 1993;110:1278–1279. doi: 10.1111/j.1476-5381.1993.tb13954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUNO H., UEMATSU T., UMEMURA K., TAKIGUCHI Y., WADA K., NAKASHIMA M. Effects of vapiprost, a novel thromboxane receptor antagonist, on thrombus formation and vascular patency after thrombolysis by tissue-type plasminogen activator. Br. J. Pharmacol. 1992;106:533–538. doi: 10.1111/j.1476-5381.1992.tb14370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENYS V.C., BELCHER P.R., NOBLE M.I., EVANS R.D., DROSSOS G.E., PILLAI R., WESTABY S. Macroaggregation of platelets in plasma, as distinct from microaggregation in whole blood (and plasma), as determined using optical aggregometry and platelet counting respectively, is specifically impaired following cardiopulmonary bypass in man. Thromb. Haemost. 1994;72:511–518. [PubMed] [Google Scholar]
- NICOLINI F.A., NICHOLS W.W., MEHTA J.L., SALDEEN T.G., SCHOFIELD R., ROSS M., PLAYER D.W., POHL G.B., MATTSSON C. Sustained reflow in dogs with coronary thrombosis with K2P, a novel mutant of tissue-plasminogen activator. J. Am. Coll. Cardiol. 1992;20:228–235. doi: 10.1016/0735-1097(92)90164-i. [DOI] [PubMed] [Google Scholar]
- OZAKI Y., SATOH K., YATOMI Y., YAMAMOTO T., SHIRASAWA Y., KUME S. Detection of platelet aggregates with a particle counting method using light scattering. Anal. Biochem. 1994;218:284–294. doi: 10.1006/abio.1994.1180. [DOI] [PubMed] [Google Scholar]
- RUGGERI Z.M. Mechanisms initiating platelet thrombus formation. Thromb. Haemost. 1997;78:611–616. [PubMed] [Google Scholar]
- SCHAFER A.I. Antiplatelet Therapy. Am. J. Medicine. 1996;101:199–209. doi: 10.1016/s0002-9343(96)80077-5. [DOI] [PubMed] [Google Scholar]
- SMITH J.B., WILLIS A.L. Aspirin selectively inhibits prostaglandin production in human platelets. Nature. 1971;231:235–237. doi: 10.1038/newbio231235a0. [DOI] [PubMed] [Google Scholar]
- TOHGI H., TAKAHASHI H., WATANABE K., KUKI H., SHIRASAWA Y. Development of large platelet aggregates from small aggregates as determined by laser-light scattering: effects of aggregant concentration and antiplatelet medication. Thromb. Haemost. 1996;75:838–843. [PubMed] [Google Scholar]
- TOPOL E.J. Novel antithrombotic approaches to coronary artery disease. Am. J. Cardiol. 1995;75:27B–33B. doi: 10.1016/0002-9149(95)80007-f. [DOI] [PubMed] [Google Scholar]
- WHITE B.P., SULLIVAN A.T., LUMLEY P. Prevention of intra-coronary thrombosis in the anaesthetised dog: the importance of thromboxane A2 and thrombin. Thromb. Haemost. 1994;71:366–374. [PubMed] [Google Scholar]


