Abstract
Vasoconstriction of carotid arteriovenous anastomoses may be involved in the therapeutic action of acutely acting antimigraine agents, including the triptans and ergot alkaloids. While 5-HT1B/1D receptors mediate the effect of triptans, ergotamine and dihydroergotamine also interact with α-adrenoceptors. In the present study, we investigated the potential role of α1- and α2-adrenoceptors in mediating vasoconstriction of carotid arteriovenous anastomoses in anaesthetized pigs.
Ten minute intracarotid infusions of phenylephrine (1, 3 and 10 μg kg−1 min−1) or BHT 933 (3, 10 and 30 μg kg−1 min−1) produced dose-dependent decreases in total carotid and arteriovenous anastomotic conductances; no changes were observed in the capillary fraction.
The carotid vascular effects of phenylephrine and BHT 933 were selectively abolished by prazosin (100 μg kg−1, i.v.) and rauwolscine (300 μg kg−1, i.v.), respectively. The responses to phenylephrine and BHT 933 were not affected by the selective 5-HT1B/1D receptor antagonist GR127935 (500 μg kg−1, i.v.).
These results show that both α1- and α2-adrenoceptors can mediate vasoconstriction of carotid arteriovenous anastomoses in anaesthetized pigs. Since vasoconstrictor activity in this in vivo model is predictive of anti-migraine activity, an agonist activity at particularly the α2-adrenoceptor subtypes, in view of their less ubiquitous nature, could provide migraine abortive potential. Thus, the present results may aid further understanding of the mode of action of some current anti-migraine agents and may eventually be helpful in the development of future treatment in migraine.
Keywords: α-Adrenoceptors, arteriovenous anastomoses, BHT 933, carotid artery, migraine, phenylephrine, pig, prazosin, rauwolscine, shunts
Introduction
Vasodilatation of carotid arteriovenous anastomoses may be involved in the pathophysiology of migraine headache (Heyck, 1969; Saxena, 1995). In line with this proposal, it has previously been shown that several acutely acting anti-migraine agents, including the ergots (ergotamine and dihydroergotamine) and triptans, potently vasoconstrict porcine carotid arteriovenous anastomoses predominantly via 5-HT1B/1D receptors (De Vries et al., 1999; Villalón et al., 1999). Interestingly, the carotid vasoconstrictor effect of ergot alkaloids involves, in addition to 5-HT1B/1D receptors, unidentified receptor mechanisms (De Vries et al., 1998, 1999). Since the ergot derivatives display high affinity at α-adrenoceptors (Leysen, 1985), their therapeutic efficacy may be partly explained by an action mediated via these receptors. Indeed, we recently reported that canine external carotid vasoconstriction by ergot alkaloids also involves α-adrenoceptors (Villalón et al., 1999). In this context, the possible involvement of α-adrenoceptors in the porcine carotid vasculature has been hampered, mainly on the basis that porcine carotid arteriovenous anastomoses were described to be insensitive to sympathetic nerve stimulation or intracarotid infusions of noradrenaline (Verdouw et al., 1984). This discrepancy is striking since in conscious pigs arteriovenous anastomoses are under a vasoconstrictor sympathetic tone (Hales, 1974; Van Woerkens et al., 1990; Den Boer et al., 1993). In fact, sympathetic nerve stimulation as well as exogenously administered noradrenaline causes α-adrenoceptor-mediated vasoconstriction of arteriovenous anastomoses in the hind limb of several species (Folkow & Sivertsson, 1964; Spence et al., 1972; Hales, 1974; Baker et al., 1978; Hales et al., 1982).
In the light of the above, the present study was designed to investigate the potential role of α1- and α2-adrenoceptors in the vasoconstriction of carotid arteriovenous anastomoses in anaesthetized pigs. For this purpose, we made use of the selective agonists, phenylephrine (α1) and BHT 933 (6-ethyl-5, 6, 7, 8-tetrahydro-4H-oxazolo[4,5-d]azepin-2-amine dihydro-chloride; (α2), and the selective antagonists, prazosin (α1), rauwolscine (α2) (see Ruffolo et al., 1991; Piascik et al., 1996), in a well-defined in vivo model predictive of anti-migraine activity (Saxena, 1995; De Vries et al., 1999). In addition, GR127935 (N-[methoxy-3-(4-methyl-1-piperazinyl) phenyl]-2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl) [1,1-biphenyl]-4-carboxamide was used to exclude the possible role of 5-HT1B/1D receptors (Clitherow et al., 1994; De Vries et al., 1996; Skingle et al., 1996). The results obtained may provide useful information concerning the mode of action of some currently used anti-migraine agents and, possibly, new avenues for the development of anti-migraine agents.
Methods
General
After an overnight fast, 40 domestic pigs (Yorkshire×Landrace; 10–14 kg) were anaesthetized with azaperone (120 mg, i.m.), midazolam hydrochloride (5 mg, i.m.) and sodium pentobarbitone (600 mg, i.v.). After tracheal intubation, the animals were connected to a respirator (BEAR 2E, BeMeds AG, Baar, Switzerland) for intermittent positive pressure ventilation with a mixture of room air and oxygen. Respiratory rate, tidal volume and oxygen supply were adjusted to keep arterial blood gas values within physiological limits (pH: 7.35–7.48; pCO2: 35–48 mmHg; pO2: 100–120 mmHg). Anaesthesia was maintained with a continuous i.v. infusion of sodium pentobarbitone (20 mg kg−1 h−1). It may be pointed out that this anaesthetic regimen, together with bilateral vagosympathectomy (see below), leads to an increase in heart rate and vasodilatation of arteriovenous anastomoses due to a loss of parasympathetic and sympathetic tone, respectively. Indeed, basal arteriovenous anastomotic blood flow is considerably higher in sodium pentobarbitone-anaesthetized pigs (70–80% of carotid blood flow) than in those under fentanyl/thiopental anaesthesia (∼19% of carotid blood flow) (Den Boer et al., 1993). A high basal carotid arteriovenous anastomotic flow is particularly useful for investigating the effects of drugs that vasoconstrict arteriovenous anastomoses.
A catheter was placed in the inferior vena cava via the left femoral vein for infusion of antagonists and sodium pentobarbitone. Another catheter was placed in the aortic arch via the left femoral artery for the measurement of arterial blood pressure (Combitrans disposable pressure transducer; Braun, Melsungen, Germany) and arterial blood withdrawal for the measurement of blood gases (ABL-510; Radiometer, Copenhagen, Denmark). Subsequently, the right common carotid artery and the external jugular vein were dissected free and the accompanying vagosympathetic trunks were cut between two ligatures in order to prevent a possible influence via baroreceptor reflexes on agonist-induced carotid vascular responses. The right external jugular vein was catheterized for withdrawal of venous blood samples for determining blood gases (ABL-510; Radiometer, Copenhagen, Denmark). Two hub-less needles, each connected to a polyethylene tube, were inserted into the right common carotid artery and used for agonist (phenylephrine or BHT 933) infusion and radioactive microspheres injection, respectively. The microspheres were injected against the direction of blood flow for uniform mixing.
Blood flow was measured in the right common carotid artery with a flow probe (internal diameter: 2.5 mm) connected to a sine-wave electromagnetic flow meter (Transflow 601-system, Skalar, Delft, The Netherlands). Heart rate was measured with a tachograph (CRW, Erasmus University, Rotterdam, The Netherlands) triggered by electrocardiogram signals. Arterial blood pressure, heart rate and right common carotid blood flow were continuously monitored on a polygraph (CRW, Erasmus University, Rotterdam, The Netherlands). During the experiment, body temperature was kept around 37°C and the animal was continuously infused with saline to compensate for fluid loss.
Distribution of carotid blood flow
The distribution of common carotid blood flow was determined with 15.5±0.1 μm (s.d.) diameter microspheres labelled with 141Ce, 113Sn, 103Ru, 95Nb or 46Sc (NEN Dupont, Boston, U.S.A.). For each measurement, about 200,000 microspheres, labelled with one of the radioisotopes, were mixed and injected into the right common carotid artery. At the end of the experiment, the animal was killed by an overdose of sodium pentobarbitone and the heart, lungs, kidneys and all ipsilateral cranial tissues were dissected out, weighed and put in vials. The radioactivity in these vials was counted for 10 min in a γ-scintillation counter (Packard, Minaxi autogamma 5000), using suitable windows to discriminate the different isotopes (141Ce: 120–167, KeV, 113Sn: 355–435 KeV, 103Ru: 450–548 KeV, 95Nb: 706–829 KeV and 46Sc: 830–965 KeV). All data were processed by a set of specially designed programs (Saxena et al., 1980). The fraction of carotid blood flow distributed to the different tissues was calculated by multiplying the ratio of tissue and total radioactivity of each radioisotope by the total common carotid blood flow at the time of the injection of the microspheres labelled with the respective isotope. Since little or no radioactivity was detected in the heart and kidneys, all microspheres trapped in lungs reached this tissue from the venous side after escaping via carotid arteriovenous anastomoses. Therefore, the amount of radioactivity in the lungs was used as an index of the arteriovenous anastomotic fraction of the common carotid blood flow (Saxena & Verdouw, 1982). Vascular conductance (10−2 ml min−1 mmHg−1) was calculated by dividing blood flow (ml min−1) by blood pressure (mmHg), multiplied by hundred.
Experimental protocol
After a stabilization period of about 60 min, baseline values of heart rate, mean arterial blood pressure, common carotid blood flow and its distribution, as well as arterial and jugular venous blood gases were measured. Thereafter, the animals were divided into four groups receiving i.v. infusions (0.5 ml min−1 for 10 min) of either distilled water (vehicle; n=14), prazosin (100 μg kg−1; n=10), rauwolscine (300 μg kg−1; n=10) or GR127935 (500 μg kg−1; n=6). After a waiting period of 15 min, all variables were reassessed. Subsequently, each group was divided into two subgroups receiving 10-min intracarotid infusions (0.1 ml min−1) of phenylephrine (cumulative doses: 1, 3, and 10 μg kg−1 min−1) or BHT 933 (cumulative doses: 3, 10 and 30 μg kg−1 min−1). The number of animals in the different groups receiving phenylephrine and BHT 933 infusions were: vehicle (n=7 each), prazosin (n=7 and 3, respectively), rauwolscine (n=3 and 7, respectively) and GR127935 (n=3 each). All variables were collated again 10 min after the start of each agonist infusion.
It may be pointed out here that GR127935, which is a potent and selective 5-HT1B/1D receptor antagonist (Clitherow et al., 1994; De Vries et al., 1996; Skingle et al., 1996), was used to rule out the remote possibility that the effects of phenylephrine and/or BHT 933 were mediated via 5-HT1B/1D receptors. The effectiveness of the blockade of 5-HT1B/1D receptors by GR127935 was confirmed by evaluating decreases in the total carotid blood flow and conductance by sumatriptan (De Vries et al., 1996). Sumatriptan (30, 100 and 300 μg kg−1, i.v.; every 10 min over a period of 5 min each) was administered in animals treated with GR127935 after a near complete recovery from the effects of the last dose of phenylephrine or BHT 933 had been achieved (∼90 min).
Data presentation and statistical analysis
All data have been expressed as the mean±s.e.mean. The per cent changes from baseline (i.e. after treatment with vehicle, prazosin, rauwolscine and GR127935) values caused by the different doses of phenylephrine or BHT 933 within each group of animals were calculated. A two-way repeated measures ANOVA with Bonferroni's correction (SigmaStat 1.0, Jandel Corporation, Chicago, IL, U.S.A.) was used to establish whether these changes were statistically significant (P<0.05, two-tailed) when compared with the baseline in each group as well as with the corresponding agonist dose in the vehicle-treated group.
Drugs
Apart from the anaesthetics azaperone (Stresnil®; Janssen Pharmaceuticals, Beerse, Belgium), midazolam hydrochloride (Dormicum®; Hoffmann La Roche b.v., Mijdrecht, The Netherlands) and sodium pentobarbitone (Apharmo, Arnhem, The Netherlands), the compounds used in this study were: prazosin hydrochloride (Bufa Chemie b.v., Castricum; The Netherlands), GR127935, sumatriptan succinate (both from GlaxoWellcome, Ware, Herts, U.K.; courtesy: Dr H.E. Connor), phenylephrine hydrochloride, BHT 933 and rauwolscine dihydrochloride (all from Sigma-Aldrich Chemie b.v., Zwijndrecht, The Netherlands). Finally, heparin sodium (Leo Pharmaceutical Products, Weesp, The Netherlands) was used to prevent clotting of blood in the catheters. All drugs were dissolved in distilled water. For prazosin, rauwolscine and GR127935 a short period of heating was needed. The doses of the drugs refer to their respective salts.
Ethical approval
The local ethics committee dealing with the use of animals in scientific experiments approved the protocol.
Results
Systemic and carotid haemodynamic effects of vehicle, prazosin, rauwolscine and GR127935
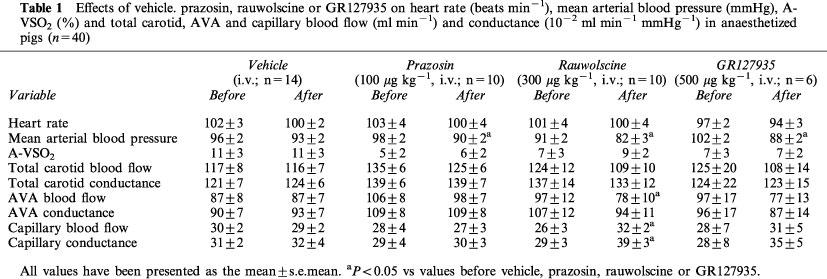
As shown in Table 1, the administration of vehicle (5 ml of distilled water) did not produce any change in systemic or carotid haemodynamic variables. On the other hand, prazosin (100 μg kg−1, i.v.), rauwolscine (300 μg kg−1, i.v.) and GR127935 (500 μg kg−1, i.v.) decreased mean arterial blood pressure by 7±2, 9±3 and 13±2%, respectively, which only in the case of rauwolscine was accompanied by a significant decrease in arteriovenous anastomotic blood flow by 18±5%. Additionally, rauwolscine increased capillary blood flow and conductance by 24±7% and 38±9%, respectively. These effects of rauwolscine were noticed in several tissues (ear, skin, muscle, bones, salivary gland, fat and tongue; data not shown). No other significant changes were noticed with prazosin, rauwolscine or GR127935.
Table 1.
Effects of vehicle. prazosin, rauwolscine or GR127935 on heart rate (beats min−1), mean arterial blood pressure (mmHg), A-VSO2 (%) and total carotid, AVA and capillary blood flow (ml min−1) and conductance (10−2 ml min−1 mmHg−1) in anaesthetized pigs (n=40)
Systemic haemodynamic changes by phenylephrine and BHT 933
Systemic haemodynamic variables measured before and after the two agonists in animals treated with vehicle, prazosin, rauwolscine or GR127935 are shown in Table 2. In animals treated with vehicle, phenylephrine elicited an immediate and dose-dependent increase in heart rate by up to 29±6%. This increase was not affected by treatment with prazosin, rauwolscine or GR127935. BHT 933 did not cause any change in heart rate. Moreover, no changes in mean arterial blood pressure were observed after infusions of phenylephrine or BHT 933.
Table 2.
Changes in heart rate (beats min−1), mean arterial blood pressure (mmHg) and A-VSO2 (%) induced by intracarotid phenylephrine or BHT 933 in anaesthetized pigs treated i.v. with vehicle (5 ml), prazosin (100 μg kg−1), rauwolscine (300 μg kg−1) or GR127935 (500 μg kg−1)
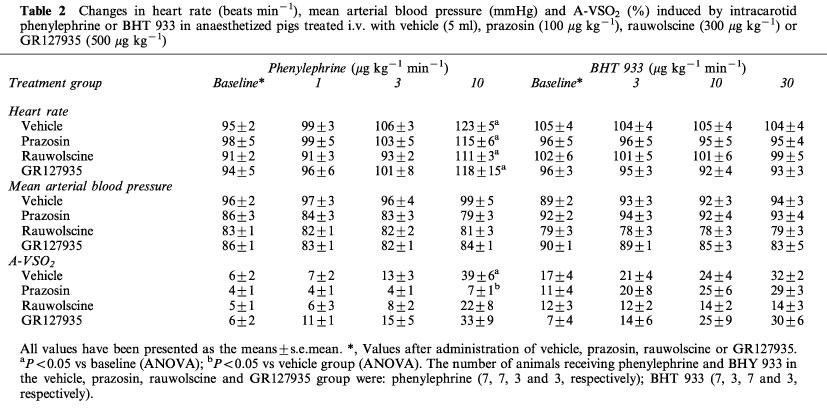
In vehicle-treated animals, both phenylephrine and BHT 933 showed a trend to increase the difference between arterial and jugular venous oxygen saturation (A-VSO2) by up to 1036±325 and 254±140%, respectively (Table 2). However, statistical significance was achieved only with the highest dose of phenylephrine. The phenylephrine-induced response was selectively antagonized by treatment with prazosin.
Carotid haemodynamic responses to phenylephrine and BHT 933
Absolute values of total carotid vascular conductance and its arteriovenous anastomotic and capillary fractions before and after infusions of phenylephrine and BHT 933 in the four groups of animals treated with vehicle, prazosin, rauwolscine or GR127935 are shown in Figures 1 and 2, respectively. In vehicle-treated animals, both phenylephrine and BHT 933 caused dose-dependent decreases in total carotid conductance (maximum change: 74±4 and 59±4%, respectively). These decreases were solely due to changes in arteriovenous anastomotic conductances, since capillary (nutrient) vascular conductance remained unchanged. Phenylephrine-induced changes were absent in animals treated with prazosin, but not in those treated with rauwolscine or GR127935 (Figure 1). On the contrary, BHT 933-induced effects were absent in animals treated with rauwolscine, but not in those treated with prazosin or GR127935 (Figure 2).
Figure 1.
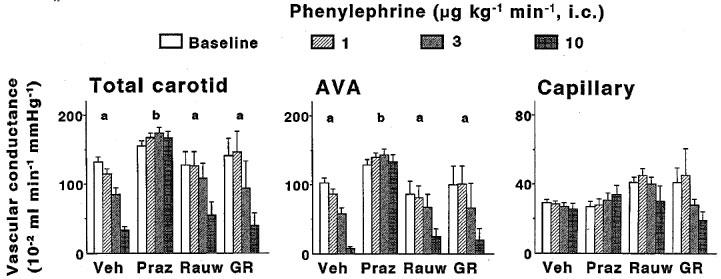
Effects of 10-min intracarotid infusions of phenylephrine on total carotid, arteriovenous anastomotic (AVA) and capillary conductances in anaesthetized pigs treated i.v. with vehicle (Veh; n=7), prazosin (Praz; 100 μg kg−1; n=7), rauwolscine (Rauw; 300 μg kg−1; n=3) or GR127935 (GR; 500 μg kg−1 n=3). All data are presented as mean±s.e.mean. aP<0.05 vs baseline (ANOVA); bP<0.05 vs vehicle group (ANOVA).
Figure 2.
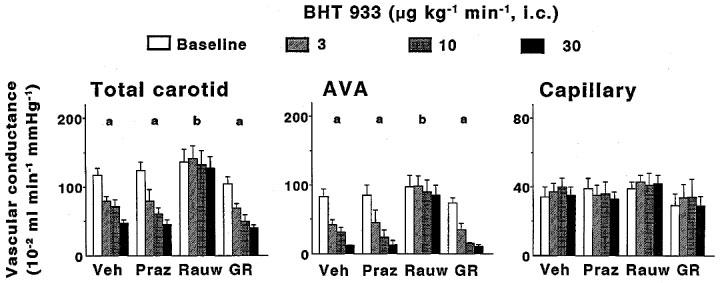
Effects of 10-min intracarotid infusions of BHT933 on total carotid, arteriovenous anastomotic (AVA) and capillary conductances in anaesthetized pigs treated i.v. with either vehicle (Veh; n=7), prazosin (Praz; 100 μg kg−1; n=3), rauwolscine (Rauw; 300 μg kg−1; n=7) or GR127935 (GR; 500 μg kg−1 n=3). All data are presented as mean±s.e.mean. aP<0.05 vs baseline (ANOVA); bP<0.05 vs vehicle group (ANOVA).
In Figure 3 per cent changes (from baseline) observed with phenylephrine and BHT 933 in carotid arteriovenous anastomotic conductance have been compared in the four groups of animals. It is clearly shown that prazosin and rauwolscine selectively blocked the decreases in carotid arteriovenous anastomotic conductance induced by, respectively, phenylephrine and BHT 933. The responses to the two agonists were not affected in animals treated with the potent and selective 5-HT1B/1D receptor antagonist GR127935.
Figure 3.
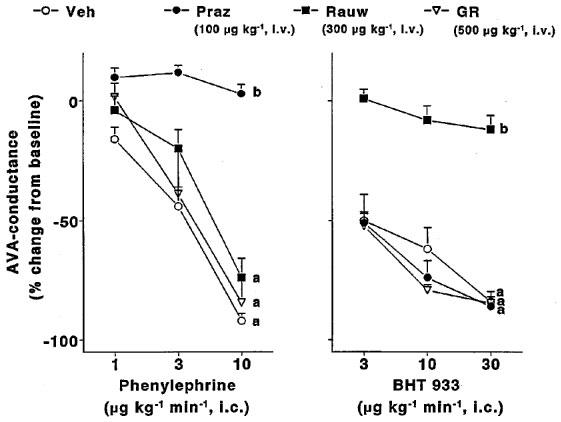
Per cent changes in arteriovenous anastomotic (AVA) conductance induced by 10-min intracarotid infusions of phenylephrine or BHT933 in anaesthetized pigs treated i.v with vehicle (Veh), prazosin (Praz; 100 μg kg−1), rauwolscine (Rauw; 300 μg kg−1) or GR127935 (GR; 500 μg kg−1). All data are presented as mean±s.e.mean. aP<0.05 vs baseline (ANOVA); bP<0.05 vs vehicle group (ANOVA). The number of animals receiving phenylephrine and BHT933 in the vehicle, prazosin, rauwolscine and GR127935 group were: phenylephrine (7, 7, 3 and 3, respectively); BHT933 (7, 3, 7 and 3, respectively).
Figure 4 shows the effects of phenylephrine and BHT 933 on tissue vascular conductances in animals treated with vehicle. Neither phenylephrine nor BHT 933 induced any change in vascular conductance in the ear, skin, salivary gland, fat and tongue. On the other hand, phenylephrine decreased vascular conductance in bone, eye and dura mater. These phenylephrine-induced conductance decreases were absent in animals treated with prazosin (data not shown). In vehicle-treated animals (Figure 4), but not in rauwolscine-treated animals (data not shown), BHT 933 increased muscle and brain vascular conductances. The responses to the two agonists were similar in GR127935-treated animals as in animals treated with vehicle (data not shown).
Figure 4.
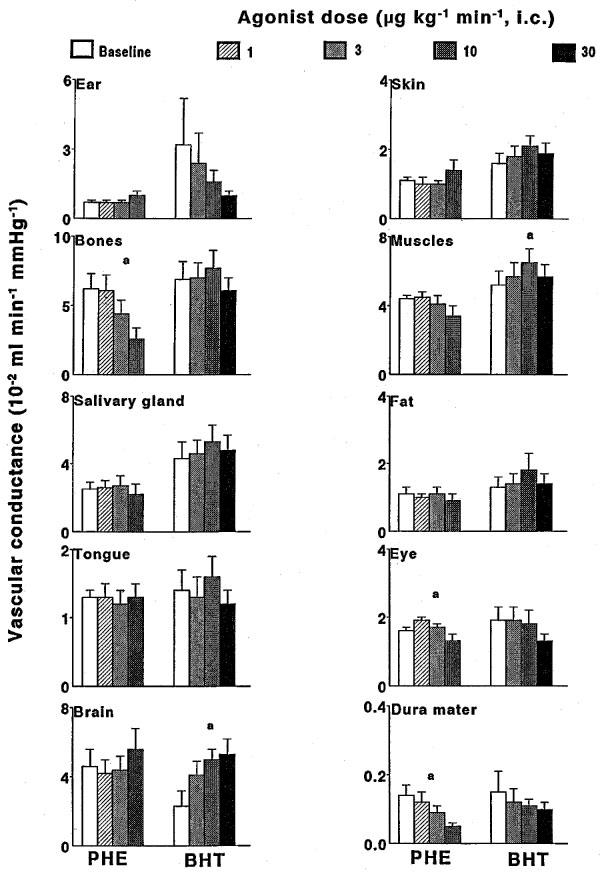
The effect of 10-min intracarotid infusions of phenylephrine (PHE) or BHT933 (BHT) on the distribution of total carotid conductance in different ipsilateral cranial tissues in anaesthetized pigs treated with vehicle (n=7 each). All data are presented as mean±s.e.mean. aP<0.05 vs baseline (ANOVA); bP<0.05 vs vehicle group (ANOVA).
Since in the present experiments mean arterial blood pressure was not affected by intracarotid infusion phenylephrine or BHT 933 (Table 2), the above described agonist-induced changes in vascular conductances were qualitatively and quantitatively similar to those observed in blood flow (data not shown).
Carotid vascular responses to sumatriptan in animals treated with GR127935
As reported earlier (De Vries et al., 1996), sumatriptan (30, 100 and 300 μg kg−1, i.v.) did not decrease either total carotid blood flow (% changes: −4±3, −5±3 and −6±3, respectively) or conductance (% changes: 8±4, 5±4 and 3±3, respectively) in animals treated with GR127935. In several previous publications from our laboratory (for references, see De Vries et al., 1998), we have shown that these doses of sumatriptan consistently cause dose-dependent decreases in the total carotid conductance. For example, the changes in the carotid vascular conductance reported by De Vries (1996) with 30, 100 and 300 μg kg−1, i.v. of sumatriptan in vehicle-treated pigs (n=5) were −22±4, −42±5 and −49±7, respectively.
Discussion
General
Several in vitro studies show that both α1- and α2-adrenoceptors mediate vascular effects in carotid vessels, including those of the dog (Kawai et al., 1988) and pig (Ohgushi et al., 1993). Nevertheless, very few studies have investigated whether these receptors are operative in the carotid circulation in vivo. Verdouw et al. (1984) reported that intracarotid bolus injections of noradrenaline elicited short-lasting and phentolamine-sensitive decreases in carotid and arteriovenous anastomotic conductance in pigs, whereas intracarotid infusions of noradrenaline were devoid of carotid vasoconstriction. Notwithstanding, the mechanisms involved in this response to noradrenaline were not further analysed, particularly in terms of different subtypes of α-adrenoceptors. Apart from the implications discussed below, the present study clearly shows that both α1- and α2-adrenoceptors can mediate vasoconstriction of carotid arteriovenous anastomoses in anaesthetized pigs.
Systemic and carotid haemodynamic effects of vehicle, prazosin and rauwolscine
Prazosin and rauwolscine produced moderate decreases in mean arterial blood pressure, most likely due to their blocking properties at, respectively, α1- and α2-adrenoceptors (Massingham & Hayden, 1975; Hoffman & Lefkowitz, 1996; Terrón et al., 1996). Importantly, no changes in carotid or arteriovenous anastomotic conductance were observed after treatment with prazosin or rauwolscine. This observation seems to contradict the results of Den Boer et al. (1993), who reported that prazosin causes a potent vasodilatation of carotid arteriovenous anastomoses. However, in contrast to their study where fentanyl/thiopentane was employed as anaesthetic, we used sodium pentobarbitone, which attenuates sympathetic tone and potently vasodilates carotid arteriovenous anastomoses (see also the Method section). Interestingly, rauwolscine, but not prazosin, increased capillary conductance, which was noticed in the ear, skin, muscle, bones, salivary gland, fat and tongue. Although we do not have a clear explanation, it may be that α2-adrenoceptors mainly maintain vascular tone in these cranial tissues. Alternatively, rauwolscine may have activated 5-HT1B/1D receptors, at which the compound displays partial agonist property (Shimamoto et al., 1993). 5-HT1B/1D receptors can indeed mediate vasodilatation in porcine coronary arteries (Schoeffter & Hoyer, 1990) and cranial tissues (De Vries et al., 1996).
Systemic haemodynamic effects of phenylephrine and BHT 933
No changes in blood pressure or heart rate were observed after BHT 933. In contrast, phenylephrine produced a moderate increase in heart rate, which remained essentially unchanged in animals treated with prazosin, rauwolscine or GR127935. Since this tachycardia was absent after propranolol (500 μg kg−1, i.v., n=3; unpublished observations), it would appear that phenylephrine has a direct action at cardiac β-adrenoceptors, as shown earlier (Bassett et al., 1968).
Carotid haemodynamic changes: role of α1- and α2-adrenoceptors
Intracarotid infusions of phenylephrine and BHT 933 dose-dependently decreased total carotid conductance, which in both cases was exclusively caused by vasoconstriction of carotid arteriovenous anastomoses. Consistent with the closure of arteriovenous anastomoses (Saxena, 1987), both phenylephrine and BHT 933 showed a trend towards increasing A-VSO2; this was however only significant after phenylephrine. The activity of the above agonists implies, but does not categorically prove, that α1- and α2-adrenoceptors are involved.
The involvement of α1-adrenoceptors in the vasoconstriction of carotid arteriovenous anastomoses is strengthened by the finding that prazosin completely blocked the effects of phenylephrine. It should be noted that in the dose employed prazosin acted selectively at α1-adrenoceptors, since the drug did not modify BHT 933-induced carotid vasoconstriction. α1-Adrenoceptors also mediate vasoconstriction within the canine external carotid vascular bed (Terrón et al., 1996) and seem to maintain vascular tone in porcine carotid arteriovenous anastomoses (Den Boer et al., 1993). On similar grounds, the fact that rauwolscine completely antagonized BHT 933-induced responses indicates that α2-adrenoceptors also mediate carotid arteriovenous anastomotic vasoconstriction. This conclusion is substantiated by previous results demonstrating that clonidine vasoconstricts porcine carotid arteriovenous anastomoses (Verdouw et al., 1984) and that α2-adrenoceptors partly mediate canine external carotid vasoconstriction by ergotamine and dihydroergotamine (Villalón et al., 1999). Furthermore, prazosin selectively attenuated the phenylephrine-induced increases in A-VSO2.
Taken together, the results of the present study clearly show that activation of both α1- and α2-adrenoceptors results in a vasoconstriction of carotid arteriovenous anastomoses. Interestingly, our results seem in contradiction with earlier experiments in pigs, where intracarotid infusions of noradrenaline did not induce carotid vasoconstriction, but rather increased total carotid and arteriovenous anastomotic blood flow (Verdouw et al., 1984). The suggestion that the absence of noradrenaline-induced vasoconstrictor effect was due to the putative α1-blocking properties of the anaesthetic azaperone (Den Boer et al., 1993) seems not to be the case, as we used similar anaesthetic regimen as Verdouw et al. (1984). A more likely explanation could be that noradrenaline, when continuously and slowly infused, also induces a β-adrenoceptor-mediated vasodilator effect in the carotid circulation, which negates carotid vasoconstriction. Interestingly, Cohen & Coffman (1981) have provided evidence for the presence of β-adrenergic vasodilator mechanism in human digital arteriovenous shunts.
Possible interactions of phenylephrine and BHT 933 with 5-HT1B/1D receptors
As pointed out earlier, 5-HT1B/1D receptors mediate the potent and selective vasoconstriction of carotid arteriovenous anastomoses caused by acutely acting anti-migraine drugs in the pig (for references, see Saxena, 1995; De Vries et al., 1999). One could therefore argue that a part of the phenylephrine- and/or BHT 933-induced carotid vasoconstriction might also involve 5-HT1B/1D receptors. This possibility can however be discounted as treatment of the animals with the potent and selective 5-HT1B/1D receptor antagonist GR127935 (Clitherow et al., 1994; Pauwels, 1996; Skingle et al., 1996) did not modify carotid vascular haemodynamic changes induced by either phenylephrine or BHT 933 (see Figures 1Figures 2Figures 3).
Importantly, the dose of GR127935 used in this study (500 μg kg−1, i.v.) completely blocks sumatriptan-induced decreases in total carotid (and arteriovenous anastomotic) blood flow and conductance (De Vries et al., 1996). This was apparently also the case in our present experiments where no changes in total carotid blood flow and conductance were observed with sumatriptan in animals treated with GR127935. From these data we can exclude the possibility that 5-HT1B/1D receptors play a role in the phenylephrine- or BHT 933-induced vasoconstriction of carotid arteriovenous anastomoses in anaesthetized pigs.
Effects of phenylephrine and BHT 933 on cranial tissue conductances
Although the effects of α-adrenoceptor stimulation have been extensively studied on isolated blood vessels, the use of intracarotid injection of radiolabelled microspheres allowed us to study these effects in the different cranial tissues in vivo. Stimulation of prazosin-sensitive α1-adrenoceptors resulted in vasoconstriction in bones, dura mater and, to a lesser extent, in the eye, whereas BHT 933 was devoid of tissue vasoconstrictor effects. In fact, BHT 933 produced vasodilatation in brain vasculature, as also reported after clonidine (Verdouw et al., 1984). This may be due to an interaction with endothelial vasodilator α2-adrenoceptors as previously reported in other blood vessels (Miller & Vanhoutte, 1985; Angus et al., 1986; Ohgushi et al., 1993). Indeed, the increase in brain vascular conductance was absent in the rauwolscine-treated group.
Possible clinical implications
Lastly, we would like to consider the possible clinical relevance of the vasoconstriction of the carotid arteriovenous anastomoses induced by phenylephrine and BHT 933. It has been suggested that vasodilatation of these 'shunt' vessels may play an important role in the pathophysiology of migraine (Heyck, 1969; Saxena, 1995). Indeed, to date all migraine abortive agents vasoconstrict carotid arteriovenous anastomoses in different animal species (for references see Saxena, 1995; Saxena et al., 1997). While carotid arteriovenous anastomoses cannot be directly investigated in humans, sumatriptan has been shown to vasoconstrict arteriovenous anastomoses in the human forearm (Van Es et al., 1995). In addition, ergotamine, dihydroergotamine, clonidine and isometheptene, all of which produce vasoconstriction in the carotid vascular bed in dogs and pigs (Spierings & Saxena, 1980; Verdouw et al., 1984; De Vries et al., 1998; Villalón et al., 1999), interact with α-adrenoceptors. At least, a part of the therapeutic effect of these drugs may be related to their agonist action at these receptors. Thus, our results demonstrating the role of α1- and α2-adrenoceptor in mediating vasoconstriction of carotid arteriovenous anastomoses imply that selective agonists at, particularly, α2-adrenoceptors (in view of the less ubiquitous nature of α2-adrenoceptors compared to α1-adrenoceptors) should have potential anti-migraine properties. In this connection, further studies, which fall beyond the scope of the present investigation, will be required to ascertain the specific subtypes of α1- (α1A, α1B, α1D) and α2 (α2A, α2B, α2C) adrenoceptors mediating the vasoconstrictor response mentioned above. Eventually, these results may be helpful in the development of anti-migraine agents in the future.
Abbreviations
- AVA
arteriovenous anastomotic
- A-VSO2
difference between arterial and jugular venous oxygen saturation
- BHT 933
6-ethyl-5,6,7,8-tetrahydro-4H-oxazolo [4,5-d] azepin-2-amine dihydrochloride
- GR127935
N-[methoxy-3-(4-methyl-1-piperazinyl) phenyl]-2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl) [1,1-biphenyl]-4-carboxamide hydrochloride
- 5-HT
5-Hydroxytryptamine
References
- ANGUS J.A., COCKS T.M., SATOH K. α2-adrenoceptors and endothelium-dependent relaxation in canine large arteries. Br. J. Pharmacol. 1986;88:767–777. doi: 10.1111/j.1476-5381.1986.tb16249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER C.H., DAVIS D.L., SUTTON E.T. Control of A-V shunt and capillary circuits in the dog hindpaw. Proc. Soc. Exp. Biol. Med. 1978;157:536–540. doi: 10.3181/00379727-157-40092. [DOI] [PubMed] [Google Scholar]
- BASSETT J.R., STORY M., CAIRNCROSS K.D. The influence of orphenadrine upon the actions of a series of sympathomimetic agents. Eur. J. Pharmacol. 1968;4:198–204. doi: 10.1016/0014-2999(68)90177-5. [DOI] [PubMed] [Google Scholar]
- CLITHEROW J.W., SCOPES D.I., SKINGLE M., JORDAN C.C., FENIUK W., CAMPBELL I.B., CARTER M.C., COLLINGTON E.W., CONNOR H.E., HIGGINS G.A., BEATTIE D., KELLY H.A., MITCHELL W.L., OXFORD A.W., WADSWORTH A.H., TYERS M.B. Evolution of a novel series of [(N,N-dimethylamino)propyl]- and piperazinylbenzanilides as the first selective 5-HT1D antagonists. J. Med. Chem. 1994;37:2253–2257. doi: 10.1021/jm00041a001. [DOI] [PubMed] [Google Scholar]
- COHEN R.A., COFFMAN J.D. Beta-adrenergic vasodilator mechanism in the finger. Circ. Res. 1981;49:1196–1201. doi: 10.1161/01.res.49.5.1196. [DOI] [PubMed] [Google Scholar]
- DEN BOER M.O., VAN WOERKENS L.J., SOMERS J.A., DUNCKER D.J., LACHMANN B., SAXENA P.R., VERDOUW P.D. On the preservation and regulation of vascular tone in arteriovenous anastomoses during anesthesia. J. Appl. Physiol. 1993;75:782–789. doi: 10.1152/jappl.1993.75.2.782. [DOI] [PubMed] [Google Scholar]
- DE VRIES P., HEILIGERS J.P.C., VILLALÓN C.M., SAXENA P.R. Blockade of porcine carotid vascular response to sumatriptan by GR127935, a selective 5-HT1D receptor antagonist. Br. J. Pharmacol. 1996;118:85–92. doi: 10.1111/j.1476-5381.1996.tb15370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE VRIES P., VILLALÓN C.M., HEILIGERS J.P.C., SAXENA P.R. Characterization of 5-HT receptors mediating constriction of porcine carotid arteriovenous anastomoses; involvement of 5-HT1B/1D and novel receptors. Br. J. Pharmacol. 1998;123:1561–1570. doi: 10.1038/sj.bjp.0701770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE VRIES P., WILLEMS E.W., HEILIGERS J.P.C., VILLALÓN C.M., SAXENA P.R.Constriction of porcine carotid arteriovenous anastomoses as indicator of antimigraine activity: the role of 5-HT1B/1D, as well as unidentified receptors Migraine & Headache Pathophysiology 1999London: Martin Dunitz Ltd; 119–132.eds. Edvinsson, L. pp [Google Scholar]
- FOLKOW B., SIVERTSSON R. Aspects of the difference in vascular reactivity between cutaneous resistance vessels and A-V anastomoses. Angiologica. 1964;1:338–345. doi: 10.1159/000157600. [DOI] [PubMed] [Google Scholar]
- HALES J.R.S. Radioactive microsphere techniques for studies of the circulation. Clin. Exp. Pharmacol. Physiol. 1974;1:31–46. [Google Scholar]
- HALES J.R.S., FOLDES A., FAWCETT A.A., KING R.B. The role adrenergic mechanisms in thermoregulatory control of blood flow through capillaries and arteriovenous anastomoses in the sheep hind limb. Pflügers Arch. 1982;395:93–98. doi: 10.1007/BF00584720. [DOI] [PubMed] [Google Scholar]
- HEYCK H. Pathogenesis of migraine. Res. Clin. Stud. Headache. 1969;2:1–28. [Google Scholar]
- HOFFMAN B.B., LEFKOWITZ R.J.Catecholamines, sympathomimetic drugs, and adrenergic receptor antagonists Goodman & Gilman's The Pharmacological Basis of Therapeutics 1996New York, NY, U.S.A.: McGraw-Hill; 199–248.eds. Hardman, J.G., Limbird, L.E., Molinoff, P.B., Ruddon, R.W. & Goodman Gilman, A. pp [Google Scholar]
- KAWAI Y., KOBAYASHI S., OHHASHI T. Existence of two types of postjunctional α-adrenoceptors in the isolated canine internal carotid artery. Can. J. Physiol. Pharmacol. 1988;66:655–659. doi: 10.1139/y88-102. [DOI] [PubMed] [Google Scholar]
- LEYSEN J.E.Serotonergic binding sites Serotonin and the cardiovascular system 1985New York: Raven Press; 43–62.eds. Vanhoutte, P.M. pp [Google Scholar]
- MASSINGHAM R., HAYDEN M.L. A comparsion of the effects of prazosin and hydrallazine on blood pressure, heart rate and plasma renin activity in conscious renal hypertensive dogs. Eur. J. Pharmacol. 1975;30:121–124. doi: 10.1016/0014-2999(75)90213-7. [DOI] [PubMed] [Google Scholar]
- MILLER V.M., VANHOUTTE P.M. Endothelial α2-adrenoceptors in canine pulmonary and systemic blood vessels. Eur. J. Pharmacol. 1985;118:123–129. doi: 10.1016/0014-2999(85)90670-3. [DOI] [PubMed] [Google Scholar]
- OHGUSHI M., YASUE H., KUGIYAMA K., MUROHARA T., SAKAINO N. Contraction and endothelium dependent relaxation via alpha adrenoceptors are variable in various pig arteries. Cardiovasc. Res. 1993;27:779–784. doi: 10.1093/cvr/27.5.779. [DOI] [PubMed] [Google Scholar]
- PAUWELS P.J. Pharmacological properties of a putative 5-HT1B/D receptor antagonist GR127935. CNS Drug Rev. 1996;2:415–428. [Google Scholar]
- PIASCIK M.T., SOLTIS E.E., PIASCIK M.M., MACMILLAN L.B. α-Adrenoceptors and vascular regulation: molecular, pharmacologic and clinical correlates. Pharmacol. Ther. 1996;72:215–241. doi: 10.1016/s0163-7258(96)00117-9. [DOI] [PubMed] [Google Scholar]
- RUFFOLO R.R., JR, NICHOLS A.J., STADEL J.M., HIEBLE J.P. Structure and function of α-adrenoceptors. Pharmacol. Rev. 1991;43:475–505. [PubMed] [Google Scholar]
- SAXENA P.R.Arteriovenous anastomoses and veins in migraine research Migraine, clinical, therapeutic, conceptual and research aspects 1987London, U.K.: Chapman and Hall; eds. Blau, J.N. [Google Scholar]
- SAXENA P.R.Cranial arteriovenous shunting, an in vivo animal model for migraine Experimental headache models 1995Philadelphia, U.S.A.: Lippincott-Raven Publishers; 189–198.eds. Olesen, J. & Moskowitz, M.A. pp [Google Scholar]
- SAXENA P.R., FERRARI M.D., DE VRIES P., VILLALÓN C.M.Pharmacological overview of new 5-HT1D receptor agonists in development for the acute treatment of migraine Headache treatment: trial methodology and new drugs 1997New York, U.S.A.: Lippincott-Raven Publishers; 229–241.eds. Olesen, J. & Tfelt-Hansen, P. pp [Google Scholar]
- SAXENA P.R., SCHAMHARDT H.C., FORSYTH R.P., HOEVE J. Computer programs for the radioactive microsphere technique. Determination of regional blood flows and other haemodynamic variables in different experimental circumstances. Comput. Programs Biomed. 1980;12:63–84. doi: 10.1016/0010-468x(80)90053-7. [DOI] [PubMed] [Google Scholar]
- SAXENA P.R., VERDOUW P.D. Redistribution by 5-hydroxytryptamine of carotid arterial blood at the expense of arteriovenous anastomotic blood flow. J. Physiol. 1982;332:501–520. doi: 10.1113/jphysiol.1982.sp014427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOEFFTER P., HOYER D. 5-Hydroxytryptamine (5-HT)-induced endothelium-dependent relaxation of pig coronary arteries is mediated by 5-HT receptors similar to the 5-HT1D receptor subtype. J. Pharmacol. Exp. Ther. 1990;252:387–395. [PubMed] [Google Scholar]
- SHIMAMOTO Y., SHIMAMOTO H., KWAN C.Y., DANIEL E.E. Rauwolscine induces contraction in the dog mesenteric artery precontracted with KCl and endothelin-1: mediation via 5-hydroxytryptamine1-like receptors. J. Pharmacol. Exp. Ther. 1993;264:201–209. [PubMed] [Google Scholar]
- SKINGLE M., BEATTIE D.T., SCOPES D.I.T., STARKEY S.J., CONNOR H.E., FENIUK W., TYERS M.B. GR127935: a potent and selective 5-HT1D receptor antagonist. Behav. Brain Res. 1996;73:157–161. doi: 10.1016/0166-4328(96)00089-7. [DOI] [PubMed] [Google Scholar]
- SPENCE R.J., RHODES B.A., WAGNER H.J. Regulation of arteriovenous anastomotic and capillary blood flow in the dog leg. Am. J. Physiol. 1972;222:326–332. doi: 10.1152/ajplegacy.1972.222.2.326. [DOI] [PubMed] [Google Scholar]
- SPIERINGS E.L., SAXENA P.R. Effect of isometheptene on the distribution and shunting of 15 microM microspheres throughout the cephalic circulation of the cat. Headache. 1980;20:103–106. doi: 10.1111/j.1526-4610.1980.hed2002103.x. [DOI] [PubMed] [Google Scholar]
- TERRÓN J.A., RAMIREZ-SAN JUAN E., HONG E., VILLALÓN C.M. Role of α1-adrenoceptors in the reduction of external carotid blood flow induced by buspirone and ipsapirone in the dog. Life Sci. 1996;58:63–73. doi: 10.1016/0024-3205(95)02256-2. [DOI] [PubMed] [Google Scholar]
- VAN ES N.M., BRUNING T.A., CAMPS J., CHANG P.C., BLAUW G.J., FERRARI M.D., SAXENA P.R., VAN ZWIETEN P.A. Assessment of peripheral vascular effects of anti-migraine drugs in humans. Cephalalgia. 1995;15:288–291. doi: 10.1046/j.1468-2982.1995.1504288.x. [DOI] [PubMed] [Google Scholar]
- VAN WOERKENS L.J., DUNCKER D.J., HUIGEN R.J., VAN DER GIESSEN W.J., VERDOUW P.D. Redistribution of cardiac output caused by opening of arteriovenous anastomoses by a combination of azaperone and metomidate. Br. J. Anaesth. 1990;65:393–399. doi: 10.1093/bja/65.3.393. [DOI] [PubMed] [Google Scholar]
- VERDOUW P.D., DUNCKER D.J., SAXENA P.R. Poor vasoconstrictor response to adrenergic stimulation in the arteriovenous anastomoses present in the carotid vascular bed of young Yorkshire pigs. Arch. Int. Pharmacodyn. Ther. 1984;272:56–70. [PubMed] [Google Scholar]
- VILLALÓN C.M., DE VRIES P., RABELO G., CENTURIÓN D., SÁNCHEZ-LÓPEZ A., SAXENA P.R. Canine external carotid vasoconstriction to methysergide, ergotamine and dihydroergotamine: a role of 5-HT1B/1D receptors and α2-adrenoceptors. Br. J. Pharmacol. 1999;126:585–594. doi: 10.1038/sj.bjp.0702324. [DOI] [PMC free article] [PubMed] [Google Scholar]


