Abstract
Background
The Onchocerciasis Control Program (OCP) in West Africa has been closed down at the end of 2002. All subsequent control will be transferred to the participating countries and will almost entirely be based on periodic mass treatment with ivermectin. This makes the question whether elimination of infection or eradication of onchocerciasis can be achieved using this strategy of critical importance. This study was undertaken to explore this issue.
Methods
An empirical approach was adopted in which a comprehensive analysis was undertaken of available data on the impact of more than a decade of ivermectin treatment on onchocerciasis infection and transmission. Relevant entomological and epidemiological data from 14 river basins in the OCP and one basin in Cameroon were reviewed. Areas were distinguished by frequency of treatment (6-monthly or annually), endemicity level and additional control measures such as vector control. Assessment of results were in terms of epidemiological and entomological parameters, and as a measure of inputs, therapeutic and geographical coverage rates were used.
Results
In all of the river basins studied, ivermectin treatment sharply reduced prevalence and intensity of infection. Significant transmission, however, is still ongoing in some basins after 10–12 years of ivermectin treatment. In other basins, transmission may have been interrupted, but this needs to be confirmed by in-depth evaluations. In one mesoendemic basin, where 20 rounds of four-monthly treatment reduced prevalence of infection to levels as low as 2–3%, there was significant recrudescence of infection within a few years after interruption of treatment.
Conclusions
Ivermectin treatment has been very successful in eliminating onchocerciasis as a public health problem. However, the results presented in this paper make it almost certain that repeated ivermectin mass treatment will not lead to the elimination of transmission of onchocerciasis from West Africa. Data on 6-monthly treatments are not sufficient to draw definitive conclusions.
Background
Ivermectin has been in operational use for more than a decade, and the question of the required duration of treatment is receiving renewed attention. In the ivermectin trials that were undertaken over a decade ago, the effectiveness of ivermectin treatment for morbidity control was demonstrated [1-3]. Transmission trials showed that mass treatment with ivermectin resulted in a major reduction in transmission of onchocerciasis in Africa [3]. However, after the first three rounds of treatment significant transmission continued. Simulation models that were calibrated using these early trial data predicted that interruption of transmission by ivermectin treatment alone would be difficult to achieve and that ivermectin treatment needed to be planned for a period of decades [4]. Hence, ivermectin treatment programs were established with a long time frame in mind, with the exact duration to be determined on the basis of further evaluation of the long-term impact of mass treatment on transmission and the parasite reservoir.
With the closing down of the Onchocerciasis Control Program (OCP) in West Africa at the end of 2002, all subsequent onchocerciasis control will be transferred to the participating countries, and will almost entirely be based on periodic mass treatment with ivermectin using the strategy of Community Directed Treatment with Ivermectin (CDTI) [5]. Questions still to be answered are where this strategy should be implemented, at what frequency, and for how long.
The question of the duration of treatment entails the question whether elimination of transmission – or preferably world wide eradication – with ivermectin treatment alone is possible. If elimination of transmission in this way turns out to be impossible, then, to avoid recrudescence, control has to continue indefinitely or until alternative interventions (e.g. macrofilaricidal drugs [6]) become available. Studies using the stochastic microsimulation program ONCHOSIM [7], carried out by Winnen et al. [4], indicate that prolonged high-coverage treatment programs with ivermectin would have a high probability of achieving elimination. However, many uncertainties remain about these model predictions, and much effort is needed to refine them. Therefore, at this moment, practical examples of successes of attempts to eliminate transmission would be much more cogent arguments in favour of the hypothesis that this is possible by using ivermectin alone.
The question of the duration of ivermectin treatment programs was raised during the meetings of the Joint Program Committee of the Onchocerciasis Control Program in West Africa (OCP) and the Joint Action Forum of the African Program for Onchocerciasis Control (APOC), held in Yaounde, Cameroon, in December 2000. It was recognised that the main experience with large-scale ivermectin treatment sustained over a long period of time in Africa is in the OCP. It was therefore recommended to undertake an in-depth analysis of the relevant OCP data, and to use the results of this to update ONCHOSIM model predictions of the long term impact of ivermectin treatment on onchocerciasis transmission, and of the feasibility to eliminate transmission of onchocerciasis with ivermectin treatment alone. A detailed analysis plan was developed during the annual internal operational research meeting of the OCP in March 2001, and the Center for Decision Sciences in Tropical Disease Control of the Erasmus University Rotterdam was contracted to undertake the analysis and the corresponding ONCHOSIM simulations. Results of the analysis were discussed during a meeting of onchocerciasis experts, held from 3–5 October 2001 at the Erasmus University Rotterdam, the Netherlands, and sponsored by OCP and by the UNDP / World Bank / WHO Special Program for Research and Training in Tropical Diseases (TDR). In this paper, these results are reported. The second phase of the work involved a recalibration of the ONCHOSIM model on the basis of the observed epidemiological trends in the different river basins selected for analysis. This work will be reported separately. The objectives of this study were:
1. to describe in detail the impact of ivermectin on epidemiological and entomological parameters: prevalence of microfilariae (mf), Community Microfilarial Load (CMFL), Annual Biting Rate (ABR), and Annual Transmission Potential (ATP), and
2. to assess the potential to eliminate transmission of onchocerciasis by ivermectin treatment alone, and under which conditions this can be achieved.
The same questions and answers are relevant for most of the rest of Africa where onchocerciasis is endemic (APOC countries), keeping in mind the absence of vector control in APOC countries. As yet, eradication is not the objective of APOC but evidence that ivermectin mass treatment could lead to eradication might impact on its objectives and strategies. Thus, the conclusions drawn in this paper may equally apply to APOC.
Materials and methods
Data
A total of 15 study areas were included in this study. 14 River basins / foci from the countries participating in the OCP were selected on the basis of their history of ivermectin treatment. These areas were divided into three groups, depending on the control measures taken: 1) areas that had ivermectin treatment only, 2) areas that had ivermectin treatment and vector control in parallel, and 3) areas that had ivermectin treatment after vector control. Areas which only had larviciding done or were under ivermectin treatment for too short a time, were excluded. Variation in the epidemiological background and treatment history were also important in selecting these areas. Data from the following 14 river basins / foci were included in the analysis:
1. Ivermectin treatment only:
A. River Gambia, Mako focus, Senegal and Guinea. 6-Monthly ivermectin treatment since 1989.
B. Rio Corubal, Guinea Bissau. 3-Monthly ivermectin treatment from 1991 until 1996.
C. Rio Gêba, Guinea Bissau. 6-Monthly ivermectin treatment from 1989 until 1996.
D. Falémé, Senegal and Mali. Annual ivermectin treatment since 1989.
E. Bafing, Mali and Guinea. Annual ivermectin treatment since 1989.
F. Bakoye, Mali and Guinea. Annual ivermectin treatment since 1989.
G. Baoulé, Mali. Annual ivermectin treatment since 1989.
2. Ivermectin treatment and (incomplete) vector control:
H. Tienfala focus, Mali. Annual ivermectin treatment since 1987 and (ground) larviciding since 1994.
I. Bui Gorge focus, Ghana. Annual ivermectin treatment since 1987, 3-monthly ivermectin treatment from 1994–1996, and aerial larviciding from 1975–1996.
J. Titira and Kouporgou focus, Togo. Annual ivermectin treatment since 1988, and larviciding since 1977.
K. Milo and Sankarani, Guinea. Annual ivermectin treatment since 1989, and aerial larviciding since 1989.
L. Asubende focus, Ghana. Annual ivermectin treatment since 1987, and larviciding since 1990.
M. Dienkoa, Burkina Faso. Annual ivermectin treatment since 1988, aerial larviciding since 1975 with some interruptions, ground larviciding since 1990.
3. Ivermectin treatment after vector control:
N. Bougouriba, Burkina Faso. 4-Monthly ivermectin treatment since 1996, larviciding from 1975–1990.
In addition, one non-OCP study area was included, namely Vina Valley in Cameroon. This was an area in which only ivermectin treatment was used. In Figure 1, a map is shown of the geographical locations of the 14 OCP areas.
Figure 1.
Map of the 14 study areas within the area covered by the OCP.
All geographical, epidemiological, and entomological data obtained between 1975 and 2001 were taken from the OCP databases as available in February 2002. Analyses of trends over time were made of the following epidemiological and entomological variables:
1. The sex and age standardized prevalence of microfilariae (mf) in people aged five years and older, directly standardized using the OCP standard population [8]. This variable indicates the percentage of individuals with positive skin snip on either one of the two iliac crests,
2. The community microfilarial load (CMFL), calculated as the geometric mean of the number of microfilariae per skin snip in adults 20 years and older [9]. As this index includes those with a mf count of zero, the mean was calculated after a log(n + 1) transformation, where n = the number of mf/snip. The CMFL is an indicator of the intensity of infection in a community.
3. The annual biting rate (ABR); the estimated number of Simulium bites a subject at a catching point would receive per year. This index is presumably not affected by ivermectin treatment, but is an indicator of human exposure to Simulium fly bites.
4. The crude annual transmission potential (ATP); the estimated number of Onchocercal L3 larvae which would have been transmitted to a subject at a catching site per year. The crude ATP includes all Onchocerca species. It is an indicator of human exposure to infectious Simulium bites, and therefore directly measures ongoing transmission.
5. The O. volvulus specific annual transmission potential (ATPO. volv.); the ATP due to O. volvulus exclusively.
In addition to data from OCP databases, use was made of spreadsheets supplied by OCP that contained information on CDTI geographical and therapeutic coverage. This information was compiled by OCP from notebooks kept by the Community Distributors, that was in several areas cross-checked by independent (non-OCP) assessors as well as by OCP Vector Control Unit field staff not directly involved with ivermectin treatment. Therapeutic coverage as used in the OCP is defined as the percentage of subjects treated out of the total census population. The eligible population consisted of all people older than 5 years, excluding pregnant women and those too ill to take treatment. Geographic coverage was defined as the percentage of communities in a district that received treatment out of the total number of eligible communities in that district.
Methods
The 14 study areas were first drawn on a paper map by hand and were subsequently transferred to a digital format appropriate for the geographic information system MapInfo version 6 (Maplnfo Corporation, One Global View, Troy, New York 12180–8399, USA), that was used for all geographical manipulations. Villages and capture points were selected using a geographical query in Maplnfo. Essentially, a village or a capture point was selected if its coordinates fell within one of the study areas. Consequently, survey villages or capture points that were actually located in one of the study areas but of which the coordinates were not known (missing values for the coordinates in the OCP databases) were not included. Additional capture points were identified by taking all nearest capture points of the selected villages, as specified in the OCP databases. Thereby, nearest capture points that were (just) outside one of the study areas could be included. This was done to strengthen the relationship between entomological and epidemiological variables. As some study areas bordered each other, nearest capture points could exist that were also nearest capture points of one or more villages in another adjacent area. Data from these capture points were used in the plots for both study areas. Capture points without associated entomological data were omitted.
Some of the study areas had, because of their close geographical proximity, a similar geography. These basins have been grouped together to ease presentation. The Rio Corubal and the Rio Gêba are discussed in one section, and so are the Falémé, Bafing, Bakoye, and Baoulé rivers. Because identical treatment schedules were used in these last four basins, and because epidemiological and entomological results were similar, only data aggregated over these last four basins are presented.
The analyses were done using SPSS version 10 (SPSS Inc., 233 S. Wacker Drive, Chicago, Illinois 60606, USA) and Splus version 2000 (Insightful Corporation, 1700 Westlake Avenue North, Suite 500, Seattle, Washington 98109-3044, USA). All plots were made with Splus 2000.
Results
In Table 1 the numbers of survey villages and capture points in the fourteen study areas from the OCP are shown. This table also includes the number of epidemiological and entomological observations available for each area, aggregated over villages, respective capture points. In all selected areas there were more villages than capture points, while in the total dataset from the whole area covered by OCP there was much more balance in this respect. This probably relates to the relative absence of vector control from these foci. In the absence of vector control fewer capture points are needed. In many areas, only small numbers of entomological observations were available, especially in the seven areas in which ivermectin treatment was used exclusively. The numbers of villages, capture points, and observations were not available for Vina Valley, Cameroon.
Table 1.
Number of survey villages and capture points and the total number of epidemiological and entomological observations per area in the 14 study areas from the OCP
| Area | Villages | Capture Points | ||
| # Vil. | # Obs. | #Pts. | # Obs. | |
| Ivermectin distribution only: | ||||
| - River Gambia, Mako focus (A) | 16 | 79 | 8 | 26 |
| - Rio Corubal (B) | 67 | 115 | 6 | 14 |
| - Rio Gêba (C) | 18 | 38 | 7 | 11 |
| - Falémé (D), Bafing (E), Bakoye (F), Baoulé (G) | 163 | 341 | 72 | 233 |
| Ivermectin and incomplete vector control: | ||||
| - Tienfala focus (H) | 7 | 18 | 5 | 47 |
| - Bui Gorge focus (I) | 18 | 47 | 6 | 87 |
| - Titira and Kouporgou focus (J) | 19 | 59 | 25 | 278 |
| Ivermectin and vector control in parallel: | ||||
| - Milo and Sankarani (K) | 46 | 170 | 37 | 267 |
| - Asubende focus (L) | 7 | 45 | 1 | 24 |
| - Dienkoa (M) | 25 | 52 | 15 | 151 |
| Recrudescence control with ivermectin: | ||||
| - Bougouriba (N) | 48 | 111 | 6 | 50 |
Ivermectin distribution only
River Gambia, Mako focus (A)
Background
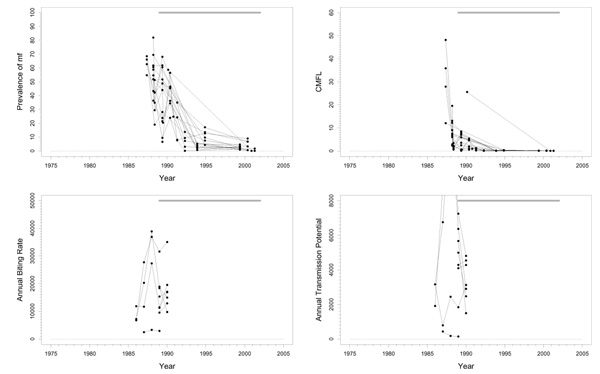
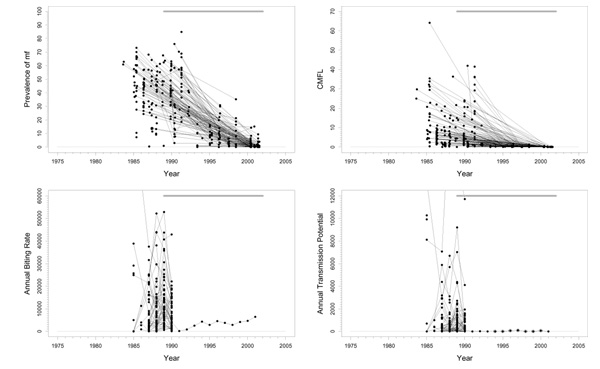
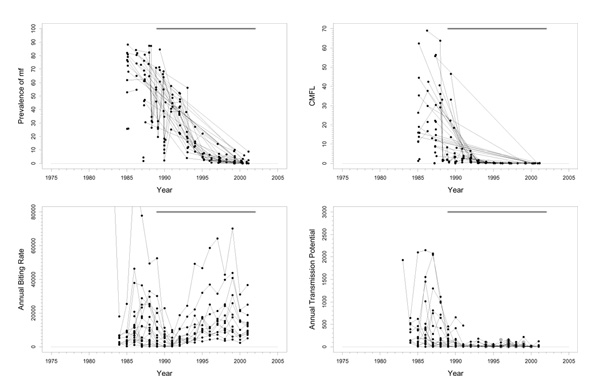
This basin is situated completely in the savannah area in the southeastern part of Senegal and the northeastern part of Guinea (Figure 1). In the northwestern part of the focus, along the Niokolo river, there is a large national park that extends to the north bank of the River Gambia. There is important human migration from south to north, and from south to west. Only savannah vectors are present, mostly S. sirbanum. The area is entomologically relatively isolated. However, as migration of flies follows the dominant winds, and relates to the proximity of the river basins, immigrating flies may come from the Falémé in the east during the Harmattan period. DNA probe identification of onchocercal larvae from collected flies started in 1992. In identified samples, collected and preserved in the period 1986–1992, 23% savannah strains of O. volvulus and 77% non-O. volvulus parasites (mostly O. ochengi) were found. Before onchocerciasis control, this was a hypo-/mesoendemic area. Pre-control prevalence ranged between 60% and 80%, and pre-control CMFL values were > 30 mf/s in only two survey villages (Figure 2). The prevalences in this focus differed considerably from those in the surrounding areas. From a transmission point of view, the rest of Senegal should be taken into account.
Figure 2.
Prevalence of microfilariae (mf), community microfilarial load (CMFL), annual biting rate (ABR), and crude annual transmission potential (ATP) against time in the River Gambia / Mako (A) study area. The thick grey lines at the top indicate the period over which ivermectin mass treatment was given. Note that the top two panels refer to survey villages, and the bottom two panels to capture points.
Experimental larviciding was carried out in 1989 for 20 weeks during the rainy season. Large-scale ivermectin treatment started in 1989, with a 6-monthly treatment schedule. Entomological studies were carried out for only two years in preparation of treatment. Too few data were recorded during these studies to obtain confident conclusions.
Trends
The prevalence of mf and CMFL declined dramatically to very low values, although low prevalences seem to persist in a few villages (Figure 2). This suggests that the parasite reservoir is close to suppression. The entomological data is very limited. The very large fluctuations in ABR values around an overall average value of 16900 may have been the result of random sampling error, or of year-to-year fluctuations because of, for instance, rainfall. The small number of ATP measurements indicated highly variable values around the rather high mean value of 4300, averaged over capture points and time. In addition to the data presented in Figure 2 there is some recent pool screening data wherein no infective flies have been detected, suggesting that current ATP values may be close to zero. However, this data involved only 3000 flies, which may be too few to decide whether transmission is really interrupted. No ATPO. volv. measurements were available. Therapeutic treatment coverage was high (around 80%). In a recent independent evaluation a geographic coverage of 100% was reported. Therefore, coverage in this basin seems to have been good.
Problems and comments
The results suggest that ivermectin treatment may have interrupted transmission in this area. However, this needs to be confirmed by more detailed entomological studies with full dissection of flies. If interruption of transmission is confirmed in these studies, it could be considered to stop treatment after a few more years (when the prevalence in all villages has fallen close to zero), while still keeping the surrounding areas under control, and observe whether transmission remains interrupted.
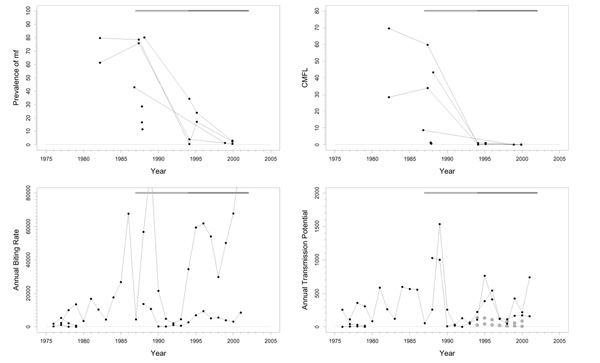
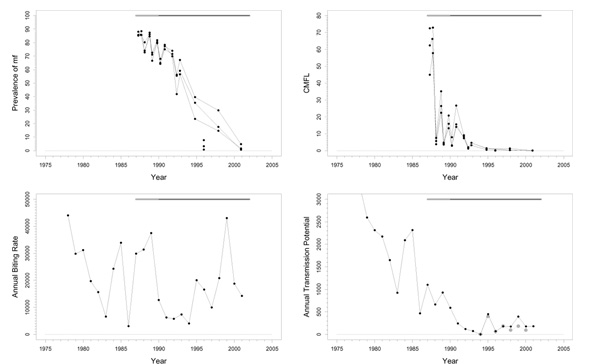
Rio Corubal (B) and Rio Gêba (C)
Background
The Rio Corubal is situated on the border between Guinea and Guinea Bissau (Figure 1), while the Rio Gêba is almost entirely within Guinea Bissau on the border with Senegal (Figure 1). Both areas are close to the River Gambia, Mako focus. Rio Corubal used to be a mesoendemic area, while the Rio Gêba had a very low pre-control endemicity. Vector control has never been applied in these areas, but some capturing and dissection was done for a period of four years in Rio Corubal (1989–1992) and two years in Rio Gêba (1989–1990). Only the savannah vector species S. sirbanum is present. DNA probe examinations of parasites in 46 infective flies from both basins for the period 1993–2000 showed 6.5% O. volvulus and 93.5% non-O. volvulus. In Rio Corubal, 3-monthly ivermectin treatment started in 1991, but ended in 1996 because of civil strife in the area. In Rio Gêba, 6-monthly ivermectin treatment was started in 1989, and also stopped in 1996.
Trends in Rio Corubal
Pre-control prevalence in the Rio Corubal basin varied from almost zero to 73% (Figure 3), and CMFL levels were mostly < 30 mf/s. Prevalence showed a favourable trend, and decreased in most villages to values close to zero in 1997 when ivermectin was no longer distributed. However, recently collected data strongly suggests that at least in some villages prevalence is increasing again from 0–12% in 1997 to values between 12% and 36% in 2001. The plot of CMFL values shows a large heterogeneity at the start of the treatment program. Some villages were meso- or even highly endemic, but the majority had rather low values. After the start of ivermectin distribution CMFL diminished to values close to zero around 1997 and after. Unfortunately, almost all entomological data pertains to the early intervention period, and has, therefore, little bearing on the current situation. Only one more recent observation, made in the capture point in the village Cabuca, was available. ABR was not extremely high, excepting a few high points observed in Cabuca, and seems compatible with a mesoendemic situation. Crude ATP either was not very high, except again at capture point Cabuca. No ATPO. volv. values were available. In entomological studies carried out in July 1997 and July 2001 at Cabuca, 1 fly infected with O. volvulus was found among 1054 and 1030 dissected flies respectively. Raw infectivity declined from 60.61 inf/1000 p (infective flies per 1000 parous flies) in July 1989 to 6.92 inf/1000 p in July 1997 and then increased again to 45.73 inf/1000 p in July 2001 at Cabuca. Therapeutic coverage increased from around 60% at the start of the program to good values around 80% in 1996. No data on geographic coverage was available.
Figure 3.
Prevalence of microfilariae (mf), community microfilarial load (CMFL), annual biting rate (ABR), and crude annual transmission potential (ATP) against time in the Rio Corubal (B) study area. The thick grey lines at the top indicate the period over which ivermectin mass treatment was given. Note that the top two panels refer to survey villages, and the bottom two panels to capture points.
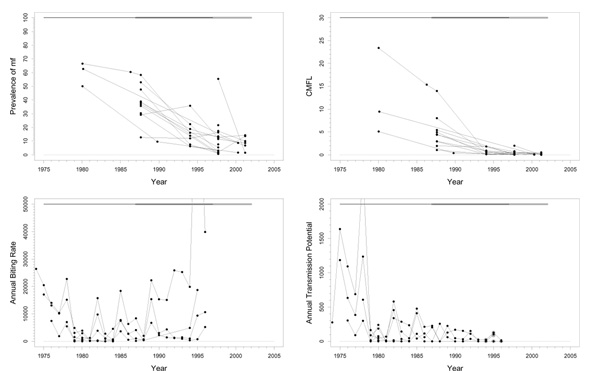
Trends in Rio Gêba
At the start of the program, prevalences in the Rio Gêba basin varied from almost zero to 28%, and CMFL values were < 5 mf/s. These low values decreased further to values near zero in 1997 (Figure 4). In 2001, when the last epidemiological data was collected, only zero prevalences and CMFL values were observed. ABR (range 0–7393) and ATP (range 0–245) were quite low, and consistent with the epidemiological parameters. O. volvulus specific ATPO. volv. measurements were not available. In entomological studies carried out in July 1997 and July 2001 at Station Medidora no flies infected with O. volvulus were found among 38 and respectively 331 dissected flies. Raw infectivity at that capture point declined from 37.74 inf/1000 p in July 1990 to 8.10 inf/1000 p in July 1997, and to 0.0 inf/1000 p in July 2001. ABR and ATP at Station Medidora were 134 and 0 respectively in 2001 (Figure 4). Therapeutic coverage, although available for only three villages, appears to have been good (80%). Geographic coverage is not known.
Figure 4.
Prevalence of microfilariae (mf), community microfilarial load (CMFL), annual biting rate (ABR), and crude annual transmission potential (ATP) against time in the Rio Gêba (C) study area. The thick grey lines at the top indicate the period over which ivermectin mass treatment was given. Note that the top two panels refer to survey villages, and the bottom two panels to capture points.
Problems and comments
The reason for the high frequency treatment regimen in these areas was that OCP wanted to study in an area that was hypo- to mesoendemic whether it was possible to interrupt transmission by supplying a large quantity of ivermectin at short intervals. This was feasible because at that time OCP used mobile treatment. The favourable figures on therapeutic coverage show that this treatment schedule was totally acceptable to the population.
Trends in the Rio Corubal area seem similar to those in the River Gambia focus, but later follow-up showed evidence of significant recrudescence. After cessation of control, prevalence was low but increased in four years time to a serious high level under a low level of transmission – as deduced from the available entomological data. After about 20 rounds of ivermectin treatment in a mesoendemic situation this presents a situation of concern.
If the criterion of CMFL values < 10 mf/s had been applied, the Rio Gêba basin should not have been included in the program at all. Despite the limited data available, it seems that in this area onchocerciasis is now eliminated. It is unclear whether this is the result of ivermectin treatment or whether onchocerciasis would have died out by itself in this low endemic area.
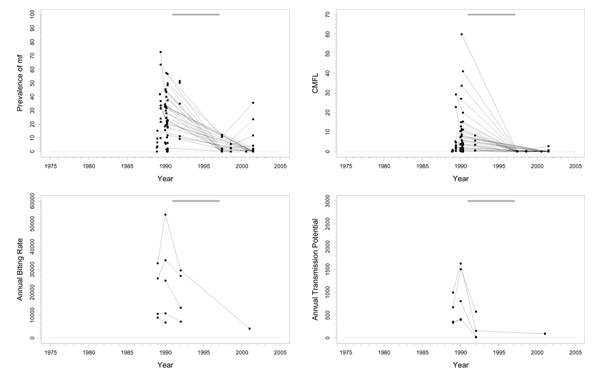
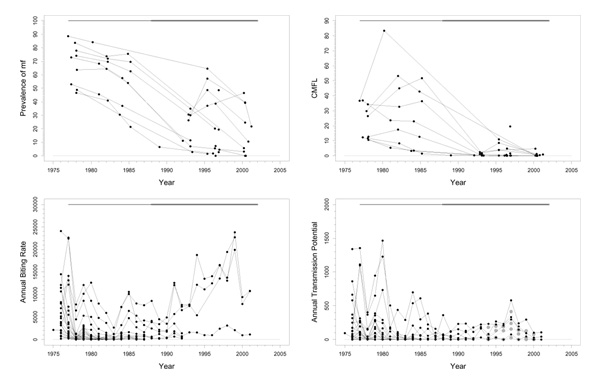
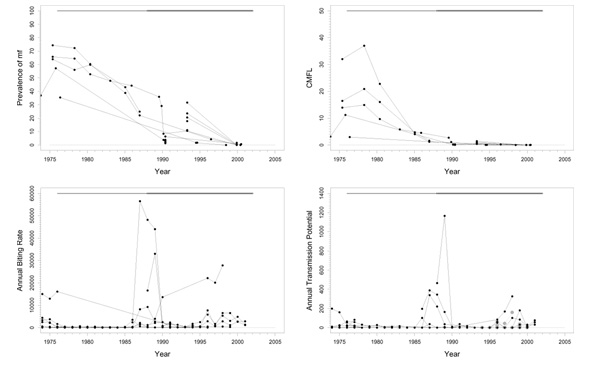
Falémé (D), Bafing (E), Bakoye (F), and Baoulé (G)
Background
These basins are located across the borders between Mali, Senegal, and Guinea in the western extension area of the program (Figure 1). Because of their geographical similarity and comparable control regimens, their epidemiological and entomological data was aggregated and presented in one series of plots (Figure 5). The Falémé, a tributary of the Senegal, is situated on the border between Senegal and Mali (Figure 1), and has a complex river system with many tributaries. It was not a high prevalence area and only just qualified for inclusion in the program. The Bafing basin is situated in Mali and Guinea and had a medium pre-control endemicity. The Bakoye is also situated in Mali and Guinea, on the eastern side of the Bafing basin (Figure 1). Pre-control endemicity levels in this basin were very variable. The Baoulé study area is situated entirely in Mali. Pre-control endemicity levels indicated that this was a mesoendemic focus. The lower parts of the Falémé, Bafing, Bakoye, and Baoulé were medium risk areas for blindness, as they were situated in a zone with little or no onchocercal blindness. The middle part of the Bakoye was, in terms of CMFL, a high risk area for blindness. Only savannah vector species are present, mostly S. sirbanum (100% in Falémé basin, 66.5% in Bafing, 85.6% in Bakoye, and 82.7% in Baoulé), complemented by small percentages of S. damnosum s.s., S. dieguerense and S. squamosum. Annual ivermectin treatment was introduced in these four basins in 1989.
Figure 5.
Prevalence of microfilariae (mf), community microfilarial load (CMFL), annual biting rate (ABR), and crude and O. volvulus specific annual transmission potentials (ATP and ATPO. volv.) against time in the Falémé(D), Bafing (E), Bakoye (F), and Baoulé (G) study areas. The thick grey lines at the top indicate the period over which ivermectin mass treatment was given. Note that the top two panels refer to survey villages, and the bottom two panels to capture points. ATPO. volv. values are denoted by the grey symbols in the plot for ATP.
Trends in the Falémé basin
Pre-control prevalence in 1986/1987 ranged from 13% to 64% (Figure 5). Since then, there has been a good downward trend towards values below 5% in 2001. Pre-control CMFL values were mostly low (< 10 mf/s), with the exception of one or two villages. CMFL also had a favourable downward trend reaching values close to 0 mf/s in 2001 (range 0.0–0.13). Therapeutic coverage was good (around 80%). Very high ABRs (40000–50000) were observed in some places, but most capture points had much lower values. The median ABR over all capture points and over the total period of follow-up amounted to 8070. ATP also was high at some sites (over 7000), but low at most others (median ATP over time and capture points was 366). In 2000, 21% savannah strain O. volvulus were found in DNA probe identification of 14 parasites. No entomological data are available after 1990.
Trends in the Bafing basin
Most villages had pre-control CMFL values < 23 mf/s (Figure 5), and prevalences ranged from 0.4% to 68% with an average of 32%. Prevalence and CMFL had good trends towards very low values in 2001 (< 9% for prevalence and < 0.2 mf/s for CMFL). Therapeutic coverage data was available for only two villages, and these data indicate that coverage might not have been good (20%-80%). The ABR was not so high, and was typically < 10000, with a median value over all capture points and over time of 4600. This was lower than in the Falémé basin, although these two basins were comparable in terms of prevalence. Entomological data was scarce after 1990. Extensive follow-up until 2000 was available for only one capture point with an ABR that rose slowly from 180 in 1990 to 6455 in 2001. ATP was also not very high (< 1000 in most villages). The recent not yet zero ATP and ATPO. volv. values (0–100) suggest that there is still some transmission going on. In 2000, no O. volvulus savannah strain were found in DNA probe identification of 9 parasites.
Trends in Bakoye basin
Pre-control prevalence in this basin ranged from 7% to 73%. The trends in prevalence were good (Figure 5), with the exception of one village that had a prevalence of 35% in 1998 after which follow-up ceased. CMFL values before control ranged from values near 0 mf/s to 35 mf/s, that subsequently dropped to very low values. One village, that was not followed up, had a pre-control CMFL of 64.1 mf/s. Therapeutic coverage was – with values slightly above 80% – very good. No entomological data was collected after 1990, but the ABRs and ATPs collected before that year were compatible with a mesoendemic situation. Median ABR over the whole period of follow-up and over all capture points was 12400; median ATP over the whole period and capture points was 771. There were occasional much higher ABR (> 40000) and ATP (> 4000) values. 12% O. volvulus savannah strain were found in DNA probe identification of 190 parasites in 2000.
Trends in the Baoulé basin
Pre-control prevalences in this area ranged from 32% to 63% (Figure 5). Prevalences decreased to values near 0% in 2001 (range 0–4%). CMFL values before the start of treatment were in the range of 0.3–32 mf/s, and decreased to zero values in 2000 and 2001. Therapeutic coverage was good (70–80%). No entomological data was collected after 1990 in this basin also. ABR seemed somewhat lower than in the other basins in this group, but was otherwise consistent with a mesoendemic situation. Overall median ABR was 4275 (range 475–25700). ATP was low with a median value over all capture points and over time of 350. There was one considerably outlying capture point among these data, with a much higher ATP (10271) and ABR value (25705) in 1985. This point seems to have been visited up to 1985 only. In 2000, 39% O. volvulus savannah strain were found using DNA probe identification among 31 parasites.
Problems and comments
Based on data collected in the 1960s and 1970s these four basins were highly endemic and vector control was planned for them. Subsequently, the whole area was badly affected by drought in the 1970s and the beginning of the 1980s. When OCP carried out epidemiological surveys before starting the western extension, endemicity appeared to be much lower than expected, and especially the blinding form of the disease had disappeared from the north of the area. Now the period of drought is over, and rainfall and river discharge have returned to normal values, the entomological parameters may resemble those of the 1960s and 1970s.
Geographical coverage may not have been very good. Because these basins are far from urban centers and have very bad roads making communication difficult, they are difficult to treat. The figures from the external evaluations may, therefore, be inaccurate.
From the point of view of morbidity control, the situation in the whole area is good, and onchocerciasis is no longer a public health problem. The latest epidemiological results are similar to those for the Gambia basin in spite of the different treatment interval (annual vs. 6-monthly). The lack of recent entomological data makes it impossible to state whether transmission has been interrupted. Because prevalences are still close to 10% in some villages, stopping ivermectin distribution is, however, not indicated.
Vina Valley, Cameroon
Background
The river Vina runs from west to east in the center of Cameroon close to the borders with Chad and the Central African Republic. The north of Cameroon is a sudano-savannah region and is amongst the most hyperendemic foci of the blinding savannah type onchocerciasis. The Vina du Nord Valley is located between the sudano-savannah focus in the north and the grassland further south. In the grassland, endemicity of human onchocerciasis is low, despite very high fly biting intensities and quite high O. volvulus transmission. This is probably due to cattle zoöprophylaxis and cross-protective immunity by O. ochengi transmission [10].
The area along the river Vina is geographically isolated by mountain ranges on the north and on the south. Following the civil upraise in the year 1928, a new main road was built along the valley, and the villagers in the study area around Sora Mboum were moved from their traditional settlements uphill on the mountains south of the river towards this road. There are now 63 villages in the valley, almost all of which lie on the road that runs from west to east roughly parallel to the river. It is likely that the human population came into much closer contact with Simulium vectors and onchocerciasis after they moved to the road. Wherever the road approached the river closely, there was a very high hyperendemicity, with CMFL's in excess of 200 or 300 mf/s. Substantial proportions of the villagers had severe eye lesions (7.1% in Touboro, and 19% in Bonandinga [11]).
Transmission is effected mainly by S. damnosum s.s.. A small proportion of flies belong to S. sirbanum and S. squamosum. Pre-control ABR increased from 24250 at the upstream part of the river in the west to 47450 at the downstream part in the east. Pre-control ATP also increased in the downstream direction from 1055 to 4190. Just over one half to about three quarters of the infective larvae were O. volvulus. Annual ivermectin treatment was started by the Centre Pasteur in 1987 in the most eastern part of the area. This area was expanded twice in 1988 to cover the whole basin. Since 1995 all of north Cameroon has been treated with ivermectin under control of APOC.
The percentage of flies infected with O. volvulus L3 decreased from a pre-treatment level of 0.0171% in 1987–1988 to about 0.004% in 1992–1993 and then increased again to 0.007% in 1996–1998. The percentage of non-O. volvulus (O. ochengi and O. ramachandrini) declined slightly from 0.013% in 1987–1988 to 0.010% in 1992–1996 and then increased to 0.016% in 1996–1998. Thus, O. ochengi actually increases over the whole period, presumably as a result of the increasing number of herdsmen coming into the area from the north, driven south by drought.
Trends
CMFL in Sora Mboum, in the center of the area, diminished quickly from about 50 mf/s in 1987/1988, to 1 in 1996/1997 [12]. Sora Mboum was not the most highly endemic village in the area. Prevalence decreased from 80% in 1987/1988 to just over 20% in 1996/1997. O. volvulus ATP decreased from 1221 in 1987/1988 to values between 200 and 500 in the following years. Levels fluctuated following the trend in ABR. A very low number of 29 was reached in 2000, but then it went up again to > 293. ATP due to O. ochengi increased constantly, while there was also an increase in ATP due to O. ramachandrini from the warthog.
Ivermectin was distributed free of charge during the early phase of treatment in this area, but later on a system of partial cost-recovery was applied. Treatment coverage varied between 42% and 76% in two evaluated villages along the river [13], but was most likely much lower in the people from nomadic tribes. Therapeutic coverage was thus not very high. Geographic coverage on the other hand was high because almost all the population lived in the villages along the road. The only exceptions being the nomadic herdsmen who stayed in the bush. No coverage data is available after 1994, but it is likely that values have remained approximately the same. Treatment is now carried out by APOC.
Problems and comments
The main conclusion is that there was a significant decrease in transmission due to 12 years of ivermectin treatment in this area but certainly no interruption. However, the now increasing proportion of bloodmeals on cattle and the predominance of O. ochengi in the ATP is supposed to have an overall beneficial synergic effect on the reduction of human onchocerciasis [10,12].
Ivermectin and vector control in parallel
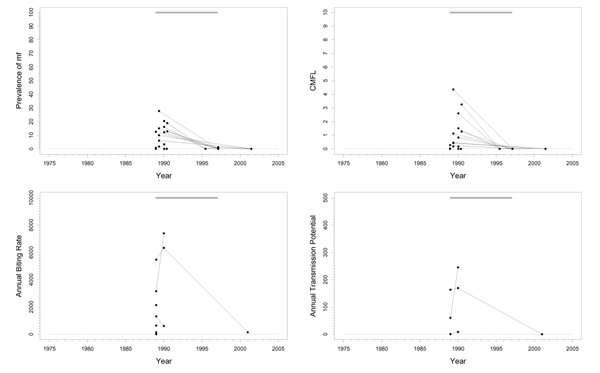
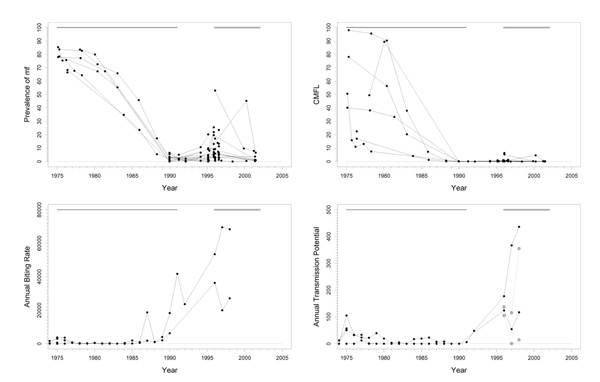
Tienfala focus (H)
Background
This is a highly endemic area, situated in southern Mali on the river Niger, just on the border of the original program area and the western extension (Figure 1). The area has a complex history of larviciding. Aerial larviciding started in 1977 in the dry season, but was suspended in the rainy season because of very high river discharges (> 2000 m3/sec). No larviciding was done from 1977 until 1985. From 1986 until 1993 aerial and ground treatment were used, but there were also periods in which treatment was suspended. From 1994 to the present only ground larviciding (by boat) was used on the river Niger. The huge size of the complex of breeding sites makes vector control extremely difficult in this area. One of the objectives is to protect the people on the riverbanks against nuisance. Transmission is effected by S. sirbanum exclusively. In 2001, the percentages of O. volvulus strain differed considerably between sites: 5% in Tienfala, 12% in Faya, 23% in Dylamba, and 32% in Sanankoro (Fie). Annual ivermectin treatment was started in 1987. In the early 1980's nodulectomies were performed in one village in this area in connection with research on the population dynamics of O. volvulus [14] until this research was stopped.
Trends
The trend in prevalence was good with levels before the start of ivermectin treatment varying from 11% to 80% in 1987 decreasing to values ≤ 3% in 2000 (Figure 6). The trend in CMFL was similarly good, with pre-ivermectin control levels ranging from 9 to 70 mf/s dropping to near zero values reached around 1995. Therapeutic coverage rose from 60% in 1988 to 80% in 1998. ABR was generally high and very variable, and sometimes even exceptionally high (ranging from 0 to > 80000). ATP was mostly below 700 with a few exceptional years with higher values. ATPO. volv. values, available from 1994 onwards, were generally much lower, and varied between 7 and 135. ATP did not seem to decrease very much, which seems to be consistent with the fact that this ATP contained such a large percentage non-O. volvulus.
Figure 6.
Prevalence of microfilariae (mf), community microfilarial load (CMFL), annual biting rate (ABR), and crude and O. volvulus specific annual transmission potentials (ATP and ATPO. volv.) against time in the Tienfala (H) study area. The thick grey lines at the top indicate the period over which ivermectin mass treatment was given, while thin black lines indicate the period of vector control. Note that the top two panels refer to survey villages, and the bottom two panels to capture points. ATPO. volv. values are denoted by the grey symbols in the plot for ATP.
Problems and comments
The dramatic decline in parasite loads in this holoendemic focus is illustrative of good morbidity control. It is unclear what the relative contributions of vector control and ivermectin distribution have been. However, as the pattern of decline in epidemiological parameters was similar to that observed in basins where only ivermectin distribution was used, and as the main part of the improvement seems to have occurred after the introduction of ivermectin treatment, it seems plausible that the contribution of ivermectin was dominant. Apart from this, it should be noted that there might have been a contribution from the nodulectomies carried out in one of the villages, but this is unlikely to have been very important.
Bui Gorge focus (I)
Background
Bui Gorge is located in Ghana on the lower Black Volta (Mouhoun) river (Figure 1). This area has the largest continuous string of breeding sites in the program running from Tagadi in the north to Tain-Aboi in the south. The southern limit of the focus is not clearly defined. Most of the flies in this area are savannah flies (S. damnosum s.s. and S. sirbanum). Aerial larvidicing started in 1975 and was stopped 22 years later on December 31, 1996. Some nodulectomies were performed in a few places, but this did not seem to have had much effect. Annual ivermectin treatment was started in 1987. A 3-monthly treatment schedule was used from 1994 to 1996.
Trends
Real pre-control epidemiological parameters are not available. Prevalences dropped from around 60% in 1980 to low, but certainly not zero, values in 2001 (Figure 7). In one village, a very high prevalence was observed in 1997. After 1996, CMFL values were not high, indicating that 22 years of larviciding might have had an effect. Therapeutic coverage was initially not good (50% in 1987) but was much improved after 1995 (> 80% in 1997). ABR was very variable and decreased from values in the range 7430–26400 in 1975 to rather low values in the period 1980–1985 but then increased again to be in the range 5202–39850 in 1995 and later with an occasional extremely high value (> 50000). ATP was high in some sites but not in others and dropped to low levels around 1996. ATPO.volv. measurements, available for 1994 and later, were similar to the crude ATP values.
Figure 7.
Prevalence of microfilariae (mf), community microfilarial load (CMFL), annual biting rate (ABR), and crude and O. volvulus specific annual transmission potentials (ATP and ATPO.volv.) against time in the Bui Gorge (I) study area. The thick grey lines at the top indicate the period over which ivermectin mass treatment was given, while thin black lines indicate the period of vector control. Note that the top two panels refer to survey villages, and the bottom two panels to capture points. ATPO.volv. values are denoted by the grey symbols in the plot for ATP.
Problems and comments
It is difficult to link entomological and epidemiological data in this area. The most recent entomological data from 1996 suggest that transmission is very low or even absent, while the epidemiological data from 1996 and later indicate that transmission is still on-going. There may be several explanations for this discrepancy. The first is human migration. Sociological studies indicated that there was a certain amount of population movement to the south. In the south-eastern part there was still some amount of transmission and treatment. Because of the low ATP levels and high prevalences ivermectin treatment was introduced. A second explanation might be that at Bui Dam Site there is a population of fishermen who came from the south where not all areas are treated.
The Ghanian government has planned to build a dam at Bui-Damsite. The lake that will result from this project will flood a lot of upstream breeding sites, which will probably solve most of the problems in this area.
The situation in this basin is complicated by many factors. Although larviciding had an effect, 22 years of vector control did not interrupt transmission. Ivermectin distribution has improved the epidemiological parameters but has equally not interrupted transmission.
Titira and Kouporgou focus (J)
Background
This is a meso- to hyperendemic area located in the eastern part of the program in Togo (Figure 1). It is a mountainous area with many huge breeding sites in the rainy season. The core area consists of the Oti, Keran, Kara, and Mo river basins. The Keran river runs through Togo and Benin. Most of the flies are of the savannah species; there are very few forest flies. It is likely that there is migration of Simulium flies from the Oti to the Keran rivers. There is much human migration in this area. Thousands of gold miners migrate during the dry season from Ghana, Togo, Benin, and Nigeria to the Keran river. It is also possible that there are migrants from the south of Togo.
Larviciding started in 1977, but results were poor until in 1987 larviciding was started in the south. Annual ivermectin distribution started in 1988. Aerial treatment was suspended from 1993 until 1996 on the Oti, and on the lower Keran and lower Kara rivers.
In 1997, the situation in this area became very problematic when larviciding appeared to be ineffective. In Titira, after 20 years of vector control, ATP was still about 400. This lead some participants of the expert advisory committee meeting of 1998 to believe it was better to stop. The recommendations that were made were to continue larviciding and to increase, if possible, first the coverage of larviciding and then the coverage of the ivermectin distribution. These recommendations were strictly followed and this has led to improvement. However, the situation in this focus is still far from elimination of transmission.
Trends
Prevalence decreased from very high values (between 53% and 89%) in 1977 to still high values (range 0–47%) in 2000 (Figure 8). CMFL has declined substantially from values around 25 mf/s to very low values, but is in some places not yet zero. Therapeutic coverage was reported to be good (80%) with equally high geographic coverage (96%). ABR has risen considerably in recent years to values around 15000, but this observation was based on data from two capture points only, which had high values throughout the whole period. ATP decreased from values around 1000 at some catching points to much lower values, but remained above 100. Only in the last two years may there have been an improvement in ATP towards values below 100. Similar trends were observed in the ATPO. volv. measurements, although on a somewhat lower level. It is unclear as to what extent this improvement is attributable to vector control or to ivermectin treatment.
Figure 8.
Prevalence of microfilariae (mf), community microfilarial load (CMFL), annual biting rate (ABR), and crude and O. volvulus specific annual transmission potentials (ATP and ATPO. volv.) against time in the Titira and Kouporgou (J) study area. The thick grey lines at the top indicate the period over which ivermectin mass treatment was given, while thin black lines indicate the period of vector control. Note that the top two panels refer to survey villages, and the bottom two panels to capture points. ATPO. volv. values are denoted by the grey symbols in the plot for ATP.
Problems and comments
It is clear that there are still problems in this area. The epidemiological results are far from satisfactory, and significant transmission continues. Recent CMFL values are low, but the prevalences are still 0% to 47%. The entomological data suggests that there has been improvement in the last two years, but this is over a very short period only.
There may be several explanations for this. One is that there is somehow a coverage problem with the ivermectin distribution, despite that this is not confirmed by the independent evaluations. It should be noted that these last evaluations were made in static villages only and did not include the migrants, many of which (gold miners) are extraordinarily hard to reach. Furthermore, ivermectin distribution is difficult in this mountainous area with bad roads and villages that are consequently difficult to reach. This casts doubt on whether geographic coverage was really good enough. A further explanation may be that there is transmission of disease going on in groups that do not partake in the epidemiological monitoring (the taking of skin snips), but there are no data to substantiate this. A good coverage and reinvasion affecting the vectors could also explain the situation. Finally, parasites could have been brought in by migrants, but there is also no data that supports this. In all, the epidemiological and entomological data seem consistent. It is not possible to stop ivermectin distribution in this basin.
Milo and Sankarani (K)
Background
The Milo and Sankarani are both tributaries of the Niger river and belong to the intermediate zone between forest and savannah. This zone is characterized by an intense seasonal migration of forest and savannah flies from the south-west to the north-east and vice versa with the help of the monsoon and harmattan winds. A compilation of weekly fly captures from 1988 to 1999 yielded that the highest fly densities occur during the harmattan period (the dry period including January, February, and March). Furthermore, the maximum numbers of infective female flies are found in this period of the year.
The Milo river is situated entirely in Guinea (Figure 1). The Sankarani is almost entirely located in the eastern part of Guinea; a small part extends to Cote d'lvoire. There is sudano-guinean savannah in the medium and lower parts of the basins. The lower parts are mountainous with more vegetation. Maximum river discharge is in September and October. The human populations are involved in agro-pastoral activities and fishing. Other important activities are diamond and gold mining, mainly done during the dry season.
In the lower part of the Milo basin there is a predominance of savannah vectors (S. sirbanum and S. damnosum s.s.) throughout the year. In the medium part, there is a predominance of savannah vectors (S. sirbanum and S. damnosum s.s.) during the dry season, and forest vectors (S. squamosum essentially) in the rainy season. In the upper part of the basin, there is a predominance of forest vectors (S. squamosum and S. yahense) throughout the year. The proportions of parasite strains found in 35 infective flies from 1993 to 2000 were 52% O. volvulus savannah, 14% O. volvulus forest, 32% non-O. volvulus. The pre-control situation in the Milo basin was hypo- to hyperendemic with prevalences ranging from 4.4% to 87% in villages evaluated from 1985 to 1987 (Figure 9). Anti-reinvasion larviciding was done in 1987 and 1988, and full-scale larviciding was started in 1989. Large scale annual ivermectin treatment started in 1989.
Figure 9.
Prevalence of microfilariae (mf), community microfilarial load (CMFL), annual biting rate (ABR), and crude and O. volvulus specific annual transmission potentials (ATP and ATPO. volv.) against time in the Milo and Sankarani (K) study area. The thick grey lines at the top indicate the period over which ivermectin mass treatment was given, while thin black lines indicate the period of vector control. Note that the top two panels refer to survey villages, and the bottom two panels to capture points. ATPO. volv. values are denoted by the grey symbols in the plot for ATP.
In the lower part of the Sankarani basin there is a predominance of savannah vectors (S. sirbanum and S. damnosum s.s.) throughout the year. In the medium and upper parts, there is a predominance of savannah vectors (S. sirbanum and S. damnosum s.s.) during the dry season and forest vectors (S. squamosum) during the rainy season. The proportions of parasite strains found in 36 infective flies collected between 1993 to 2000 were 47% O. volvulus savannah strain, 11% O. volvulus forest strain, and 42% non-O. volvulus strain. Pre-control endemicity levels indicated that this was a meso- to hyperendemic area with prevalences varying from 26% to 88% in villages evaluated between 1985 and 1987 (Figure 9). Anti-reinvasion and experimental larviciding were done in 1984 and 1988. Full-scale larviciding started in 1989. Large scale annual ivermectin treatment also started in 1989.
Trends
There were favourable trends in the epidemiological data. Prevalence dropped from very high values around 80% in 1985 to values < 10% but not yet zero in 2001 (Figure 9). There was a similar trend in CMFL, that diminished from very high values in some places (around 70 mf/s) to values near zero. This had occurred already in many villages from 1993 onwards. There was an enormous pre-control heterogeneity in CMFL values ranging from 1.3 mf/s to 69 mf/s. Therapeutic coverage decreased from 75% in 1989 to around 20% in 1997, which can be explained by the fact that only those with a positive skin snip were treated. There seems to be no information on geographical coverage. ABR was generally very high and increased slowly from an average of 5430 in 1984, to an average of 13900 in 2001, with extremely high values (> 50000) occurring occasionally at some sites. These high values may be the result of reinvasion or of treatment failures. The rise in ABR values may be partially explained by the suspension of larviciding in the northern part of the OCP. ATP and ATPO. volv. values were more favourable, and decreased from values between 89 and 1930 in 1984 to values within the range 0–120 in 2001.
Problems and comments
Good trends were observed in the epidemiological data from these two basins. The entomological data seem consistent with this, despite the high ABRs in some places. The only logical conclusion seems to be that this favourable situation is the result of the additional effect of ivermectin treatment. To allow OCP to test whether the strategy of 12 years of combined vector control and ivermectin treatment is indeed sufficient to eliminate the risk of transmission it might be considered to stop vector control in a good basin and implement very close monitoring.
Asubende focus (L)
Background
This is a hyperendemic focus located in the eastern part of the southern extension on the Pru river in Ghana (Figure 1). The focus consists of a continuous string of breeding sites along the river with the village Asubende in the center. From 1994 until 2000 almost all flies caught were of the savannah strain; less than 1% were forest flies. In the period January 1993 until December 2000 the proportions of flies infected with the various parasite strains were: 48% O. volvulus savannah, 20% O. volvulus forest, and 32% non-O. volvulus. Vector control in the southern extension was started in 1988. On the Pru it was suspended during the rainy seasons in 1988 and 1989 because of xenodiagnostic studies, and because of studies of the impact of ivermectin on transmission. Normal vector control has been done on the lower part of the Pru river since 1990. Annual ivermectin treatment was started in 1987.
Trends
There were favourable trends in the epidemiological data (Figure 10). Prevalence decreased from values slightly under 90% in 1987 to values below 5% at the end of 2000. CMFL declined from very high values, slightly over 70 mf/s in one village in 1987, to near zero values in 2000. Therapeutic coverage was high (around 70%). In a recent evaluation, a geographic coverage of 80% was found, and a therapeutic coverage of 76%. Thus, geographic coverage was good in Asubende, although it was poor in Ghana as a whole. The ABR was high and very variable over the years with maximum values above 40000 in 1978 and 1999, and minimum values between 3000 and 7500 in 1983, 1986 and in the period 1991–1994. ATP dropped from values above 3000 in 1978 to values around 200 in about 1991, and remained at that level until 2001. When they became available, ATPO. volv. measurements were almost equal to the ATP values, but were substantially lower in more recent years.
Figure 10.
Prevalence of microfilariae (mf), community microfilarial load (CMFL), annual biting rate (ABR), and crude and O. volvulus specific annual transmission potentials (ATP and ATPO. volv.) against time in the Asubende (L) study area. The thick grey lines at the top indicate the period over which ivermectin mass treatment was given, while thin black lines indicate the period of vector control. Note that the top two panels refer to survey villages, and the bottom two panels to capture points. ATPO. volv. values are denoted by the grey symbols in the plot for ATP.
Problems and comments
Despite the fact that the Asubende focus is a relatively small area where much research has been done, the situation in this basin is unclear and complicated by many factors.
First, there may be problems with the borders. No larviciding was done in the area to the south and on the upper stream where before 1988 mostly forest flies were found. Entomological studies indicated, however, that there was little impact from immigrating vectors.
A second point concerns human migration and the resulting composition of the population. In December 2001, a sociodemographic study was conducted on the Pru river. One of the findings of this study was that the Pru basin inhabitants are clustered in eleven villages along the bank of the river. Many of the settlers are either indigenous to the area, economic migrants or refugees (deprived population) from the northern ethnic conflict. Other people came from Volta, Upper West, Ashanti, or Brong Ahafo regions [15].
A further important issue concerns the effectiveness of vector control in this area. Current biting rates are similar to pre-control values. Thus, vectors abound, and, as indicated by the entomological data, there is still significant transmission.
Finally, the change from mobile treatment to CDTI in 1996 turned out to be difficult to implement in Ghana. In 1996, ivermectin treatment was late for various reasons, and the villagers complained about it. At that time, Ghana had the lowest coverage of all the countries. This presented a situation of concern because Asubende was an important area with a very high prevalence. Therefore, much effort was expended in providing an extra treatment in a form somewhat different from regular CDTI. Thereafter, the area was normally treated with CDTI.
The entomological data clearly indicate that significant transmission continues. It is therefore impossible to cease ivermectin treatment. The prevalence and parasite population are declining nicely but slowly, which appears to be consistent with significant transmission in addition to treatment. It is difficult to determine the impact of ivermectin on transmission.
Dienkoa (M)
Background
This is a small but severely affected focus, located in the southwestern part of Burkina Faso on the Dienkoa river near the border with Mali (Figure 1). The basin is in the sudano-guinean zone with perennial medium sized rivers and savannah vector species. The area is very fertile and characterised by numerous hamlets and intensive migration. The many cattle in the area result in some O. ochengi strain among collected parasites. Data from before 1974 (not shown in the plots), when control started, indicated that this was a hyperendemic focus with an ABR of more than 9000, an ATP of 1200, a prevalence of 68%, a rather low CMFL of 13.9 mf/s, and a blindness rate of 2.8%.
The area has a complex history of treatment and monitoring. Aerial larviciding was started in 1975. Because it was difficult to select good sites representative of the epidemiological situation, the regularly visited capture points were changed with time. The network was restructured in 1978/1979, when the number of capture points was reduced as a result of serious financial difficulties in the program. Better sites were selected in 1985, 1986 and 1988, and three of these were regularly visited from 1986 onwards. From 1979 to 1989, larviciding turned out to be very difficult, and was carried out irregularly. This resulted in a resumption of transmission, and also, in 1983 and 1985, in the detection of a number of newly infected children. No larviciding was done in the period 1987–1988 because of studies on the impact of ivermectin on transmission. Annual ivermectin distribution started in 1988 with mobile treatment, and was switched to Community Based Treatment with Ivermectin (CBTI) in 1996, and finally to CDTI. Because of the poor results and new infections found, and because some capture points indicated that transmission was still continuing, systematic and widespread ground larviciding was started in 1990. Ground larviciding was easy to implement and gave good results, leading to better epidemiological results from 1994 to the present.
Trends
Over the whole period, prevalence diminished from rather high values in 1975 (average 60%) to values near zero in 2000 (Figure 11). CMFL was quite variable at the start, ranging from 3 mf/s to just over 30 mf/s, and dropped to values near zero around 1993, after which this level was maintained. Therapeutic coverage was 50% at the start in 1988, rising to 85% in 2000. Geographic coverage was poor at the start of treatment, (about 5 villages treated), but improved gradually until over 60 villages were treated from 1997-onwards, although with a serious relapse in 2000 (31 villages). ABR decreased from a average value of 3700 at the start of the program in 1974 to an average of 1750 at the start of 2001, with a sudden increase in 1986 when larviciding was stopped. When vector control was resumed it diminished again, but in some places problems with high values remained. ATP followed the same pattern, and was generally low during the period of vector control, but in recent years values above 100 were observed in some places. ATPO. volv. values appeared substantially lower than ATP measurements, but was at least in one capture point above 100.
Figure 11.
Prevalence of microfilariae (mf), community microfilarial load (CMFL), annual biting rate (ABR), and crude and O. volvulus specific annual transmission potentials (ATP and ATPO. volv.) against time in the Dienkoa (M) study area. The thick grey lines at the top indicate the period over which ivermectin mass treatment was given, while thin black lines indicate the period of vector control. Note that the top two panels refer to survey villages, and the bottom two panels to capture points. ATPO. volv. values are denoted by the grey symbols in the plot for ATP.
Problems and comments
The situation in this basin is complex, involving many factors outside vector control and ivermectin treatment. The area forms part of the large area in Burkina Faso that benefited from good vector control. It seems likely therefore, that if vector control hadn't been interrupted to investigate the effect of ivermectin treatment, similar results would have been obtained as in the other areas. Also, the focus was not clearly defined, and was expanded by including new villages when problems turned out to be larger than previously thought. There is also much migration in this area. Because the surrounding regions are free of onchocerciasis, migration from within Burkina Faso should not matter much, but the large number of people recently driven out of Cote d'lvoire is important. Lastly, there were the problems with the changes in capture points, and with an initially poor geographic coverage that improved after the introduction of CDTI. The impact of ivermectin on transmission in this basin is, therefore, difficult to ascertain.
Ivermectin treatment after vector control
Bougouriba (N)
Background
The Bougouriba is a tributary of the Mouhoun (Black Volta), and is located in the southwestern part of Burkina Faso, bordering Ghana (Figure 1). It is in the sudano-guinean savannah zone with forest galleries. The area has non-permanent medium sized rivers, taking into account that, for a considerable part of the period studied here, there was drought. A number of national parks are located along the river. There was intensive migration across the border with Cote d'lvoire. The savannah vector species is predominant. Pre-control data (not shown in the plots) collected before 1974 indicated a hyperendemic situation, with a prevalence of 83.7% at some places, a CMFL of 98 mf/s, a blindness prevalence of 11.6%, an ABR of more than 6000, and an ATP >600.
Regular larviciding was done on the main river and on important tributaries during the period 1975–1990. The capture points on the main river were established at the beginning of the program in 1974 but were reduced in 1978. There were many difficulties in accessing the rivers. Because of turmoil in the area, ground larviciding was impossible. Prospections during the rainy seasons were done by helicopter to complement ground prospections during the dry seasons. On some tributaries, and in particular on the Naimo, larviciding stopped in 1987. There was a complete cessation of larviciding in 1990, when the usual evaluation sites indicated good results or good trends in the preceding two years.
In the period 1976–1991, there was an organised installation of populations in the basin by the government, in particular in the Naimo area. These migrants modified the environment and thereby created new artificial breeding sites. This new situation was not recognized at the time when it was decided to stop larviciding. In 1992, post-control entomological data collected at Batie on the Bambassou river, also a tributary of the Mouhoun (Black Volta) and close to the Bougouriba, indicated a deteriorating situation. In 1994, newly infected children were found with an incidence > 1.8% in some villages. Even in the areas where in 1991 a low infectivity rate (0.6%) was found, an increase to 5.9% was observed in 1995. In 1996, after this had been discovered, large scale ivermectin treatment on a 4-monthly schedule was started in the Bougouriba basin for the purpose of recrudescence control.
Some ground larviciding was done in 1997 and 1998 to protect the population of a village close to the Naimo at breeding sites that were created as a result of the activities of this population. This larviciding was done by the villagers themselves with technical support of the national team.
From 1991 until 1998, upward trends in infectivity rates, calculated from flies caught during the rainy season, were found at Nabere and Zambo. In Nabere, calculated infectivity rates were 0.39 (1991), 1.25 (1996), 2.33 (1997), and 8.65 (1998); in Zambo these rates were 0.61 (1991), 5.22 and 2.94 (both in 1996), and 5.77 (1998). The reasons for these poor results are unknown. In 2000, the local population caught flies that were analysed using poolscreening. This yielded zero values for infectivity rates at both sites, which seemed to be good. However, these numbers are not reliable as not many flies were received. A new evaluation is planned in which enough flies will be caught for a proper analysis.
Trends
Prevalences declined from a pre-control average of 80% to low values in 1990, after which they rose again to values around 20% in some villages in 1996 (Figure 12). In the villages for which data was available after the start of ivermectin treatment, prevalence was below 10% again in 2001. CMFL diminished to low values in 1990 and then stayed low with the exception of some newly included survey villages. Because of the frequent ivermectin treatment schedule epidemiological examination was difficult, and only limited follow-up was available after 1996. Therapeutic coverage was quite good (70–80%). Geographic coverage left much to be desired, because it was not exactly known how many villages were to be treated. Furthermore, after an initial strong effort to get a good coverage, the number of villages treated dropped from around 180 per year to values below 100 per year after 1998. After cessation of vector control in 1990, ABR rose to very high levels after 1995 ranging from 19900 to 69500, and ATP rose to over 400 in one capture point in 1998. Trends in the scarce ATPO. volv. measurements mimicked those in the ATP. In one of the two capture points ATPO. volv. became low in 1998, but in the other it rose to over 350.
Figure 12.
Prevalence of microfilariae (mf), community microfilarial load (CMFL), annual biting rate (ABR), and crude and O. volvulus specific annual transmission potentials (ATP and ATPO. volv.) against time in the Bougouriba (N) study area. The thick grey lines at the top indicate the period over which ivermectin mass treatment was given, while thin black lines indicate the period of vector control. Note that the top two panels refer to survey villages, and the bottom two panels to capture points. ATPO. volv. values are denoted by the grey symbols in the plot for ATP.
Problems and comments
The Bougouriba is a very problematic area in which a much faster post-vector control recrudescence has occurred than was anticipated from model simulations.
Discussion
We have presented a detailed description and analysis of trends in prevalence of mf, CMFL, ABR, and ATP observed between 1975 and 2001 in 14 river basins from the area covered by the OCP, and in an area from the north of Cameroon, with a view to assess the impact of ivermectin mass treatment on epidemiological and entomological parameters, and to assess the potential of ivermectin treatment for elimination of transmission of onchocerciasis in West Africa.
If elimination of transmission is feasible and reasonably cost-effective, it is worth attempting to pursue that objective. If it is not feasible, control should either continue indefinitely or until other forms of treatment, more effective towards elimination, become available. As has already been mentioned in the introduction, simulation studies using ONCHOSIM [7], carried out by Winnen et al., [4] indicate that prolonged – often for several decades – high-coverage treatment programs, especially based on 6-monthly treatment intervals, have a high probability of achieving elimination. Several uncertainties remain, however, about these simulation results. Firstly, it is questionable whether such high coverage rates (in excess of 80% in highly endemic foci) in all transmission foci would be sustainable. Secondly, it was assumed in the simulations that drug-resistance would not emerge. Thirdly, not all aspects of reality may have been adequately captured by the assumptions underlying the simulations. For example, vector properties (e.g. zoöphily) may have been misspecified for some foci, or vector longevity may differ from that specified in the model.
Therefore, we argued that empirical examples of successes of attempts to eliminate transmission would be much more cogent arguments in favour of the hypothesis that elimination is possible by ivermectin treatment alone. For example, if in a holoendemic focus, prolonged ivermectin treatment has been sustained at high levels and has actually achieved (near) elimination this would constitute strong evidence that transmission can be sufficiently interrupted at practicable coverage levels, and that elimination of transmission by means of ivermectin mass treatment programs alone is possible. If, despite sustained high coverage rates and adequate frequency, elimination of transmission seems not in sight in any example of a highly endemic focus, this may raise doubt about the feasibility of elimination by ivermectin alone. Successes achieved in mesoendemic areas are, of course, less informative. Elimination of transmission may well be possible in mesoendemic areas, but difficult to attain in highly endemic foci.
The main results in the 15 areas studied for this report are presented in Table 2, where the impact of ivermectin mass treatment on four aspects of the disease problem have been summarised for each basin. The first aspect is the impact of ivermectin treatment on the intensity of infection as measured by the Community Microfilarial Load (CMFL). The CMFL is an index of the public health importance of the disease. Onchocerciasis is considered a public health problem when the CMFL exceeds 5–10 mf/s. The second aspect is the impact of ivermectin on the prevalence of infection. The third aspect concerns whether or not ivermectin distribution has interrupted transmission. The fourth and last aspect is whether elimination of the parasite has been achieved to the extent that control can be stopped without risk of renewed transmission.
Table 2.
Impact of ivermectin distribution on four aspects of onchocerciasis infection and transmission.
| River Basin | Intervention Strategy | Impact of ivermectin treatment (Status in 2001) | |||
| PH problem eliminated (CMFL= 0) | Prevalence of infection very low (< 10%) | Transmission interrupted (Rx ongoing) | Elimination (control ceased) | ||
| Ivermectin only: | |||||
| - River Gambia focus (A) | 6-Monthly ivm.1 since 1989 | √ | √ | ? | – |
| - R. Corubal (B) (up to 1996) | 3-Monthly ivm. 1991–1996 | √ | √ | ? | – |
| - R. Corubal (B) (from 1996 onwards) | No ivm. since 1996 | ? | Increasing prevalence | Transmission ongoing | – |
| - Rio Gêba (C) | 6-Monthly ivm. since 1989 and no treatment since 1996 | √ | √ | ? | ? |
| - Falémé (D), Bafing (E), Bakoye (F), Baoulé (G) | Annual ivm. since 1989 | √ | √ | ? | – |
| - Vina Valley (Cameroon) | Annual ivm. since 1987 | √ | Prev. mf ≈ 20% in 1999 | Transmission ongoing | – |
| Ivermectin + vector control: | |||||
| - Tienfala focus (H) | Annual ivm. since 1987, and (ground) larv.2 since 1994 | √ | √ | ? | – |
| - Bui Gorge focus (I) | Annual ivm. since 1987 (3-monthly from 1994–1996), and larv. from 1975–1996 | √ | Prev. mf up to 55% in 1998 | ? | – |
| - Titira and Kouporgou focus (J) | Annual ivm. since 1988, and larv. since 1977 | √ | Prev. mf up to 50% in 1998 | Transmission ongoing | – |
| - Milo and Sankarani (K) | Annual ivm. since 1989, and larv. since 1989 | √ | √ | √ | – |
| - Asubende focus (L) | Annual ivm. since 1987, and larv. since 1990 | √ | √ | Transmission ongoing | – |
| - Dienkoa (M) | Annual ivm. since 1988, and larv. since 1975 (with interruptions) | √ | √ | √ | – |
| Ivermectin treatment after vector control: | |||||
| - Bougouriba (N) | 4-Monthly ivm. since 1996, and larv. from 1975–1990 | √ | √ | Transmission ongoing | – |
1ivm.: ivermectin treatment 2larv.: larviciding
As indicated in the third and fourth columns of Table 2, evidence from all 15 study areas clearly shows that ivermectin treatment has been uniformly successful in controlling onchocerciasis as a public health problem. CMFL values are everywhere (near) zero, and, in most basins, prevalence of infection is generally quite low.
Interruption of transmission is a more difficult issue. In some areas transmission is certainly continuing after 10–12 years of ivermectin treatment. Problems in these areas relate to, amongst others, stopping treatment too early (Rio Corubal), shortcomings in therapeutic and/or geographic treatment coverage (Vina Valley, Titira, Bougouriba), human migration (Titira, Asubende, Bougouriba), migration of flies (Asubende), or to overlooking a number of problem spots (Bougouriba).
There are also a few areas where transmission may have been interrupted. However, there are still many uncertainties involved, and further monitoring and research is required to remove all doubt.
An important point involves the third and fourth columns of Table 2. Stopping ivermectin distribution when low levels of infection (low CMFL values), and low, but not yet zero, prevalences are attained may result in very serious problems. This is illustrated by the results from the Rio Corubal area where the most recent data show a serious and unexpected recrudescence. Therefore, even if interruption of transmission has been achieved for some time, when the parasite reservoir is not entirely eliminated, it is unclear whether ivermectin treatment can be stopped.
Interruption of transmission in an area should be reflected by low and declining levels of observed epidemiological and entomological indices (prevalence of mf, CMFL, ABR, ATP) [16]. Inconsistencies between these two types of data may make it hard to draw definitive conclusions. Therefore, the apparent discrepancies between epidemiological and entomological variables observed in several study areas, where low CMFL measurements are compatible with an absence of transmission, while at the same time moderately high (crude) ATP measurements indicate transmission is going on, need explanation. A first possible explanation might be that there was a positive correlation between participation in skin snipping and reception of ivermectin treatment. Those who did not participate in ivermectin treatment (and the data suggest that this may concern a substantial number of persons) might have been the same persons whose skin was never examined. These individuals may act as a reservoir for continued transmission that can better be ascertained from entomological than from epidemiological parameters. A second possibility is that the entomological situation at the capture points was not very representative of the associated survey villages. In this case, one should be aware of a possible bias in the data. In the presence of non-zero ATP values, one cannot conclude that transmission has been interrupted, unless it is established that occasional reinvasion of infected flies accounts for the observed ATP, and few individuals get reinfected by both male and female parasites.
The third, and probably most plausible, explanation is that the crude ATP, used in the graphs, includes transmission due to both human and animal parasite strains. Therefore, this ATP may substantially overestimate ATP due to O. volvulus strains alone, especially in areas with many cattle and/or a highly zoöphilic behavior of the flies. Vastly more detailed and important information on transmission can be obtained now that recently developed PCR techniques permit the separate assessment of ATP due to O. volvulus exclusively (ATPO. volv.), and ATP due to O. ochengi from cattle. That crude ATP overestimates O. volvulus specific ATP in at least some capture points is illustrated in the plots by the ATPO. volv. values, that are often significantly lower than the corresponding ATP measurements. Unfortunately, ATPO. volv. measurements were only available for a small percentage of the selected capture points, mostly from areas with vector control and ivermectin mass treatment in parallel, and only for recent years.
Transmission on non-O. volvulus species is thought to influence intensity of infection in two ways. First, it reduces the vectorial capacity of blackfly populations for O. volvulus (zoöprophylaxis) [17]. And secondly, inoculation in humans of infective filarial larvae of animal origin that cannot in this way develop into adult worms is hypothesized to provide some degree of cross-protective immunity acting against O. volvulus. This is particularly true for the species O. ochengi from cattle, which is closely related to O. volvulus, in situations where the ATP due to O. ochengi is considerably higher than the ATP due to O. volvulus [10,12]. In view of the available data, however, it seems difficult to separate effects of zoöprophylaxis and cross-protective immunity from those of ivermectin treatment.
Overall, therapeutic ivermectin treatment coverage (i.e. the coverage in villages reached by the program) was quite high. This is favourable, implying that communities have a positive attitude towards it. On the other hand, geographical treatment coverages are a cause for concern. These also appear to have been somewhat irregular, with some rounds of treatment reaching much higher levels of geographical coverage than others. How this all works out in terms of effective treatment coverage is not easy to estimate. One could define, for operational purposes, the 'effective ivermectin coverage' by the fraction of bites that flies take from individuals 'covered' by ivermectin treatment program, i.e. from persons who have taken ivermectin less than a specified time ago. As ABRs of treated and untreated villages are not well known, actual effective ivermectin coverage is not precisely estimable. However, it may have been much lower than the therapeutic coverages when important – high transmission – villages were not covered. If effective coverage rates were much lower than therapeutic coverage rates, then it is hard to draw definite conclusions about the potential impact of ivermectin. Also, the question of whether higher effective coverage rates are attainable, and whether these would have led to a different conclusion, i.e. that these would indeed lead to elimination of transmission, cannot be answered with certainty on the basis of available empirical data.
As regards the frequency of treatment, we note the following. The Titira region had both (incomplete) vector control and ivermectin distribution (approximately once per year). Yet, elimination of transmission seems to be a remote prospect. ATPs of up to 411 have recently been observed. This suggests that annual ivermectin treatments (sometimes even in the presence of imperfect vector control) in highly endemic areas, as actually practised, does not interrupt transmission, at least not quickly. If transmission continues at reasonably high levels, then elimination of infection would be impossible. However, it is unknown whether this intervention, if sustained for a more prolonged period would succeed in eliminating transmission.
As regards 6-monthly treatments, the Bougouriba focus would be able to provide important information on controlling recrudescence in formerly hyperendemic areas [2]. We recommend that detailed studies of this area be undertaken. Such studies should include, in addition to epidemiological and entomological data, precise ivermectin coverage levels and treatment histories per individual for all individuals in all villages where transmission may take place. This would make it possible to assess whether, on the individual level, participation in the treatment program is random over time, or whether there are groups of individuals that participate always and groups that participate seldom or never. If a group of the latter type exists, it would form a serious threat for future reinfection, especially if it is geographically clustered.
A final point regarding the frequency of ivermectin distribution is the following. As far as elimination of onchocerciasis as a public health problem is concerned, annual treatment has been very effective and there appears to be no need for shorter treatment intervals. A question then is whether annual treatment should be continued in this form or whether less frequent treatment schedules should be considered, because when interruption turns out to be impossible, treatment should be maintained for a very long time. Implementing other treatment schedules, and especially less frequent ones, may have important operational consequences as well as implications for the development of ivermectin resistance. This last issue should be further investigated, e.g. by incorporating ivermectin resistance in the ONCHOSIM simulation model.
Conclusions
Both actual observations and ONCHOSIM predictions [4] indicate that control of onchocerciasis by annual or more frequent ivermectin treatment is possible when sustained sufficiently long. However, the results presented in this paper make it almost certain that repeated ivermectin mass treatment will not lead to the elimination of onchocerciasis from West Africa. This most likely implies that treatment will have to be continued indefinitely, which might ultimately result in development of ivermectin resistance. The latter forms a strong argument in favour of upgrading research for a macrofilaricide effective against O. volvulus because it is imperative that, should resistance develop, a second line treatment is available (notwithstanding the urgent need for a macrofilaricide that is safe enough to be used on a large scale under rural conditions in tropical Africa). Furthermore, elimination of transmission will be elusive if reinfection (by immigrating infective flies or infected humans) takes place. A precondition therefore appears to be a very wide geographical coverage. Yet, even if elimination of transmission appears to be nearly impossible, annual ivermectin treatments can lead to very low prevalence and CMFL values. The objective of eliminating onchocerciasis as a public health problem by annual ivermectin treatments – as long as it retains its effectiveness – seems therefore feasible.
Acknowledgments
Acknowledgment
We are grateful to all past and current members of OCP epidemiological and entomological survey teams, to all past and current vector collectors, to all those who are or have been involved in the initiation of ivermectin treatment programs and in ivermectin distribution, to all non-OCP technicians that are or have been involved in the program, and to all other current and former OCP employees involved in collecting and archiving of the data used in this report, without whose work over more than 25 years this publication would have been impossible.
We also thank all non-OCP participants of the 2001 Rotterdam OCP/TDR meeting for their comments and discussion, in particular Prof. A. Abiose (Sight Care International Medical and Surgical Center, Kaduna, Nigeria), Prof. M. Homeida (Academy of Medical Sciences and Technology, Khartoum, Sudan), Dr. M.-G. Basáñez (Department of Infectious Disease Epidemiology, Imperial College Faculty of Medicine, London, United Kingdom), Dr. J.-M. Hougard (Institut de Recherche pour le Developpement, Montpellier, France), Dr. B. Philippon (Institut de Recherche pour le Developpement, Paris, France), Dr. M. Alleman (Mectizan Donation Program, Decatur, Georgia, USA), Dr. T. Mancero (OEPA, Ecuador), Dr. M. Karam (WHO/CPE), and Dr. B. Liese (Health Services Department, World Bank, Washington, USA).
Contributor Information
Gerard JJM Borsboom, Email: g.borsboom@erasmusmc.nl.
Boakye A Boatin, Email: boatinb@oncho.oms.bf.
Nico JD Nagelkerke, Email: N.J.D.Nagelkerke@lumc.nl.
Hyacinthe Agoua, Email: yaneagoua@hotmail.com.
Komlan LB Akpoboua, Email: akpobouaa@oncho.oms.bf.
E William Soumbey Alley, Email: soumbeye@afro.who.int.
Yeriba Bissan, Email: bissany@oncho.oms.bf.
Alfons Renz, Email: Alfons.Renz@uni-tuebingen.de.
Laurent Yameogo, Email: yameogol@oncho.oms.bf.
Jan HF Remme, Email: remmej@who.ch.
J Dik F Habbema, Email: j.d.f.habbema@erasmusmc.nl.
References
- De Sole G, Awadzi K, Remme J, Dadzie KY, Ba O, Giese J, Karam M, Keita FM, Opoku NO. A community trial of ivermectin in the onchocerciasis focus of Asubende, Ghana. II. Adverse reactions. Trop Med Parasitol. 1989;40:375–382. [PubMed] [Google Scholar]
- Remme J, De Sole G, Dadzie KY, Alley ES, Baker RH, Habbema JD, Plaisier AP, van Oortmarssen GJ, Samba EM. Large scale ivermectin distribution and its epidemiological consequences. Acta Leiden. 1990;59:177–191. [PubMed] [Google Scholar]
- Remme J, Baker RH, De Sole G, Dadzie KY, Walsh JF, Adams MA, Alley ES, Avissey HS. A community trial of ivermectin in the onchocerciasis focus of Asubende, Ghana. I. Effect on the microfilarial reservoir and the transmission of Onchocerca volvulus. Trop Med Parasitol. 1989;40:367–374. [PubMed] [Google Scholar]
- Winnen M, Plaisier AP, Alley ES, Nagelkerke NJD, van Oortmarssen G, Boatin B, Habbema JDF. Can ivermectin mass treatments eliminate onchocerciasis in Africa? Bull World Health Organ. 2002;80:384–390. [PMC free article] [PubMed] [Google Scholar]
- Remme JHF. The African programme for onchocerciasis control: preparing to launch. Parasitol Today. 1995;11:403–406. doi: 10.1016/0169-4758(95)80017-4. [DOI] [Google Scholar]
- Alley WS, van Oortmarssen G, Boatin B, Nagelkerke N, Plaisier A, Remme HJ, Lazdins J, Borsboom GJ, Habbema JD. Macrofilaricides and onchocerciasis control, mathematical modelling of the prospects for elimination. BMC Public Health. 2001;1:12. doi: 10.1186/1471-2458-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisier AP, van Oortmarssen GJ, Habbema JD, Remme J, Alley ES. ONCHOSIM: a model and computer simulation program for the transmission and control of onchocerciasis. Comput Methods Programs Biomed. 1990;31:43–56. doi: 10.1016/0169-2607(90)90030-D. [DOI] [PubMed] [Google Scholar]
- Moreau JP, Prost A, Prod'hon J. Essai de normalisation de la méthodologie des enquêtes clinico-parasitologiques sur l'onchocercose en Afrique de l'ouest. Méd Trop. 1978;38:43–51. [PubMed] [Google Scholar]
- Remme J, Ba O, Dadzie KY, Karam M. A force-of-infection model for onchocerciasis and its applications in the epidemiological evaluation of the Onchocerciasis Control Programme in the Volta River basin area. Bull World Health Organ. 1986;64:667–681. [PMC free article] [PubMed] [Google Scholar]
- Wahl G, Enyong P, Ngosso A, Schibel JM, Moyou R, Tubbesing H, Ekale D, Renz A. Onchocerca ochengi: Epidemiological evidence of cross-protection against Onchocerca volvulus in man. Parasitology. 1998;116:349–362. doi: 10.1017/S003118209700228X. [DOI] [PubMed] [Google Scholar]
- Renz A, Wenk P, Anderson J, Fuglsang H. Studies on the dynamics of transmission of onchocerciasis in a sudan-savanna area of North Cameroon V. What is a tolerable level of annual transmission potential? Ann Trop Med Parasitol. 1987;81:263–274. doi: 10.1080/00034983.1987.11812119. [DOI] [PubMed] [Google Scholar]
- Seidenfaden R, Fischer A, Bonow I, Ekale D, Tanya V, Renz A. Combined benefits of annual mass treatment with ivermectin and cattle zooprophylaxis on the severity of human onchocerciasis in northern Cameroon. Trop Med Int Health. 2001;6:715–725. doi: 10.1046/j.1365-3156.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- Boussinesq M, Prod'hon J, Chippaux JP. Onchocerca volvulus: Striking decrease in transmission in the Vina valley (Cameroon) after eight annual large scale ivermectin treatments. Trans R Soc Trop Med Hyg. 1997;91:82–86. doi: 10.1016/s0035-9203(97)90406-5. [DOI] [PubMed] [Google Scholar]
- Karam M, Schulz-Key H, Remme J. Population dynamics of Onchocerca volvulus after 7 to 8 years of vector control in West Africa. Acta Trop. 1987;44:445–457. [PubMed] [Google Scholar]
- Sena AK. A report on the socio-demographic study in the Pru basin. WHO-ONCHO control programme document Ouagadougou. 2001. p. 45.
- Remme J, Dadzie KY, Rolland A, Thylefors B. Ocular onchocerciasis and intensity of infection in the community. I. West African savanna. Trop Med Parasitol. 1989;40:340–347. [PubMed] [Google Scholar]
- Renz A, Barthelmess C, Eisenbeiβ W. Vectorial capacity of Simulium damnosum s.l. populations in Cameroon. Trop Med Parasitol. 1987;38:344–345. [Google Scholar]