Abstract
Eotaxin is a novel C-C chemokine with selective chemoattractant activity for eosinophils. We determined whether eotaxin could be produced by human airway smooth muscle (HASM) cells in culture and examined its regulation by interleukin-10 (IL-10) and the corticosteroid, dexamethasone.
Stimulation of the cells with interleukin-1β (IL-1β) or tumour necrosis factor (TNFα) each at 10 ng ml−1 induced the release of eotaxin protein with maximal accumulation by 24 h. Interferon-γ (IFNγ) alone at 10 ng ml−1 had no effect and there was no synergy between these cytokines on the release of eotaxin.
Reverse phase high performance liquid chromatographic (HPLC) analysis of supernatents from cells treated with TNFα (10 ng ml−1 for 96 h showed immunoreactivity to eotaxin which eluted with the expected retention time of 34.5–35 min.
Both IL-1β and TNFα-induced release of eotaxin was not inhibited by dexamethasone (1 μM), however IL-10 (10 ng ml−1) had a significant inhibitory effect. Dexamethasone and IL-10 did not inhibit the induction of eotaxin mRNA induced by IL-1β or TNFα.
Thus, human airway smooth muscle cells can release eotaxin and could be an important source of chemokine production during airway inflammatory events.
Keywords: Eotaxin, airway smooth muscle cells, interleukin-10, interleukin-1β, interferon-γ, tumour necrosis factor-α
Introduction
Asthma is characterized by reversible airway narrowing and inflammation of the airway wall leading to epithelial cell damage, mucus plugging, stimulation of neural reflexes and infiltration of eosinophils, macrophages and lymphocytes (Djukanovic et al., 1990; Azzawi et al., 1990; Bousquet et al., 1990). Structural changes in the airway wall such as increased smooth muscle content and subepithelial fibrosis associated with matrix deposition, are also present (Ebina et al., 1993; Roche et al., 1989) and may contribute to persistent airways obstruction and bronchial hyperresponsiveness (James et al., 1989). Until recently, airway smooth muscle was regarded to be solely contractile because its ability to shorten in response to many inflammatory mediators leads to a reduction in airway calibre. However, it is now known that airway smooth muscle cells are also capable of responding to cytokines to release other inflammatory mediators, in particular chemokines such as RANTES and interleukin-8 (IL-8; John et al., 1997; 1998), Airway smooth muscle may therefore act as an effector cell in perpetuating airway inflammation by releasing chemoattractants for various inflammatory cells.
Eotaxin is a newly described CC chemokine that was first isolated from lung lavage fluid of sensitized guinea-pigs following allergen exposure (Jose et al., 1994). Amongst eosinophil-active chemoattractants, eotaxin specifically attracts and activates eosinophils as demonstrated in vitro for both mouse and human eotaxin using assays for chemotaxis and calcium release (Rothenberg et al., 1996; Forssmann et al., 1997). Eotaxin has also been demonstrated to selectively induce eosinophil recruitment to the airways and to the skin in vivo (Rothenberg et al., 1995; Jose et al., 1994; Collins et al., 1995). Eotaxin is partly important in recruiting eosinophils to the airways following allergen challenge, and the time-course of its appearance coincides with that of eosinophil recruitment to the airways (Rothenberg et al., 1997). However, in mutant mice in which the eotaxin gene has been disrupted, lung eosinophilia induced by sephadex beads or by ovalbumin aerosol exposure was not affected (Yang et al., 1998). Eotaxin is expressed by several cell types in the inflamed airways of asthmatic patients including epithelial and endothelial cells, T-lymphocytes, macrophages and eosinophils (Ying et al., 1997; Lamkhioued et al., 1997; Mattoli et al., 1997). In the present study, we determined whether primary cultures of human airway smooth muscle (HASM) cells have the capacity to express eotaxin since they can also produce other chemokines (John et al., 1997; 1998). We therefore examined whether the pro-inflammatory cytokines interleukin-1β (IL-1β), tumour necrosis factor α (TNFα) and interferon-γ (IFNγ) could induce eotaxin mRNA expression and release from airway smooth muscle cells in vitro, and we determined the modulatory effects of the anti-inflammatory cytokine, interleukin 10 (IL-10), and of the corticosteroid, dexamethasone.
Methods
Human airway smooth muscle cell culture
HASM cells were obtained from tracheas or main bronchi of healthy donors from either heart/heart and lung transplantation donors (four male, two female, aged 11–47 years). The smooth muscle was dissected out under sterile conditions and placed in culture as previously described (Belvisi et al., 1997; Hirst et al., 1992). Once in culture, the cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with foetal calf serum (FCS, 10% v−1v), sodium pyruvate (1 mM), L-glutamine (2 mM), non essential amino acid mixture (1×), and antimicrobial agents as previously described. All cultures were maintained in a humidified atmosphere at 37°C in air/CO2 (95:5% v−1v). Fresh medium was replaced every 72 h. Using immunofluorescence techniques for both smooth muscle actin and myosin, more than 95% of the cells displayed the characteristics of smooth muscle cells in culture.
Cell stimulation
Cells were plated onto either 24 well plates with an initial seeding density of 8000 cells/well and 6 well plates (Costar U.K. Ltd, High Wycombe, U.K.) with an initial seeding density of 30 000 cells/well for eotaxin release experiments and Northern blot analysis respectively. Sub-confluent human airway smooth muscle cells (passage 3–8) were growth arrested by being placed in supplemented DMEM void of FCS for 24 h. Cells were stimulated in fresh FCS-free medium containing TNFα, IL-1β or IFNγ in a concentration- and time-dependent manner. The effect of combinations of these pro-inflammatory cytokines was also investigated. Furthermore, the ability of IL-10 (10 ng ml−1) or dexamethasone (1 μM) to inhibit any effects produced by these cytokines was examined. In these experiments, dexamethasone and IL-10 were preincubated for 2 h prior to the addition of TNFα, IL-1β or IFNγ. Following the treatment period, the supernatants were harvested, clarified by centrifugation and stored at −20°C until assayed. The cells were lysed by freeze-thawing once in 1 ml of 0.08% trifluoroacetic acid. After centrifugation of the lysate, the soluble fraction was freeze-dried, resuspended in 500 μl of assay buffer and adjusted to pH 7.20 for measurement of eotaxin protein by a sandwich ELISA.
Preparation of eotaxin cDNA probe
The eotaxin probe was generated by reverse transcription polymerase chain reaction (RT–PCR). Total cellular RNA was extracted from airway smooth muscle cells stimulated with TNFα using a modification of the method of Chomczynski & Sacchi (1987). Following two phenolchloroform extractions and isopropanol precipitation, RNA samples were precipitated overnight and washed twice with 75% ethanol and dissolved in RNAse-free water. Reverse transcription was performed as follows: 1 μg of the RNA and oligo dT15 primer (0.4 μg) were incubated at 65°C for 10 min then placed on ice for 5 min. AMV-reverse transcriptase 15 u, 1 mM of dATP, dCTP, dGTP and dTTP, RNAse inhibitor 30 u, MgCl2 5 mM, KCl 50 mM, Tris-HCl (pH 9.0) 10 mM and 0.1% Triton X-100 were added in a total volume of 40 μl to the samples and incubated at 42°C for 60 min followed by 10 min at 85°C. The cDNA was subsequently diluted to a final volume of 400 μl in nuclease-free water. PCR was performed with 10 μl of the cDNA solution using 7.5 pM of forward and reverse primers, dATP, dGTP, dTTP, dCTP at a final concentration of 0.2 mM each, Taq polymerase 1.5 U, MgCl2 1.5 mM, KCl 50 mM, Tris-HCl (pH 9.0) mM and 0.1% Triton X-100 in a final volume of 30 μl. The primers for eotaxin were: 5′-CTCGCTGGGCCA-GCTTCTGTC-3′ and 5′-GGCTTTGGAGTTGGAGAT-TTTTGG-3′ giving a product of 227 base-pairs. PCR was carried out in a Techne multiwell thermocycler (Techne, Cambridge, U.K.) at 95°C for an initial 5 min followed by 30 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 60 s. Final extension was 10 min at 72°C. The product was sequenced and confirmed to be human eotaxin. Following phenol/chloroform/isoamyl alcohol (50 : 49 : 1) extraction and ethanol precipitation, the product (10 ng ml−1) was labelled using [32P]dCTP using a DNA labelling kit according to the manufacturer's instructions (Pharmacia, St Albans, Herts, U.K.).
Northern blot analysis
After a 24 h treatment period total cellular RNA was extracted from adherent cells using a modification of the method of Chomczynski & Sacchi (1987). Briefly, cell monolayers (approximately 1×106/extraction) were washed twice with ice cold Hanks' balanced salt solution (HBSS) before addition of solution D (4 M guanidinium thiocyanate, 2-mercaptoethanol, 0.5% sodium sarcosyl) directly to each well. RNA was then isolated with a phenol:chloroform; isoamyl alcohol (50 : 49 : 1) extraction in the presence of 0.4 M sodium acretate (pH 4.0). RNA was precipitated in an equal volume of propan-2-ol and pelletted by centrifugation at full speed in a microcentrifuge for 15 min at 4°C. Residual salt was removed by a further wash in 75% ethanol. Denatured RNAs (20 μg) were size-fractionated by gel electrophoresis on 1% agarose/formaldehyde gels containing 20 mM morpholinosulphonic acid (MOPS), 5 mM sodium acetate and 1 mM EDTA (ph 7.0) before Northern blotting to ‘Magna' nylon membranes (msi, Westborough, U.S.A.) by capillary action.
Prehybridization and hybridization were carried out at 42°C with [32P]dCTP-labelled probes (approximately 1.5×106 cpm−1ml) in a buffer containing 50% formamide, 50 mM Tris-HCl (pH 7.50), 5×Denhardts solution, 0.1% sodium dodecyl sulphate (SDS), 5 mM EDTA and 250 μg−1ml denatured salmon sperm DNA. Following hybridization, the blots were washed to stringency of 0.2×SSC, 0.1% SDS at 60° before exposure to Kodak X-OMAT film. After suitable exposure times, autoradiographs were analysed by laser densitometry (Protein & DNA Imageware System, Discovery Series, New York, NY, U.S.A.). Probes were stripped by incubating the blot in a 50% formamide solution at 70°C for 2 h before hybridization with a [32P]-labelled 1272-base pair rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe. Specific RNA levels were calculated as a ratio of eotaxin to GAPDH mRNA then expressed as a percentage of the control response.
Eotaxin immunoassay
Eotaxin was measured by a sandwich ELISA using monoclonal capture and polyclonal detector antibodies as described previously (Evans et al., 1998). The limit of sensitivity was 2 pM.
Reversed phase HPLC separation of eotaxin
The culture supernatant of human airway smooth muscle cells was collected after 96 h stimulation with TNFα (10 ng ml−1). Conditioned medium (22 ml) was acidified to pH 2.0 with trifluoroacetic acid (TFA), filtered (0.45 μm) and applied to C18 reversed phase SepPaks (Waters: 2×840 mg cartridges in series, pre-wetted with 0.1% TFA/acetonitrile and equilibrated in 0.1% TFA/water). After washing the cartridges with 0.1% TFA/water, the bound material was eluted with 0.1% TFA/acetonitrile and the solvents removed using a Savant SpeedVac. The sample was applied to a 300Å C18 HPLC column (Vydac: 4.6×250 mm fitted with guard column 4.6×20 mm) in 0.08% TFA and eluted at 1 ml min−1 with acetonitrile gradients in 0.08% TFA whilst collecting 0.5 min fractions (0–50% acetonitrile over 50 min followed by 50–80% acetonitrile over 6 min).
Materials
All cytokines were purchased from R&D Laboratories (Oxford, U.K.). Phenol was obtained from Rathburn Chemicals (Perthshire, U.K.). Ethanol and propan-2-ol was from BDH (Poole, U.K.) and [32P]dCTP was purchased from Amersham International (Amersham, Bucks, U.K.). AMV-reverse transciptase, dATP, dCTP, dGTP, dTTP, RNAse inhibitor, MgCl2, KCl, Tris-HCl Triton X-100 was purchased from Promega (Southampton, U.K.). All other reagents were obtained from Sigma Chemical Company (Poole, U.K.).
Data analysis
All data are reported as s.e.mean of n determinations from HASM cells obtained from at least three patients. Data generated from Northern blot analysis was calculated as a ratio of eotaxin mRNA to GAPDH mRNA and then expressed as a percentage of the control response. Comparison between groups was performed using the non-parametric one-way analysis of variance (ANOVA) Kruskall-Wallis test followed by the Dunn's multiple comparison test. A P value of <0.05 was considered to be significant.
Results
Release of eotaxin protein by TNFα, IL-1β & IFNγ
HASM cells stimulated with TNFα, IL-1β or IFNγ (10 ng ml−1 for each cytokine) did not cause significant release of eotaxin at 4 h. However, TNFα and IL-1β induced maximal release of eotaxin into the supernatants at 24 h (210±22.1, n=5, P<0.05 compared to time-point control and 151.7±37.7 fmol per well, n=5, P<0.01 compared to time-point control respectively). There was no further increase at 48 h (Figure 1a). The levels of eotaxin in the cytosolic fraction of airway smooth muscle cells did not change significantly at 4 and 24 h after stimulation with either TNFα or IL-1β (TNFα: 14.2±5.0 and 10.7±3.5; IL-1β: 21.2±5.4 and 15.4±2.6 fmol−1 well, respectively; n=3), when compared to control levels (12.1±4.7 and 22.7±5.0). This indicates that newly-synthesized eotaxin was released into the supernatant fraction. There was no evidence of additive effects on eotaxin release when the cells were stimulated with TNFα and IL-1β, or with IFNγ and TNFα together (Figure 1b).
Figure 1.
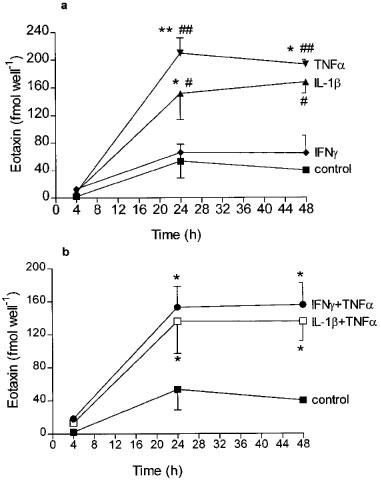
Time-course of eotaxin release into the supernatants of airway smooth muscle cells in culture stimulated with either (a) TNFα, IL-1β or INFγ (10 ng ml−1 each) alone or (b) IL-1β and TNFα together, and TNFα and INFγ together (all at 10 ng ml−1). There was a significant release at 24 and 48 h following stimulation with either TNFα or IL-1β alone. Cells stimulated with the combination of cytokines did not release eotaxin in an additive or synergistic manner. Results shown as s.e.mean of smooth muscle cultures derived from three different donors. *P<0.05, **P<0.01 compared to control at similar time-points; #P<0.05, ##P<0.01 compared to the 4 h time-point.
To investigate the concentration-dependent response of IL-1β, TNFα and IFNγ on eotaxin release, cells were stimulated with each of these cytokines at concentrations of 1, 3 and 10 ng ml−1 for 24 h. IFNγ had no significant effect up to 10 ng ml−1, while IL-1β and TNFα significantly induced the release of eotaxin at 10 ng ml−1 (151.7±37.8, n+5, P<0.05 and 210.0±22.1 fmol per well, n=5, P<0.01 respectively; Figure 2) compared to control unstimulated cells.
Figure 2.
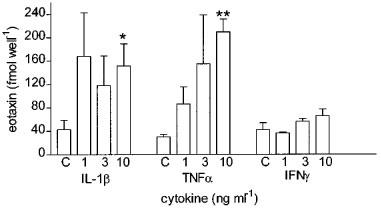
Effect of increasing concentrations (1, 3 and 10 ng ml−1) of IL-1β, TNFα and INFγ on the release of eotaxin from cultured human airway smooth muscle cells measured 24 h after exposure. Both IL-1β and TNFα caused a significant increase in eotaxin release at 10 ng −1 but INFγ had no effect. Data shown as s.e.mean of smooth muscle cultures derived from three different donors. *P<0.05, **P<0.01 compared to control values.
HPLC analysis of supernatants from HASM cells
Immunoractivity to eotaxin eluted with the expected retention time of 34.5–35 min and a minor tailing peak at 36–36.5 min which was not further characterized (Figure 3). Both peaks eluted before RANTES (37–37.5 min), which is also produced by these cells (John et al., 1997).
Figure 3.
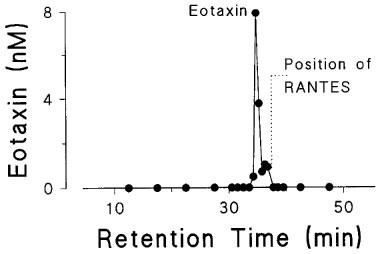
Reverse-phase HPLC separation of eotaxin from supernatants of stimulated airway smooth muscle cells. The supernatant was separated by C18 HPLC and 0.5 min fractions were collected. Immunoreactive eotaxin was detected with a major peak at 34.5–35 min and a minor peak at 36–36.5 min. The position for RANTES is shown for comparison.
Effects of IL-10 and dexamethasone on eotaxin release
To investigate the effects of IL-10 and corticosteroids on eotaxin release and expression, human airway smooth muscle cells were stimulated for 24 h with either TNFα or IL-1β alone (10 ng ml−1 each) in presence and absence of IL-10 (10 ng ml−1) or dexamethasone (1 μM). Dexamethasone had no significant effect on the release of eotaxin, while IL-10 had a suppressive effect (Figure 4a). In order to ascertain that dexamethasone was having a suppressive effect on the release of other mediators, we measured the levels of the arachidonic acid metabolite, prostaglandin E2 (PGE2) using a radioimmunoassay as previously described (Mitchell et al., 1994). IL-1β induced significant release of PGE2 from 0.7±0.6 ng ml−1 to 4.3±0.7 ng ml−1 (P<0.05). This effect was suppressed in the presence of dexamethasone (1 μM) to 0.23±0.01 ng ml−1 (Figure 4b).
Figure 4.
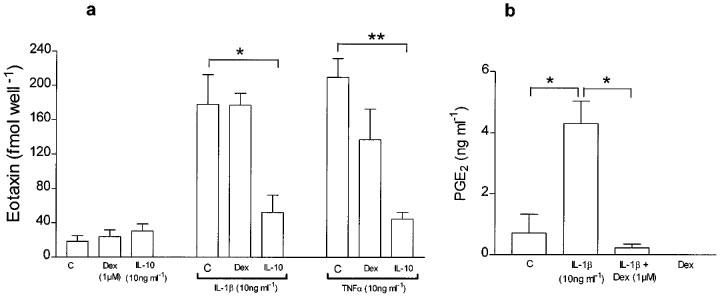
(a) Effect of IL-10 (10 ng ml−1) and dexamethasone (1 μM) on eotaxin release induced by IL-1β (10 ng ml−1) and TNFα (10 ng ml−1). Dexamethasone did not inhibit eotaxin release while IL-10 suppressed both IL-1β- and TNFα-induced release. However, dexamethasone significantly inhibited release of prostaglandin E2 release induced by IL-1β (b). Results shown as s.e.mean of 3–4 different donors. *P<0.05, **P<0.001.
Effects of IL-10 and dexamethasone on IL-1β and TNFα-induced eotaxin mRNA expression
Preliminary experiments showed that eotaxin mRNA induction appeared by 4 h and was optimal after stimulation of HASM cells with IL-1β or TNFα for 24 h at 10 ng ml−1 each (data not shown). Hence these conditions were used for subsequent experiments. Stimulation of HASM cells with IL-1β and TNFα (each at 10 ng ml−1) for 24 h induced the expression of eotaxin mRNA (198±31%, n=3, P<0.01 and 205±54% of control, n=4, P<0.05 respectively). This was observed as a single, distinct band of approximately 800 base pairs by Northern blot analysis (Figure 5a and Figure 5b). This transcript size is similar to that reported for eotaxin mRNA expressed in human lung epithelial cells (Lilly et al., 1997). Both dexamethasone (1 μM) and IL-10 (10 ng ml−1) had no effect on eotaxin mRNA expression induced by IL-1β or TNFα under similar conditions (Figure 5a and Figure 5b).
Figure 5.
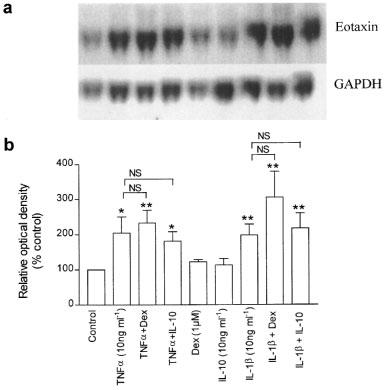
(a) Representative Northern blot analysis of eotaxin mRNA with GAPDH mRNA from airway smooth muscle cells under control unstimulated conditions, and following exposure to IL-1β, TNFα and INFγ each at 10 ng ml−1 mRNA was extracted after a 24 h exposure period to these cytokines. The effects of dexamethasone (1 μM) and IL-10 (10 ng ml−1) on the induction of eotaxin mRNA are also shown. (b) Mean densitometric measurements for eotaxin mRNA calculated as a ratio of GAPDH mRNA and expressed as percentage of control. Both IL-1β and TNFα increased eotaxin mRNA expression. These effects were not significantly affected by dexamethasone or IL-10. Results shown are s.e.mean of cells from 3–4 different donors. *P<0.05, **P<0.01 compared to control.
Discussion
We have shown that human airway smooth muscle cells in culture can be induced to express eotaxin mRNA and to release eotaxin protein when incubated with TNFα or IL-1β. IFNγ however had no effect on the release of eotaxin from these cells and there was no additive effect of any of these cytokines on eotaxin release or expression. Eotaxin produced by the cells was mostly released into the supernatants. The appearance of eotaxin mRNA at 4 h after stimulation, prior to release of eotaxin into the supernatant, indicates that the protein release is dependent on transcription. IL-10 significantly inhibited eotaxin release while corticosteroids had no effect, but neither had an effect on the induction of eotaxin mRNA. Thus, airway smooth muscle may contribute directly to airway inflammation by interacting with IL-1β and TNFα released during inflammation and by attracting eosinophils to the airway submucosa through the release of eotaxin and other chemoattractant cytokines.
The conditions under which release of eotaxin from human airway smooth muscle cells was observed were different from those of RANTES in many respects. Of the pro-inflammatory cytokines, TNFα, IL-1β and IFNγ, both TNFα and IL-1β were effective stimulators of eotaxin release, while TNFα but not IL-1β stimulated RANTES release (John et al., 1997). IFNγ was a poor stimulator of both RANTES and eotaxin release, but synergized with TNFα to cause RANTES release. However, no such synergy was observed regarding eotaxin. We also found differences when compared to IL-8 expression and release from airway smooth muscle cells (John et al., 1998). Examining the profile of eotaxin protein release and mRNA expression, however, supported the notion that these chemokines were transcriptionally regulated by the pro-inflammatory cytokines, particularly TNFα and IL-1β. In the studies of eotaxin release, we found no significant changes in cytosolic eotaxin following cytokine stimulation indicating that most of the newly-synthesized eotaxin was released extracellularly. The differences in the pattern of chemokine release observed are likely to be due to differences in signalling pathways and effector elements for transcriptional activation of each of these chemokines. Analysis of the 5′ flanking regions of the human RANTES, eotaxin and IL-8 genes have revealed a number of positive and negative transcriptional regulatory elements, including AP-1 and NF-κB elements (Garcia Zepeda et al., 1997; Hein et al., 1997). The presence of an NF-κB binding site may underlie the observed regulation of eotaxin by the pro-inflammatory cytokines TNFα and IL-1β.
Corticosteroids had no effect on the release of eotaxin from airway smooth muscle cells, which is in contrast with the inhibitory effects of corticosteroids on the release and expression of IL-8 and RANTES (John et al., 1997; 1998). In the present study, the concentration of dexamethasone used should have been effective because the release of PGE2 from stimulated cells were inhibited. The mechanism of the relative resistance of eotaxin mRNA expression to suppression by corticosteroids is unclear. This observation may however, be related to a specific property of airway smooth muscle cells since corticosteroids have been shown to be capable of inhibiting the induction of eotaxin mRNA from the epithelial cell line, A549 cells (Lilly et al., 1997). The presence of GRE sequences on the eotaxin promoter indicates that corticosteroids should inhibit transcription of eotaxin, since, for example, deletion analysis of the GRE from the IL-8 promoter revealed that this element participated in dexamethasone suppression of IL-8 (Mukaida et al., 1992). By contrast, IL-10 inhibited the stimulated release of IL-8, RANTES and eotaxin from airway smooth muscle cells (John et al., 1997; 1998). Although IL-10 also inhibited the enhanced expression of IL-8 and RANTES, it did not suppress the induction of eotaxin mRNA, indicating that IL-10 may interfere with post-transcriptional regulation of eotaxin.
Our results indicate that the airway smooth muscle should not be regarded solely as a specialized cell involved in contractile responses. Pro-inflammatory cytokines and several growth factors are capable of modulating airway smooth muscle phenotype and mitogenesis (Hirst, 1996) and the resulting increase in airway smooth muscle mass may contribute to airways obstruction and bronchial hyperresponsiveness in asthma (James et al., 1989). The additional secretory potential of airway smooth muscle, particularly in terms of eotaxin release, adds another dimension to the putative role of airway smooth muscle in airway inflammation. Because eotaxin has selective chemoattractant effects on eosinophils, airway smooth muscle could contribute directly to the recruitment of eosinophils to the airways. Whether eotaxin produced by airway smooth muscle cells could also in turn contribute to altered smooth muscle function and airway remodelling is not known. Our observations support the notion that airway smooth muscle could be a major contributor to the inflammatory and pathophysiologic features of the airways in asthma.
Acknowledgments
This study was supported by the Wellcome Trust and the National Asthma Campaign. We thank Professor Sir M. Yacoub for providing us with airway tissues.
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- HBSS
Hanks' balanced salt solution
- HASM
human airway smooth muscle
- HPLC
high performance liquid chromatography
- IFNγ
interferon-γ
- IL-1β
interleukin-1β
- IL-10
interleukin-10
- RT–PCR
reverse transcription-polymerase chain reaction
- TFA
trifluoroacetic acid
- TNFα
tumour necrosis factor α
References
- AZZAWI M., BRADLEY B., JEFFERY P.K., FREW A.J. , WARDLOW A.J., KNOWLES G., ASSOUFI B., COLLINS J.V., DURHAM S., KAY A.B. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am. Rev. Respir. Dis. 1990;142:1407–1413. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- BELVISI M.G., SAUNDERS M.A., HADDAD E-B., HIRST S.J., YACOUB M.H., BARNES P.J., MITCHELL J.A. Inductuin of cyclo-oxygenase-2 by cytokines in human cultured airway smooth muscle cells: novel inflammatory role of the cell type. Br. J. Pharmacol. 1997;142:910–916. doi: 10.1038/sj.bjp.0700963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUSQUET J., CHANEZ P., LACOSTE J.Y., BARNEON G., GHAVANIAN N., ENANDER I., VENGE P., AHLSTEDT S., SIMONY-LAFONTAINE J., GODARD P., MICHEL F.B. Eosinophilic inflammation in asthma. N. Engl. J. Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–160. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- COLLINS P.D., MARLEAU S., GRIFFITHS-JOHNSON D.A., JOSE P.J., WILLIAMS T.J. Co-operation between interleukin-5 and the chemokine, eotaxin, to induce eosinophil accumulation in vivo. J. Exp. Med. 1995;182:1169–1174. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DJUKANOVIC R., WILSON J.W., BRITTEN K.M., WILSON S.J., WALLS A.F., ROCHE W.R., HOWARTH P.H., HOLGATE S.T. Quantitation of mast cells and eosinophils in the bronchial mucosa of symptomatic atopic asthmatics and healthy control subjects using immunohistochemistry. Am. Rev. Respir. Dis. 1990;142:863–871. doi: 10.1164/ajrccm/142.4.863. [DOI] [PubMed] [Google Scholar]
- EBINA M., TAKAHASHI T., CHIBA T., MOTOMIYA M. Cellular hypertrophy and hyperplasia of airway smooth muscle underlying bronchial asthma. Am. Rev. Resp. Dis. 1993;148:720–726. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- EVANS C.A., GARCIA H.H., HARTNEL A., GILMAN R.H., JOSE P.J., MARTINEZ M., REMICK D.G., WILLIAMS T.J., FRIEDLAND J.S. Elavated concentrations of eotaxin and interleukin-5 in human neurocysticercosis. Infect. Immun. 1998;66:4525. doi: 10.1128/iai.66.9.4522-4525.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORSSMANN U., UGUCCIONI M., LOETSCHER P., DAHINDEN C.A., LANGEN H., THELEN M., BAGGIOLINI M. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J. Exp. Med. 1997;185:2171–2176. doi: 10.1084/jem.185.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA ZEPEDA E.A., ROTHENBERG M.E., WEREMOWICZ S., SARAFI M.N., MORTON C.C., LUSTER A.D. Genomic organization, complete sequence, and chromosomal location of the gene for human eotaxin (SCYA11), an eosinophil-specific CC chemokine. Genomics. 1997;41:471–476. doi: 10.1006/geno.1997.4656. [DOI] [PubMed] [Google Scholar]
- HEIN H., SCHLUTER C., KULKE R., CHRISTOPHERS E., SCHRODER J.M., BARTELS J. Genomic organization, sequence, and transcriptional regulation of the human eotaxin gene. Biochem. Biophys. Res. Commun. 1997;237:537–542. doi: 10.1006/bbrc.1997.7169. [DOI] [PubMed] [Google Scholar]
- HIRST S.J. Airway smooth muscle cell culture: application to studies of airway wall remodelling and phenotype plasticity in asthma. Eur. Resp. J. 1996;9:908–918. doi: 10.1183/09031936.96.09040808. [DOI] [PubMed] [Google Scholar]
- HIRST S.J., BARNES P.J., TWORT C.H.L. Quantifying proliferation of cultured human and rabbit airway smooth muscle cells in response to serum and platelet-derived growth factor. Am. J. Respir. Cell Mol. Biol. 1992;7:574–581. doi: 10.1165/ajrcmb/7.6.574. [DOI] [PubMed] [Google Scholar]
- JAMES A.L., PARE P.D., HOGG J.C. The mechanics of airway narrowing in asthma. Am. Rev. Respir. Dis. 1989;139:242–246. doi: 10.1164/ajrccm/139.1.242. [DOI] [PubMed] [Google Scholar]
- JOHN M., AU B.T., JOSE P.J., LIM S., SAUNDERS M., BARNES P.J., MITCHELL J.A., BELVISI M.G., CHUNG K.F. Expression and release of interleukin-8 by human airway smooth muscle cells: inhibition by Th-2 cytokines and corticosteroids. Am. J. Respir. Cell. Mol. Biol. 1998;18:84–90. doi: 10.1165/ajrcmb.18.1.2813. [DOI] [PubMed] [Google Scholar]
- JOHN M., HIRST S.J., JOSE P.J., ROBICHAUD A., BERKMAN N., WITT C., TWORT C.H.C., BARNES P.J., CHUNG K.F. Human airway smooth muscle cells express and release RANTES in response to Th-1 cytokines: regulation by Th-2 cytokines and corticosteroids. J. Immunol. 1997;158:1841–1847. [PubMed] [Google Scholar]
- JOSE P.J., GRIFFITHS-JOHNSON D.A., COLLINS P.D., WALSH D.T., MOQBEL R., TOTTY N.F., TRUONG O., HSUAN J.J., WILLIAMS T.J. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J. Exp. Med. 1994;179:881–887. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMKHIOUED B., RENZI P.M., ABI YOUNES S., GARCIA ZEPADA E.A., ALLAKHVERDI Z., GHAFFAR O., ROTHENBERG M.D., LUSTER A.D., HAMID Q. Increased expression of eotaxin in bronchoalveolar lavage and airways of asthmatics contributes to the chemotaxis of eosinophils to the site of inflammation. J. Immunol. 1997;159:4593–4601. [PubMed] [Google Scholar]
- LILLY C.M., NAKAMURA H., KESSELMAN H., NAGLER ANDERSON C., ASANO K., GARCIA ZEPEDA E.A., ROTHENBERG M.E., DRAZEN J.M., LUSTER A.D. Expression of eotaxin by human lung epithelial cells: induction by cytokines and inhibition by glucocorticoids. J. Clin. Invest. 1997;99:1767–1773. doi: 10.1172/JCI119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTOLI S., STACEY M.A., SUN G., BELLINI A., MARINI M. Eotaxin expression and eosinophilic inflammation in asthma. Biochem. Biophys. Res. Commun. 1997;236:299–301. doi: 10.1006/bbrc.1997.6958. [DOI] [PubMed] [Google Scholar]
- MITCHELL J.A., BELVISI M.G., AKARASEREENONT P., ROBBINS R.A., KWON O-J., CROXTALL J., BARNES P.J., VANE J.R. Induction of cyclo-oxygenase-2 by cytokines in human pulmonary epithelial cells: regulation by dexamethasone. Br. J. Pharmacol. 1994;113:1008–1014. doi: 10.1111/j.1476-5381.1994.tb17093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUKAIDA N., GUSSELLA G.L., KASAHARA T., KO Y., ZACHARIAE C.O., KAWAI T., MATSUSHIMA K. Molecular analysis of the inhibition of interleukin-8 production by dexamethasone in a human fibrosarcoma cell line. Immunology. 1992;75:674–679. [PMC free article] [PubMed] [Google Scholar]
- ROCHE W.R., BEASLEY R., WILLIAMS J.H., HOLGATE S.T. Subepithelial fibrosis in the airways of asthmatics. Lancet. 1989. pp. 520–522. [DOI] [PubMed]
- ROTHENBERG M.E., LUSTER A.D., LILLY C.M., DRAZEN J.M., LEDER P. Constitutive and allergen-induced expression of eotaxin mRNA in the guinea pig lung. J. Exp. Med. 1995;181:1211–1216. doi: 10.1084/jem.181.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHENBERG M.E., MACLEAN J.A., PEARLMAN E., LUSTER A.D., LEDER P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J. Exp. Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHENBERG M.E., OWNBEY R., MEHLHOP P.D., LOISELLE P.M., VAN DE RIJN M., BONVENTRE J.V., OETTGEN H.C., LEDER P., LUSTER A.D. Eotaxin triggers eosinophil-selective chemotaxis and calcium flux via a distinct receptor and induces pulmonary eosinophilia in the presence of interleukin 5 in mice. Mol. Med. 1996;2:334–348. [PMC free article] [PubMed] [Google Scholar]
- YANG Y., LOY J., RYSECK R-P., CARRASCO D., BRAVO R. Antigen-induced eosinophilic lung inflammation develops in mice deficient in chemokine eotaxin. Blood. 1998;92:3912–3923. [PubMed] [Google Scholar]
- YING S., ROBINSON D.S., MENG Q., ROTTMAN J., KENNEDY R., RINGLER D.J., MACKAY C.R., DAUGHERTY B.L., SPRINGER M.S., DURHAM S.R., WILLIAMS T.J., KAY A.B. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur. J. Immunol. 1997;27:3507–3516. doi: 10.1002/eji.1830271252. [DOI] [PubMed] [Google Scholar]


