Abstract
A number of structurally distinct superoxide dismutase (SOD) mimetics were examined to determine if they shared the ability of authentic Cu/Zn SOD to produce endothelium-dependent relaxation of rings of rat aorta by protecting basal nitric oxide from destruction by endogenously produced superoxide anion.
MnCl2 (10 nM–100 μM), CuSO4 (100 nM–1 mM) and CuDIPS (Cu [II]-[diisopropylsalicylate]2; 100 nM–30 μM) each mimicked the ability of Cu/Zn SOD (0.1–300 u ml−1) to produce relaxation of phenylephrine-precontracted aortic rings in a manner inhibited by endothelial removal or treatment with NG-nitro-L-arginine methyl ester (L-NAME, 100 μM).
In contrast, MnTMPyP (Mn [III] tetrakis [1-methyl-4-pyridyl] porphyrin; 10 nM–30 μM) augmented phenylephrine-induced contraction and this was blocked by endothelial removal or treatment with L-NAME (100 μM), consistent with destruction rather than protection of basal nitric oxide activity. Pretreatment with Cu/Zn SOD (250 u ml−1) blocked this augmentation suggesting that it arose paradoxically through destruction of nitric oxide by superoxide anion.
The spin trap agents tiron (100 nM–1 mM), tempol (100 nM–1 mM) and PTIYO (4-phenyl-2,2,5,5-tetramethyl imidazolin-1-yloxy-5-oxide; 100 nM–300 μM) all failed to promote endothelium-dependent relaxation. In fact, the last two augmented phenylephrine-induced tone and this was blocked by endothelial removal or treatment with L-NAME (100 μM), consistent with destruction of basal nitric oxide activity. This destruction was unaffected by pretreatment with Cu/Zn SOD (250 u ml−1) and probably reflected the direct ability of tempol and PTIYO to destroy nitric oxide.
Thus, the ideal SOD mimetic for protection of nitric oxide activity in conditions of oxidant stress still awaits development.
Keywords: Nitric oxide, oxidant stress, superoxide anion, superoxide dismutase, superoxide dismutase mimetic, vascular endothelium, vasodilatation
Introduction
Nitric oxide is destroyed rapidly by the reduced species of molecular oxygen, superoxide anion, leading to loss of its vasodilator action (Gryglewski et al., 1986; Rubanyi & Vanhoutte, 1986) and formation of the powerful oxidant, peroxynitrite (Beckman et al., 1990). Studies involving the use of the copper chelator, diethyldithiocarbamate, which inactivates the Cu/Zn-containing isoforms of superoxide dismutase (SOD; Cocco et al., 1981; Kelner et al., 1989) suggest that this enzyme plays a vital role in protecting nitric oxide produced by the vascular endothelium (Omar et al., 1991; Mügge et al., 1991; Mian & Martin, 1995) and nitrergic nerves (Martin et al., 1994; Lilley & Gibson, 1995). This protective action provides SOD and SOD mimetics with therapeutic potential in the treatment of pathologies associated with oxidant stress. Indeed, we and others have recently demonstrated that authentic SOD and a number of structurally distinct SOD mimetics protect, to varying degrees, the activity of endothelium- (MacKenzie & Martin, 1998a; Dudgeon et al., 1998; Fontana et al., 1999) and nitrergic nerve-derived (Mok et al., 1998) nitric oxide in models of oxidant stress. In addition to this ability to protect nitric oxide under conditions of oxidant stress, authentic SOD (Ohlstein & Nichols, 1989; Mian & Martin, 1995; Barton et al., 1997) and certain Mn-based SOD mimetics (Kasten et al., 1994; 1995) are known to produce endothelium-dependent relaxation in normal vessels. This relaxant action arises as a consequence of protection of basal nitric oxide from destruction by endogenously produced superoxide anion.
The aim of this study was to determine if the ability to induce endothelium-dependent relaxation was a general property of SOD mimetics. In order to test this, we examined the ability of a number of structurally distinct classes of SOD mimetic to induce endothelium-dependent relaxation in isolated rings of rat aorta. The SOD mimetics examined for this property were the metal-based compounds CuDIPS (Cu [II]-[diisopropylsalicylate]2; Huber et al., 1987; Sorenson, 1995) and MnTMPyP (Mn [III] tetrakis [1-methyl-4-pyridyl] porphyrin; Faulkner et al., 1994; Gardner et al., 1996), the simple metal salts CuSO4 and MnCl2 (Huber et al., 1987; Beyer & Fridovich, 1990), the nitroxide spin traps PTIYO (4-phenyl-2,2,5,5-tetramethyl imidazolin-1-yloxy-5-oxide) and tempol (4-hydroxy 2,2,6,6-tetramethylpiperidine-1-oxyl) (Mitchell et al., 1990; Ewing & Janero, 1995), and the superoxide scavenger tiron (4,5-dihydroxy-1,3-benzene-disulphonic acid; Ledenev et al., 1986). Preliminary findings from this study have already been published (MacKenzie & Martin, 1998b).
Methods
Preparation of tissues
Female Wistar rats (200–250 g) were killed by stunning and exsanguination. The thoracic aorta was then carefully removed and cleaned of fat and connective tissue and cut into transverse rings (2.5 mm wide). Care was taken not to damage inadvertently the intimal surface of the aorta. In some experiments, the endothelium was removed by gentle abrasion of the intimal surface using a moist wooden stick. Aortic rings were then mounted under 1 g resting tension on stainless steel hooks within 10 ml tissue baths and maintained at 37°C in Krebs solution (mM): NaCl 118, KCl 4.8, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 24, glucose 11, gassed with 95% O2 and 5% CO2. Tension was recorded isometrically with Grass FTO3C transducers and responses displayed and recorded on a MacLab (E Series, AD Instruments). Tissues were allowed to equilibrate for 60 min before experiments were carried out, during which time the resting tension was re-adjusted to 1 g, as required.
Experimental protocols
All experiments involving control, endothelium-containing aortic rings were conducted following induction of 40–60% of maximal phenylephrine (PE)-induced tone. This level of tone was achieved with PE at 0.1–0.3 μM. A number of the experimental procedures employed, however, affected the level of tone induced by PE. Specifically, endothelial denudation or treatment with NG-nitro-L-arginine methyl ester (L-NAME, 100 μM, for 30 min) led to loss of basal nitric oxide activity and, consequently, enhanced PE-induced contraction. In order to compensate for these changes in sensitivity, the concentration of PE employed in each individual experiment was adjusted to ensure that the level of tone was 40–60% of the maximum seen on control, endothelium-containing rings.
Cumulative concentration-response curves to authentic Cu/Zn SOD (0.1–300 u ml−1) and SOD mimetics were constructed on endothelium-containing rings following induction of PE-induced tone. The SOD mimetics examined were tiron (100 nM–1 mM), PTIYO (100 nM–300 μM), tempol (100 nM–1 mM), CuSO4 (100 nM–1 mM), CuDIPS (100 nM– 30 μM), MnCl2 (10 nM–100 μM) and MnTMPyP (10 nM–300 μM). The effects of inhibition of nitric oxide synthase with L-NAME (100 μM, 30 min) and of endothelium-denudation were examined on the effects of SOD and the SOD mimetics.
Drugs
4,5-dihydroxy-1,3-benzene-disulphonic acid (tiron), 4-hydroxy 2,2,6,6-tetramethylpiperidine-1-oxyl (tempol), phenylephrine hydrochloride, and superoxide dismutase (Cu/Zn-containing enzyme from bovine erythrocytes) were obtained from Sigma (Poole, U.K.). Cu (II)-[diisopropylsalicylate]2 (CuDIPS) and 4 - phenyl - 2,2,5,5 - tetramethyl imidazolin - 1 - yloxy - 5 - oxide (PTIYO) were obtained from Aldrich (Dorset, U.K.). Mn (III) tetrakis [1-methyl-4-pyridyl] porphyrin (MnTMPyP) was obtained from Alexis (Nottingham, U.K.), whilst CuSO4 and MnCl2 were obtained from Hopkin & Williams (Essex, U.K.). All drugs were dissolved in saline (0.9%), except for CuDIPS (3 mM stock) which was dissolved in 50% ethanol/50% tris buffer (50 mM, pH 7.4). Control experiments demonstrated that the solvent, ethanol/tris buffer, did not account for the effects observed with CuDIPS. All dilutions were made in saline (0.9%).
Analysis of data
The effects of authentic SOD and the SOD mimetics on tone are expressed as a percentage (%) of the pre-existing level (100%) of PE-induced tone. Thus, relaxations and contractions are expressed as falls and increases from this level, respectively. Results are expressed as the mean±s.e.mean of n separate experiments. Statistical comparisons were made by one-way analysis of variance followed by the Bonferroni post-test. A value of P<0.05 was considered significant. Concentration-response curves were constructed and statistical analyses performed using a computer-based programme (Graph Pad, Prism).
Results
Effects of Cu/Zn superoxide dismutase and metal-based SOD mimetics on tone of rat aorta
Following induction of submaximal tone with phenylephrine (PE, 0.1–0.3 μM) in endothelium-containing rings of rat aorta, authentic Cu/Zn superoxide dismutase (SOD, 0.1–300 u ml−1) produced concentration-dependent relaxation (Figure 1a; maximal relaxation to 26.0±5.9% of initial tone). This relaxation was almost abolished following 30 min pretreatment with the inhibitor of nitric oxide synthase, NG-nitro-L-arginine methyl ester (L-NAME, 100 μM), and by endothelial denudation.
Figure 1.
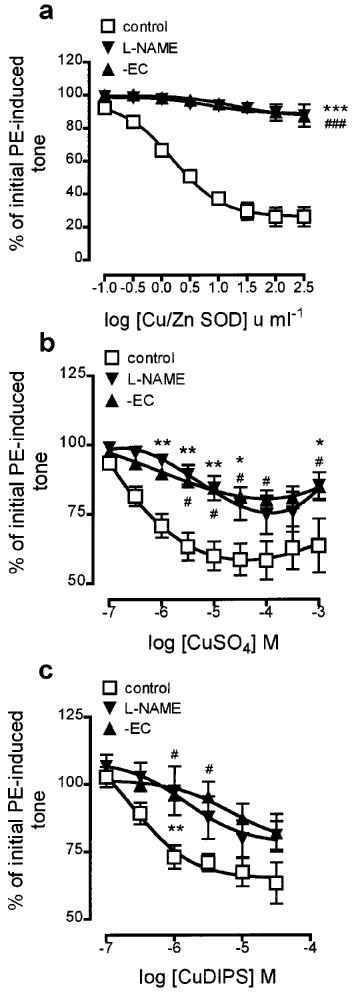
Concentration-response curves showing the changes in tone induced by (a) authentic Cu/Zn superoxide dismutase (Cu/Zn SOD), (b) CuSO4, and (c) CuDIPS on phenylephrine-contracted, endothelium-containing rings of rat aorta (control). The effects of pretreatment with L-NAME (100 μM; 30 min) and of endothelial removal (−EC) on these changes are also shown. Each point is the mean±s.e.mean of 5–8 observations. *P<0.05, **P<0.005, and ***P<0.001 indicate a significant difference following treatment with L-NAME. #P<0.05 and ###P<0.001 indicate a significant difference following endothelial removal. For clarity, levels of statistical significance shown in (a) are for the highest concentration of Cu/Zn SOD only.
As with Cu/Zn SOD, the SOD mimetics CuSO4 (100 nM–1 mM), CuDIPS (100 nM–30 μM) and MnCl2 (10 nM–100 μM) each relaxed precontracted endothelium-containing rings although the maximum relaxations obtained were somewhat smaller (to 58.3±6.9, 63.4±7.6 and 48.9±5.5% of initial tone, respectively; Figures 1b and c and 2a). Pretreatment with L-NAME (100 μM; 30 min) and endothelial denudation each impaired the relaxations induced by CuSO4, CuDIPS and MnCl2.
Figure 2.
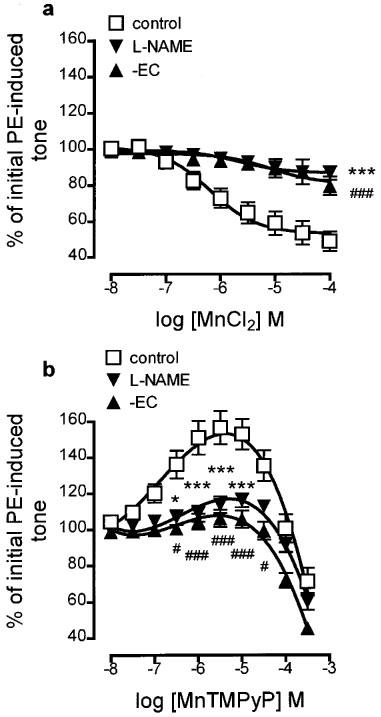
Concentration-response curves showing the changes in tone induced by (a) MnCl2 and (b) MnTMPyP on phenylephrine-contracted, endothelium-containing rings of rat aorta (control). The effects of pretreatment with L-NAME (100 μM; 30 min) and of endothelial removal (−EC) on these changes are also shown. Each point is the mean±s.e.mean of 5–8 observations. *P<0.05 and ***P<0.001 indicate a significant difference following treatment with L-NAME. #P<0.05 and ###P<0.001 indicate a significant difference following endothelial removal. For clarity, levels of statistical significance shown in (a) are for the highest concentration of MnCl2 only.
In contrast to SOD and the other metal-based SOD mimetics, the changes in tone observed following treatment of precontracted, endothelium-containing rings with MnTMPyP (10 nM–300 μM) comprised two components: an initial enhancement of tone (from 10 nM–30 μM, to a maximum of 156.6±9.2% of initial tone), followed by a relaxant component (from 100–300 μM, to 71.2±7.6% of initial tone; Figure 2b). Pretreatment with L-NAME (100 μM; 30 min) and endothelial denudation each almost abolished the enhancement of PE-induced tone (maximum levels attained were 113.7±4.5 and 106.2±4.7% of initial tone, respectively), but neither affected the relaxation induced by MnTMPyP.
Others have reported that MnTMPyP can act either as a net scavenger or generator of superoxide anion depending on the redox environment (Gardner et al., 1996). Accordingly, the possibility that the endothelium-dependent enhancement of tone by MnTMPyP might be due to destruction of basal nitric oxide activity resulting from superoxide generation was tested by pre-incubating tissues with Cu/Zn SOD. Pretreatment of endothelium-containing rings for 30 min with SOD (250 u ml−1), followed by restoration of submaximal tone with additional PE, resulted in blockade of the MnTMPyP-induced enhancement of tone: it produced a greater than 10 fold rightward shift in the concentration-response curve but did not depress the maximum enhancement observed (137.6±9.9% of initial tone; Figure 3). Experiments conducted on endothelium-denuded rings demonstrated that SOD had no effect on the relaxant actions of MnTMPyP (Figure 4).
Figure 3.
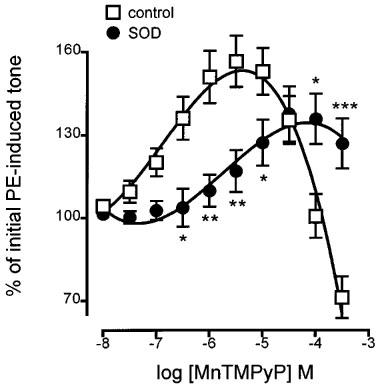
Concentration-response curves showing the changes in tone induced by MnTMPyP on phenylephrine-contracted, endothelium-containing rings of rat aorta (control) and the effects of pretreatment with Cu/Zn superoxide dismutase (SOD, 250 u ml−1, 30 min) on these changes. Each point is the mean±s.e.mean of 5–11 observations. *P<0.05, **P<0.005 and ***P<0.001 indicate a difference following treatment with superoxide dismutase.
Figure 4.
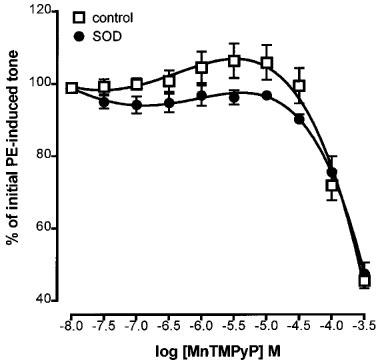
Concentration-response curves showing that the relaxation induced by MnTMPyP on phenylephrine-contracted, endothelium-denuded rings of rat aorta (control) was unaffected by pretreatment with Cu/Zn superoxide dismutase (SOD, 250 u ml−1, 30 min). Each point is the mean±s.e.mean of eight observations.
Effects of spin trap SOD mimetics on tone of rat aorta
The superoxide anion scavenger, tiron (100 nM–1 mM), had no effect on submaximal PE-induced tone either in control endothelium-containing rings, endothelium-containing rings treated with L-NAME (100 μM; 30 min), or endothelium-denuded rings (Figure 5a). In contrast, both nitroxide spin traps, PTIYO (100 nM–300 μM) and tempol (100 nM–1 mM), augmented the tone of endothelium-containing rings (to maxima of 268.3±29.4 and 163.6±14.3% of initial tone with the highest concentration of each tested, respectively; Figures 5b and c). Pretreatment for 30 min with L-NAME (100 μM) and endothelial denudation each powerfully impaired the augmentation of tone induced by PTIYO and tempol. Pretreatment for 30 min with SOD (250 u ml−1) had no effect on the augmentation of tone induced by PTIYO or tempol on endothelium-containing rings (data not shown).
Figure 5.
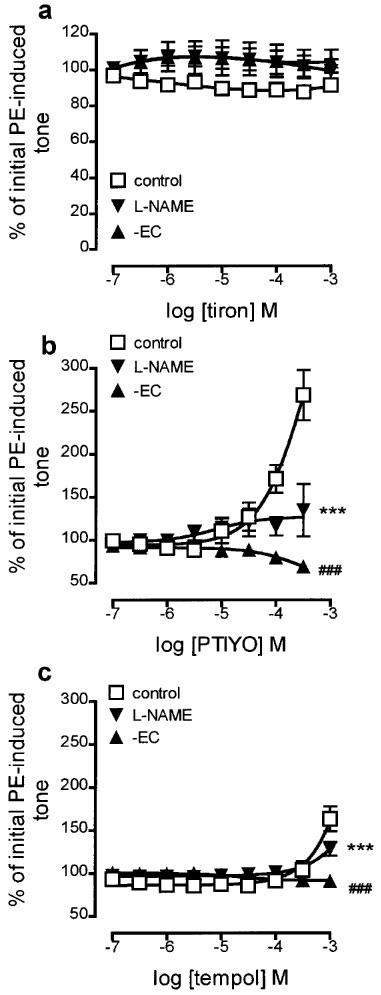
Concentration response curves showing the changes in tone induced by (a) tiron, (b) PTIYO and (c) tempol on phenylephrine-contracted, endothelium-containing rings of rat aorta (control). The effects of pretreatment with L-NAME (100 μM; 30 min) and of endothelial removal (−EC) on these changes are also shown. Each point is the mean±s.e.mean of 8–18 observations. ***P<0.001 and ###P<0.001 indicate a significant difference following treatment with L-NAME and following endothelial removal, respectively.
Discussion
It is well established that authentic Cu/Zn SOD induces endothelium-dependent vasodilatation by protecting basal nitric oxide from the destructive action of endogenously produced superoxide anion (Ohlstein & Nichols, 1989; Mian & Martin, 1995; Barton et al., 1997). Furthermore, MnCl2, which mimics the ability of the Mn-containing isoform of SOD to scavenge superoxide anion (Beyer & Fridovich, 1990), also promotes vascular relaxation (Kasten et al., 1994). Our findings with Cu/Zn SOD and MnCl2 in this study are entirely consistent with these reports and further demonstrate that CuSO4 and CuDIPS, which mimic the ability of Cu/Zn SOD to scavenge superoxide anion (Huber et al., 1987; Sorenson, 1995), each also induces relaxation of rat aortic rings in a manner that is blocked by inhibition of nitric oxide synthesis or endothelial removal. For MnCl2, CuSO4 and CuDIPS the absolute magnitude of endothelium-dependent relaxation produced was not as great as with Cu/Zn SOD itself, and each also produced a larger endothelium-independent component of relaxation. These differences may reflect additional properties seen with these agents, but lacking in Cu/Zn SOD, resulting from the presence of free metal ions. For example, CuSO4 and CuDIPS each inhibit acetylcholine-induced relaxation (Emsley et al., 1998; MacKenzie & Martin, 1998a), perhaps as a consequence of the generation of hydroxyl radical or inhibition of nitric oxide synthase (Baquial & Sorenson, 1995). Furthermore, MnCl2 is known to block calcium entry into cells and this might be expected to promote relaxation of vascular smooth muscle and inhibition nitric oxide release from endothelium (Kasten et al., 1994). These additional deleterious properties make MnCl2, CuSO4 and CuDIPS unsuitable as therapeutic agents for the protection of nitric oxide in conditions of oxidant stress. Nevertheless, the ability of Cu/Zn SOD to promote endothelium-dependent relaxation through protection of basal nitric oxide activity is certainly shared by the SOD mimetics, MnCl2, CuSO4 and CuDIPS.
In contrast, the low molecular weight Mn-porphyrin-based SOD mimetic, MnTMPyP (Faulkner et al., 1994; Gardner et al., 1996), failed to produce endothelium-dependent relaxation of rat aortic rings. Indeed, quite paradoxically, this agent produced the opposite effect, i.e. in the concentration range 10 nM–30 μM it augmented phenylephrine-induced tone; at higher concentrations it produced relaxation by an endothelium-independent action on the vascular smooth muscle. The initial augmentation of tone was almost completely abolished following endothelial removal or inhibition of nitric oxide synthesis by L-NAME. Its actions are therefore consistent with destruction rather than the anticipated potentiation of basal nitric oxide activity. This action of MnTMPyP was indeed surprising, since we and others have demonstrated previously that in conditions of oxidant stress it powerfully protects acetylcholine-induced relaxation in rabbit aorta (MacKenzie & Martin, 1998a; Fontana et al., 1999) and nitrergic transmission in the retractor penis muscle (Mok et al., 1998). Others have, however, demonstrated that MnTMPyP can be either a net scavenger or generator of superoxide anion depending upon the prevailing redox environment (Gardner et al., 1996). Specifically, when the level of superoxide is high, MnTMPyP preferentially scavenges this free radical, but when low, it participates in alternative redox reactions leading to superoxide generation. On the basis of this, we considered the possibility that the MnTMPyP-induced augmentation of tone was due to destruction of basal nitric oxide resulting from enhanced generation of superoxide. This indeed appeared to be the explanation since prior treatment with authentic Cu/Zn SOD blocked the MnTMPyP-induced augmentation of tone. Thus, despite promising indications that MnTMPyP is effective in protecting nitric oxide in conditions of oxidant stress (MacKenzie & Martin, 1998a; Mok et al., 1998; Fontana et al., 1999), its ability to destroy basal nitric oxide activity through generation of superoxide seriously compromises its potential as a therapeutic agent. This paradoxical ability of MnTMPyP to generate superoxide does not, however, appear to be a general property of Mn-based SOD mimetics. For example, the compound SC52608, produces relaxation of rat aortic rings in a manner similar to that of authentic Cu/Zn SOD (Kasten et al., 1995) and is likely, therefore, to have greater therapeutic potential. The endothelium-independent relaxation seen with high concentrations of MnTMPyP was not blocked by pretreatment with SOD and therefore did not involve superoxide anion.
The spin trap agents, tiron, PTIYO and tempol, are effective scavengers of superoxide, as demonstrated in a number of in vitro biochemical assays, but they are roughly 1000 times less potent than the metal-based SOD mimetics (Mok et al., 1998). Moreover, in this study we showed that none of these spin traps shared the ability of Cu/Zn SOD to promote endothelium-dependent relaxation of rat aortic rings; tiron did produce slight relaxation in some individual tissues but the combined data from all experiments failed to reach statistical significance. In fact, PTIYO and, to a lesser extent, tempol augmented phenylephrine-induced tone. As with MnTMPyP, this effect was inhibited following endothelial removal or blockade of nitric oxide synthesis with L-NAME, consistent with destruction of basal nitric oxide activity. Unlike with MnTMPyP, however, the augmentation of tone with PTIYO or tempol was not inhibited following treatment with Cu/Zn SOD, and so the destruction of basal nitric oxide did not involve superoxide anion. Rather, since nitric oxide itself is a free radical one would expect it to be scavenged by spin trap compounds in much the same way as superoxide anion. Indeed, the class of stable nitroxide spin traps, to which PTIYO and tempol belong, has already been shown to react with and destroy nitric oxide (Akaike et al., 1993). Moreover, the previously reported greater potency of PTIYO than of tempol to inhibit acetylcholine-induced relaxation in rabbit aorta (MacKenzie et al., 1998a) reflects the relative potencies of these two agents to augment phenylephrine-induced tone in rat aorta, presumably as a consequence of their differential ability to destroy basal nitric oxide activity. Thus, the ideal spin trap for protection of nitric oxide against oxidant stress would be one which had high affinity for superoxide anion but low affinity for nitric oxide. Tempol is clearly the better of these two nitroxide spin traps in this respect, but its affinity for nitric oxide is still too high to be considered suitable for clinical use. Nevertheless, tempol has demonstrated some utility in lowering blood pressure through augmentation of nitric oxide activity in normotensive and spontaneously hypertensive rats (Leach et al., 1998; Schnackenberg et al., 1998).
In conclusion, the ability of Cu/Zn SOD to promote endothelium-dependent relaxation of rat aortic rings through potentiation of basal nitric oxide activity is shared by the SOD mimetics, MnCl2, CuSO4 and CuDIPS. The free metal ions released from these in solution, however, preclude their use as therapeutic agents. The metal ion is, however, firmly embedded in the porphyrin ring of MnTMPyP, but this agent destroys basal nitric oxide activity by a mechanism involving the paradoxical generation of superoxide anion. The spin trap agents tiron, PTIYO and tempol also lack the ability to promote endothelium-dependent relaxation and, indeed, the last two agents augment vasoconstrictor tone by a direct ability to scavenge nitric oxide. Thus, the ideal SOD mimetic for protection of nitric oxide activity in conditions of oxidant stress still awaits development.
Acknowledgments
A. MacKenzie holds a Medical Research Council Scholarship. We are grateful to the British Heart Foundation and the Wellcome Trust for support.
Abbreviations
- CuDIPS
Cu [II]-[diisopropylsalicylate]2
- L-NAME
NG-nitro-L-arginine methyl ester
- MnTMPyP
Mn [III] tetrakis [1-methyl-4-pyridyl] porphyrin
- PE
phenylephrine
- PTIYO
4-phenyl-2,2,5,5-tetramethyl imidazolin-1-yloxy-5-oxide
- SOD
superoxide dismutase
References
- AKAIKE T., YOSHIDA M., MIYAMOTO Y., SATO K., KOHNO M., SASAMOTO K., MIYAZAKI K., UEDA S., MAEDA H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/NO through a radical reaction. Biochem. 1993;32:827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- BAQUIAL J.G.L., SORENSON J.R.J. Down-regulation of NADPH-diaphorase (nitric oxide synthase) may account for the pharmacological activities of Cu(II)2(3,5-diisopropylsalicylate)4. J. Inorg. Biochem. 1995;60:133–148. doi: 10.1016/0162-0134(95)00008-c. [DOI] [PubMed] [Google Scholar]
- BARTON M., COSENTINO F., BRANDES R.P., MOREAU P., SHAW S., LUSCHER T. Anatomic heterogeneity of vascular aging: roles of nitric oxide and endothelin. Hypertension. 1997;30:817–824. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN J., MARSHALL P.A., FREEMAN B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEYER W.F., JR, FRIDOVICH I. Superoxide dismutase mimic prepared from desferrioxamine and manganese dioxide. Methods Enzymol. 1990;186:242–249. doi: 10.1016/0076-6879(90)86115-c. [DOI] [PubMed] [Google Scholar]
- COCCO D., CALABRESE L., RIGO A., ARGESE E., ROTILIO G. Re-examination of the reaction of diethyldithiocarbamate with the copper of superoxide dismutase. J. Biol. Chem. 1981;256:8983–8986. [PubMed] [Google Scholar]
- DUDGEON S., BENSON D.P., MACKENZIE A., PAISLEY-ZYSZKIEWISCZ K., MARTIN W. Recovery by ascorbate of impaired nitric oxide-dependent relaxation resulting from oxidant stress in rat aorta. Br. J. Pharmacol. 1998;125:782–786. doi: 10.1038/sj.bjp.0702120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMSLEY A.M., WYATT M.C., JEREMY J.Y., SORENSON J.R.J., PLANE F. Inhibition of endothelium-dependent relaxation of rat isolated aortic rings by a low molecular mass complex of copper. Br. J. Pharmacol. 1998;125:19P. [Google Scholar]
- EWING J.F., JANERO D.R. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal. Biochem. 1995;232:243–248. doi: 10.1006/abio.1995.0014. [DOI] [PubMed] [Google Scholar]
- FAULKNER K.M., LIOCHEV S.I., FRIDOVICH I. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J. Biol. Chem. 1994;269:23471–23476. [PubMed] [Google Scholar]
- FONTANA L., MCNEILL K.L., RITTER J.M., CHOWIENCZYK P.J. Effects of vitamin C and of a cell permeable superoxide dismutase mimetic on acute lipoprotein induced endothelial dysfunction in rabbit aortic rings. Br. J. Pharmacol. 1999;126:730–734. doi: 10.1038/sj.bjp.0702331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDNER P.R., NGUYEN D.D.H., WHITE C.W. Superoxide scavenging by Mn(II/III) tetrakis (1-methyl-4-pyridyl) porphyrin in mammalian cells. Arch. Biochem. Biophys. 1996;325:20–28. doi: 10.1006/abbi.1996.0003. [DOI] [PubMed] [Google Scholar]
- GRYGLEWSKI R.J., PALMER R.M.J., MONCADA S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- HUBER K.R., SRIDHAR R., GRIFFITH E.H., AMMA E.L., ROBERTS J. Superoxide dismutase-like activities of copper(II) complexes tested in serum. Biochim. Biophys. Acta. 1987;915:267–276. doi: 10.1016/0167-4838(87)90309-8. [DOI] [PubMed] [Google Scholar]
- KASTEN T.P., SETTLE S.L., MISKO T.P., CURRIE M.G., NICKOLS G.A. Manganese potentiation of nitric oxide-mediated vascular relaxation. Eur. J. Pharmacol. 1994;253:35–43. doi: 10.1016/0014-2999(94)90754-4. [DOI] [PubMed] [Google Scholar]
- KASTEN T.P., SETTLE S.L., MISKO T.P., RILEY D.P., WEISS R.H., CURRIE M.G., NICKOLS G.A. Potentiation of nitric oxide-mediated vascular relaxation by SC52608, a superoxide dismutase mimic. Proc. Soc. Exp. Biol. Med. 1995;208:170–177. doi: 10.3181/00379727-208-43848. [DOI] [PubMed] [Google Scholar]
- KELNER M.J., BAGNELL R., HALE B., ALEXANDER N.M. Inactivation of intracellular copper-zinc superoxide dismutase by copper chelating agents without glutathione depletion and methemoglobin formation. Free Radical Biol. Med. 1989;6:355–360. doi: 10.1016/0891-5849(89)90079-8. [DOI] [PubMed] [Google Scholar]
- LEACH M., FRANK S., OLBRICH A., PFEILSCHIFTER J., THIEMERMANN C. Decline in the expression of copper/zinc superoxide dismutase in the kidney of rats with endotoxic shock: Effects of the superoxide anion radical scavenger, tempol, on organ injury. Br. J. Pharmacol. 1998;125:817–825. doi: 10.1038/sj.bjp.0702123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDENEV A.N., KONSTANTINOV A.A., POPOVA E., RUUGE E.K. A simple assay of the superoxide generation rate with tiron as an ESP-visible radical scavenger. Biochem. Int. 1986;13:391–396. [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Inhibition of relaxations to nitrergic stimulation of the mouse anococcygeus by duroquinone. Br. J. Pharmacol. 1995;116:3231–3236. doi: 10.1111/j.1476-5381.1995.tb15129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKENZIE A., MARTIN W. Loss of endothelium-derived nitric oxide in rabbit aorta by oxidant stress: restoration by superoxide dismutase mimetics. Br. J. Pharmacol. 1998a;124:719–728. doi: 10.1038/sj.bjp.0701899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKENZIE A., MARTIN W. A superoxide dismutase mimetic impairs basal nitric oxide activity in rat aorta. Br. J. Pharmacol. 1998b;125:17P. doi: 10.1038/sj.bjp.0702670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN W., MCALLISTER K.H.M., PAISLEY K. NANC neurotransmission in the bovine retractor penis muscle is blocked by superoxide anion following inhibition of superoxide dismutase with diethyldithiocarbamate. Neuropharmacol. 1994;33:1293–1301. doi: 10.1016/0028-3908(94)90029-9. [DOI] [PubMed] [Google Scholar]
- MIAN K.B., MARTIN W. Differential sensitivity of basal and acetylcholine-stimulated activity of nitric-oxide to destruction by superoxide anion in rat aorta. Br. J. Pharmacol. 1995;115:993–1000. doi: 10.1111/j.1476-5381.1995.tb15909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL J.B., SAMUNI A., KRISHNA M.C., DEGRAFF W.G., AHN M.S., SAMUNI U., RUSSO A. Biologically active metal-independent superoxide dismutase mimics. Biochem. 1990;29:2802–2807. doi: 10.1021/bi00463a024. [DOI] [PubMed] [Google Scholar]
- MOK J.S.L., PAISLEY K., MARTIN W. Inhibition of nitrergic neurotransmission in the bovine retractor penis muscle by an oxidant stress: effects of superoxide dismutase mimetics. Br. J. Pharmacol. 1998;124:111–118. doi: 10.1038/sj.bjp.0701809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜGGE A., ELWELL J.H., PETERSON T.E., HARRISON D.G. Release of intact endothelium-derived relaxing factor depends on endothelial superoxide dismutase activity. Am. J. Physiol. 1991;260:C219–C225. doi: 10.1152/ajpcell.1991.260.2.C219. [DOI] [PubMed] [Google Scholar]
- OHLSTEIN E.H., NICHOLS A.J. Rabbit polymorphonuclear neutrophils elicit endothelium-dependent contraction in vascular smooth muscle. Circ. Res. 1989;65:917–924. doi: 10.1161/01.res.65.4.917. [DOI] [PubMed] [Google Scholar]
- OMAR H.A., CHERRY P.D., MORTELLITI M.P., BURKE-WOLIN T., WOLIN M.S. Inhibition of coronary artery superoxide dismutase attenuates endothelium-dependent and -independent nitrovasodilator relaxation. Circ. Res. 1991;69:601–608. doi: 10.1161/01.res.69.3.601. [DOI] [PubMed] [Google Scholar]
- RUBANYI G.M., VANHOUTTE P.M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am. J. Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- SCHNACKENBERG C.G., WELCH W.J., WILCOX C.S. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic. Hypertension. 1998;35:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- SORENSON J.R.J.Pharmacological activities of copper compounds Handbook of metal-ligand interactions in biological fluids 1995New York: Marcel Dekker Inc; 1128–1139.ed. Berthon, G. pp [Google Scholar]


