Abstract
Our previous work has shown that Group I mGlu receptors participate in thalamic sensory processing in vivo. However, unequivocal demonstration of mGlu5 participation has not been possible due to the lack of specific ligands. We have therefore made a preliminary study of the in vivo actions of the agonist (R,S)-2-Chloro-5-hydroxyphenylglycine [CHPG] and the novel mGlu5 antagonist 6-methyl-2-(phenylethynyl)-pyridine [MPEP] in order to characterize their suitability for functional studies. Iontophoretically administered MPEP selectively antagonized excitatory responses of single rat thalamic neurones to CHPG compared to the broad-spectrum mGlu agonist (1S,3R)-1-aminocyclopentane-1,3-dicarboxylate. In contrast, the established mGlu1 and mGlu5 antagonist (S)-4-carboxyphenylglycine reduced responses to both agonists. These findings are the first demonstration of an in vivo action of CHPG and its antagonism by a selective mGlu5 antagonist. Furthermore MPEP appears to be a good tool for functional studies of mGlu5.
Keywords: Metabotropic glutamate receptor; mGlu5; 6-methyl-2-(phenylethynyl)-pyridine; MPEP; (R,S)-2-Chloro-5-hydroxyphenylglycine; CHPG; thalamus; sensory system; iontophoresis
Introduction
The metabotropic glutamate (mGlu) receptors is a family of receptors whose activation by L-glutamate and other excitatory amino acids is coupled to a cascade of intracellular second-messenger events. There are at least eight of these receptors, and they can be placed into three groups: Group I, mGlu1, mGlu5; Group II, mGlu2, mGlu3; Group III, mGlu4, mGlu6, mGlu7, mGlu8, on the basis of sequence homology, agonist/antagonist pharmacology and coupling to intracellular transduction mechanisms (Nakanishi, 1992; Watkins & Collingridge, 1994; Conn & Pin, 1997). Group I receptors are known to couple to postsynaptic inositol phosphate hydrolysis, and there is considerable evidence to suggest that these receptors participate in synaptic processes in several brain regions (for review see Conn & Pinn, 1997). In the rat thalamus in vivo, work from this laboratory has shown that excitatory responses to mGlu agonists can be reduced by a variety of antagonists active at Group I receptors and that these antagonists can reduce responses of thalamic neurones to noxious thermal stimulation (Eaton et al., 1993; Salt & Eaton, 1994). More recently, we have shown that an antagonist with selectivity towards mGlu1 can have similar actions (Salt & Turner, 1998). This leaves the question open as to whether there is a distinct effect of mGlu5 receptors on neuronal activity in the thalamus. Recently, the novel compound 6-methyl-2-(phenylethynyl)-pyridine (MPEP) has been described as a selective non-competitive mGlu5 antagonist which shows activity in a number of in vitro and in vivo systems (Gasparini et al., 1999). This offers the possibility of unequivocally identifying responses which may be mediated by mGlu5, and we have therefore made a preliminary evaluation of this compound on the responses of thalamic neurones to the selective mGlu5 agonist (R,S)-2-Chloro-5-hydroxyphenylglycine [CHPG] (Doherty et al., 1997) and the broad-spectrum mGlu agonist ACPD. We have compared the effects of MPEP with the activity of the established Group I antagonist (S)-4-carboxyphenylglycine [(S)-4CPG].
Methods
Experiments were carried out in adult Wistar rats anaesthetized with urethane (1.2 g kg−1 i.p.) in accordance with the U.K. Animals (Scientific Procedures) Act 1986 and associated guidelines, as detailed previously (Salt, 1987). Extracellular single-neurone recordings were made with multi-barrel iontophoretic electrodes, the outer barrels of which contained one of the following aqueous solutions: ACPD [(1S,3R)-1-aminocyclopentane-1,3-dicarboxylate] 50 mM, pH 8; CHPG [(R,S)-2-Chloro-5-hydroxyphenylglycine] 100 mM, pH 8; NMDA [N-methyl-D-aspartate] 50 mM, pH 8; (S)-4CPG [(S)-4-carboxyphenylglycine] 50 mM, pH 8; MPEP [6-methyl-2-(phenylethynyl)-pyridine] 2 mM or 10 mM in 150 mM NaCl, pH 5. In addition, one barrel contained 1 M NaCl for automatic current balancing. All drugs were ejected iontophoretically as anions (with the exception of MPEP), and prevented from diffusing out of the pipette by a retaining current (10–20 nA) of opposite polarity to the ejection current. MPEP was provided by Novartis (Basel, Switzerland), other drugs were purchased from Tocris (Bristol, U.K.). Neurones in the ventrobasal thalamus and immediately overlying thalamus were identified on the basis of their stereotaxic position and responses to somatosensory stimuli, as described previously (Salt, 1987; Salt & Eaton, 1994).
Results
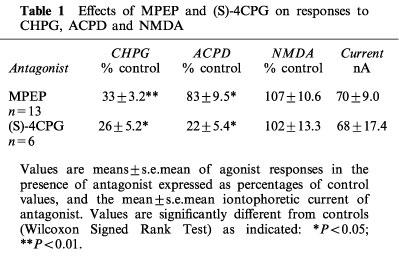
Neurones were excited by regular cyclical applications of the agonists CHPG (150–350 nA), ACPD (50–100 nA) and NMDA (20–80 nA) (Figure 1), and the resulting excitatory responses were quantified as the number of action potentials evoked. Agonist ejection parameters were adjusted so as to produce sub-maximal responses. Application of the antagonist MPEP onto 13 thalamic neurones throughout two or more agonist ejection cycles was found to reduce responses to CHPG with less effect on responses to ACPD or NMDA (Figure 1, Table 1). In contrast, application of (S)-4CPG onto six neurones (four of which were also studied with MPEP) produced a reduction of responses to both ACPD and CHPG, again with little effect on NMDA responses (Figure 1, Table 1).
Figure 1.
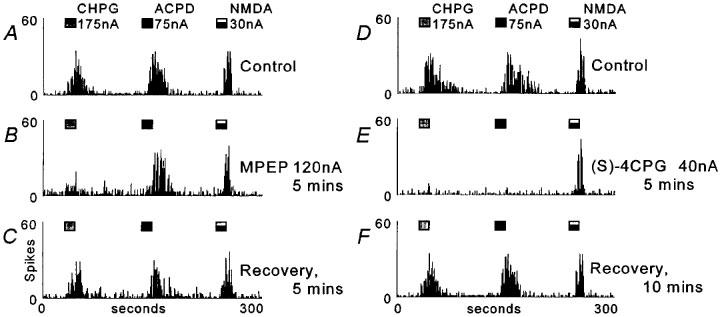
Peristimulus time histograms (PSTHs) showing responses of a thalamic neurone to iontophoretically applied CHPG, ACPD and NMDA. Each PSTH shows action potentials (‘spikes') counted into successive 1000 ms epochs, agonists were applied as indicated by the marker bars above the records. (A) Control responses. (B) Responses during the co-application of MPEP (note the selective reduction of the CHPG response). (C) Recovery following termination of the MPEP ejection. (D) Control responses. (E) Reduction of both CHPG and ACPD responses during co-application of (S)-4CPG. (F) Recovery following termination of (S)-4CPG ejection.
Table 1.
Effects of MPEP and (S)-4CPG on responses to CHPG, ACPD and NMDA
Discussion
The results of this study are consistent with previous data which show that MPEP is a selective mGlu5 antagonist (Gasparini et al., 1999) and that CHPG is a selective mGlu5 agonist (Doherty et al., 1997). The present data extend previous in vitro findings (Doherty et al., 1997) in demonstrating unequivocally for the first time that CHPG is able to activate mGlu5 receptors in vivo. It is noteworthy that MPEP was found to reduce responses to CHPG selectively compared to ACPD. This would suggest that ACPD does not exert its excitatory effects in the thalamus via mGlu5, even though this agonist has a broad spectrum of activity. In situ hybridization studies and immunohistochemistry indicate that although mGlu5 is present in the thalamus (Abe et al., 1992; Romano et al., 1996) there are considerably higher levels of mGlu1 in this brain region (Masu et al., 1991; Shigemoto et al., 1992; Martin et al., 1992). This would suggest that the excitatory action of ACPD in the thalamus is mediated via mGlu1, and this is supported by the finding that thalamic ACPD responses can be antagonized by the mGlu1-selective antagonist (+)-2-methyl-4-carboxyphenylglycine (Salt & Turner, 1998). This of course does not preclude the possibility that there is activation of mGlu5 as well as mGlu1 by ACPD but that the effects via mGlu5 are masked by the activation of mGlu1 receptors, which are numerically predominant (Masu et al., 1991; Shigemoto et al., 1992). This could explain the observed lack of effect of MPEP against ACPD. This matter could be resolved if more potent specific agonists for mGlu1 and mGlu5 become available. Interestingly, in the hippocampus, MPEP is able to antagonize excitatory responses to a broad-spectrum Group I agonist, (S)-3,5-dihydroxyphenylglycine, (Gasparini et al., 1999): this is likely to reflect the predominance of mGlu5 compared to mGlu1 in the hippocampus.
In conclusion, our results demonstrate that it is possible to selectively activate and antagonize mGlu5 receptors in the thalamus. Furthermore, the novel antagonist MPEP appears to be a powerful tool for the identification of mGlu5 synaptic contributions in forthcoming functional studies.
Acknowledgments
This work was supported by The Wellcome Trust and Novartis.
Abbreviations
- 4CPG
4-carboxyphenylglycine
- ACPD
(1S,3R)-1-aminocyclopentane-1,3-dicarboxylate
- CHPG
(R,S)-2-Chloro-5-hydroxyphenylglycine
- MPEP
6-methyl-2-(phenylethynyl)-pyridine
- NMDA
N-methyl-D-aspartate
References
- ABE T., SUGIHARA H., NAWA H., SHIGEMOTO R., MIZUNO N., NAKANISHI S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J. Biol. Chem. 1992;267:13361–13368. [PubMed] [Google Scholar]
- CONN P.J., PIN J.P. Pharmacology and functions of metabotropic glutamate receptors. Ann. Rev. Pharmacol. Toxicol. 1997;37:207–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- DOHERTY A.J., PALMER M.J., HENLEY J.M., COLLINGRIDGE G.L., JANE D.E. (R,S)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5 but not mGlu1 receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology. 1997;36:265–267. doi: 10.1016/s0028-3908(97)00001-4. [DOI] [PubMed] [Google Scholar]
- EATON S.A., BIRSE E.F., WHARTON B., SUNTER D.C., UDVARHELYI P.M., WATKINS J.C., SALT T.E. Mediation of thalamic sensory responses in vivo by ACPD-activated excitatory amino acid receptors. Eur. J. Neuroscience. 1993;5:186–189. doi: 10.1111/j.1460-9568.1993.tb00484.x. [DOI] [PubMed] [Google Scholar]
- GASPARINI F., LINGENHOEHL K., FLOR P.J., MUNIER N., HEINRICH M., VRANESIC I., BIOLLAZ M., HECKENDORN R., ALLGEIER H., VARNEY M., JOHNSON E., HESS S.D., VELICELEBI G., KUHN R. Methylphenylethynlpyridine (MPEP): a novel, potent, subtype-selective and systematically active antagonist at metabotropic glutamate receptor subtype 5. Br. J. Pharmacol. 1999;126:249P. [Google Scholar]
- MARTIN L.J., BLACKSTONE C.D., HUGANIR R.L., PRICE D.L. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992;9:259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- MASU M., TANABE Y., TSUCHIDA K., SHIGEMOTO R., NAKANISHI S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349:760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- NAKANISHI S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- ROMANO C., VAN DEN POL A.N., O'MALLEY K.L. Enhanced early developmental expression of the metabotropic glutamate receptor mGluR5 in rat brain: Protein, mRNA splice variants, and regional distribution. J. Comp. Neurol. 1996;367:403–412. doi: 10.1002/(SICI)1096-9861(19960408)367:3<403::AID-CNE6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- SALT T.E. Excitatory amino acid receptors and synaptic transmission in the rat ventrobasal thalamus. J. Physiol. 1987;391:499–510. doi: 10.1113/jphysiol.1987.sp016752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALT T.E., EATON S.A. The function of metabotropic excitatory amino acid receptors in synaptic transmission in the thalamus: studies with novel phenylglycine antagonists. Neurochem. Int. 1994;24:451–458. doi: 10.1016/0197-0186(94)90093-0. [DOI] [PubMed] [Google Scholar]
- SALT T.E., TURNER J.P. Reduction of sensory and metabotropic glutamate receptor responses in the thalamus by the novel mGluR1 selective antagonist (S) 2-methyl-4-carboxy-phenylglycine. Neuroscience. 1998;85:655–658. doi: 10.1016/s0306-4522(98)00048-7. [DOI] [PubMed] [Google Scholar]
- SHIGEMOTO R., NAKANISHI S., MIZUNO N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: An in situ hybridization study in adult and developing rat. J. Comp. Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- WATKINS J.C., COLLINGRIDGE G. Phenylglycine derivatives as antagonists of metabotropic glutamate receptors. Trends in Pharmacological Sciences. 1994;15:333–342. doi: 10.1016/0165-6147(94)90028-0. [DOI] [PubMed] [Google Scholar]


