Abstract
Previous studies have indicated a role for extracellular ATP in the regulation of epidermal homeostasis. Here we have investigated the expression of P2Y2 receptors by human keratinocytes, the cells which comprise the epidermis.
Reverse transcriptase-polymerase chain reaction (RT–PCR) revealed expression of mRNA for the G-protein-coupled, P2Y2 receptor in primary cultured human keratinocytes.
In situ hybridization studies of skin sections revealed that P2Y2 receptor transcripts were expressed in the native tissue. These studies demonstrated a striking pattern of localization of P2Y2 receptor transcripts to the basal layer of the epidermis, the site of cell proliferation.
Increases in intracellular free Ca2+ concentration ([Ca2+]i) in keratinocytes stimulated with ATP or UTP demonstrated the presence of functional P2Y receptors.
In proliferation studies based on the incorporation of bromodeoxyuridine (BrdU), ATP, UTP and ATPγS were found to stimulate the proliferation of keratinocytes.
Using a real-time firefly luciferase and luciferin assay we have shown that under static conditions cultured human keratinocytes release ATP.
These findings indicate that P2Y2 receptors play a major role in epidermal homeostasis, and may provide novel targets for therapy of proliferative disorders of the epidermis, including psoriasis.
Keywords: Epidermis, keratinocytes, skin, proliferation, extracellular nucleotides, ATP, P2Y2 receptors, in situ hybridization, intracellular calcium
Introduction
Human epidermis is a stratified squamous epithelium comprised of keratinocytes. Cell proliferation is largely confined to the basal layer of the epidermis where cells divide to generate either replacement progenitor cells, or cells committed to terminal differentiation. Differentiating cells migrate to the skin surface. The morphological and biochemical changes which occur during differentiation characterize the distinct layers of the epidermis (see Fuchs, 1990). At the skin surface, the stratum corneum is composed of dead, terminally-differentiated keratinocytes which form the main cutaneous barrier. The balance between proliferation and differentiation of basal cells, which maintains the epidermis at constant thickness, is regulated by many extracellular factors; cell proliferation and differentiation usually follow an inverse relationship. Positive growth factors include epidermal growth factor (Rheinwald, 1980), transforming growth factor α (TGFα; Barrandon & Green, 1984; Gottlieb et al., 1988) and keratinocyte growth factor (Finch et al., 1989). The best characterized negative growth regulators, and hence stimulators of differentiation, are the TGFβs (Shipley et al., 1986), extracellular Ca2+ (Hennings et al., 1980) and vitamin D (Jones & Sharpe, 1994).
Changes in keratinocyte intracellular free Ca2+ concentration ([Ca2+]i) have been recorded in response to both differentiation- and proliferation-stimulating agents, although the pattern of change is dissimilar. Differentiation is associated with a sustained increase in [Ca2+]i which requires influx of extracellular Ca2+ to be maintained (Pillai et al., 1988, 1990; Bikle et al., 1996; Sharpe et al., 1989). Recent evidence suggests that expression of the plasma membrane Ca2+-ATPase is decreased upon differentiation, and that this contributes to the increased [Ca2+]i (Cho & Bikle, 1997). In contrast to this requirement for a sustained increase in [Ca2+]i, growth stimulation is associated with a temporary rise in [Ca2+]i lasting only a few minutes, consistent with release of intracellular stores, but not Ca2+ influx (Pillai & Bikle, 1992; McGovern et al., 1995).
In the vicinity of a wound, keratinocytes will be exposed to ADP, ATP and UTP released from dead or damaged cells as well as from platelets (Boarder & Hourani, 1998). There is accumulating evidence that keratinocytes possess specific receptors for these nucleotides, which may have important effects on keratinocyte proliferation and differentiation. Extracellular nucleotides such as ATP act at P2 receptors, a family of cell-surface receptors which have been divided into two classes based on modes of signal transduction and subsequently molecular cloning: the ligand-gated ion channels, or P2X receptors; and G-protein-coupled, P2Y receptors. There are a number of subdivisions of both classes that display differential responsiveness to nucleotide agonists (see Boarder & Hourani, 1998); in the absence of specific antagonists this has formed the basis of pharmacological characterization of receptor subtypes. All mammalian P2Y receptors cloned to date are coupled to hydrolysis of phosphatidylinositol 4,5-bisphosphate and hence to inositol 1,4,5-trisphosphate- (Ins(1,4,5)P3-mediated release of intracellular Ca2+ stores (Boarder & Hourani, 1998). In addition P2Y2 receptor occupation leads to activation of the mitogen-activated protein kinase (MAPK) cascade (see Boarder & Hourani, 1998); MAPK activation is closely associated with proliferation.
The presence of P2Y receptors on primary human (Pillai & Bikle, 1992) and canine keratinocytes (Suter et al., 1991) has been indicated through recordings of increased [Ca2+]i in response to a range of extracellular nucleotides. The results of desensitization experiments in both studies suggested that ATP and UTP were competing for a single receptor, indicating that keratinocytes express P2Y2 receptors. Consistent with the reported transient nature of the ATP-evoked [Ca2+]i rise, this nucleotide was also found to induce proliferation of human keratinocytes and to inhibit differentiation (Pillai & Bikle, 1992). However, in a separate study by Cook et al. (1995) ATP was shown to have an inhibitory effect on human keratinocyte growth. This anti-proliferative effect was not mediated by P2Y2 receptors, as UTP, an equipotent agonist at this receptor, was ineffective.
To further understand the action of extracellular nucleotides on keratinocytes we have investigated the presence and function of P2Y2 receptors in human skin and cultured keratinocytes. These findings have important implications for the actions of ATP and UTP in epidermal wound repair and for the pathogenesis of hyperproliferative skin diseases. We have additionally demonstrated that under static conditions cultured human keratinocytes release ATP in significant quantities. This indicates that keratinocytes in vivo may be exposed to ATP, not only following injury, but also in the normal skin micro-environment, raising the possibility that ATP has a role in epidermal homeostasis.
Methods
Cell culture
Normal human keratinocytes were isolated from foreskins or neonatal rectoauricular skin removed during plastic surgery and cultured in MCDB153 with supplements, as described previously (Jones & Sharpe, 1994). Cells were passaged with the addition of 100 μg ml−1 bovine hypothalamic extract to aid cell attachment (Tsao et al., 1982) and used from passage 1–4.
Isolation of RNA and first strand cDNA synthesis
Total RNA was extracted from cultured keratinocytes or tissue samples as described previously (Bowler et al., 1995), treated with RNAase-free DNAse 1 (35 U μl−1) for 30 mins and stored as an ethanolic precipitate at −20°C. An aliquot of 5 μg of total RNA was used as template for first strand cDNA synthesis as described earlier (Bowler et al., 1995).
PCR analysis
PCR reactions were carried out using a 50 μl reaction volume as described earlier (Bowler et al., 1995). Following an initial denaturation step (94°C for 2 mins), the conditions for denaturation, annealing and extension were: 25, 30 or 35 cycles of 94°C for 10 s; 58°C for 30 s (60°C for glyceraldehyde phosphate dehydrogenase (GAPDH); 72°C for 30 s.
 |
In situ hybridization
Sections of skin were prepared and stored according to the protocol described earlier (Bowler et al., 1998). In situ hybridization was performed as previously (Littlewood-Evans et al., 1997; Bowler et al., 1998). Briefly, sections were rehydrated, treated with citric acid buffer, and proteinase K to allow access of the RNA probes and to decrease non-specific binding. Sections were post-fixed in paraformaldehyde to retain cellular localization of the mRNA. Remaining proteins were acetylated with acetic anhydride. Prehybridization was carried out in a humidified box at 42°C for 3 h, before addition of 300,000 c.p.m. of 500 bp, sense or anti-sense 32P-dUTP-labelled P2Y2 riboprobe per section. Hybridization was carried out overnight at 42°C. Washing of the sections and development of slides were conducted as described previously (Bowler et al., 1998). Finally, tissues were counter-stained in methylene blue, mounted and photographed.
[Ca2+]i measurements
Keratinocytes were grown to approximately 70% confluency on 22 mm diameter coverslips. The equipment and experimental protocol followed for the determination of [Ca2+]i have been described previously (Dixon et al., 1997). Measurements were made from groups of approximately 8–10 fura-2-loaded cells and addition of agonists was performed manually by pasteur pipette. Autofluoresence values were obtained in situ using ionomycin and 50 μM manganese, as described by Thomas & Delaville (1991). The ratio of fluorescence intensities at 340 nm and 380 nm after subtraction of autofluorescence was plotted as an indicator of [Ca2+]i.
Cell proliferation assay
The effect of extracellular nucleotides on proliferation of keratinocytes was assessed using a Biotrak cell proliferation ELISA system (version 2). Briefly, keratinocytes were seeded at subconfluent levels into 96 well plates and incubated in MCDB153 at 37°C in a humidified atmosphere of 5% CO2, 95% air for 48 h. Medium was removed and replaced with medium containing bromodeoxyuridine- (BrdU)-labelling reagent (1 : 1000) and the nucleotide of interest in the presence of 10% FCS. Cells were incubated for a further 24 h before fixing. Incorporation of BrdU was assessed by ELISA, according to the protocol of the manufacturer. Data were analysed by randomized block analysis of variance (ANOVA) followed by Newman Keuls procedure.
Real-time measurement of ATP release
ATP release was measured using a firefly luciferase and luciferin assay in a custom-built photon-counting luminometer described in detail in Cobbold & Lee (1991). A circular flat-bottomed stainless steel chamber at 37°C, with a capacity of approximately 300 μl was filled with HEPES buffer ((mM) HEPES 10, NaCl 121, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, CaCl2 2, NaHCO3 5, glucose 10, BSA 0.2%; pH 7.2) containing 10 μg ml−1 firefly luciferase and 10 μM luciferin. A 13 mm diameter coverslip of cells at confluency was floated gently into the full chamber to rest on its base. The chamber was then sealed with a glass coverslip and positioned immediately below the photocathode of the photomultiplier (EMI type 9789) housed in a constant-temperature incubator, held at 0–4°C to reduce electrical noise. The pulses of current generated when light hit the photomultiplier were fed into a computer fitted with an EMI photon-counting board. Data, collected in 50 ms bins, were integrated over 1 s intervals to give counts s−1 (c.p.s.).
The signals were calibrated by measuring the luciferase luminescence over a range of ATP concentrations (1 nM–1 μM). The calibration curve, plotted as log of luminescence intensity (c.p.s.) against log of [ATP], was linear over this range of concentrations.
Materials
FCS (Myoclone grade), Taq DNA polymerase and Superscript II reverse transcriptase were purchased from GIBCO BRL, Paisley, U.K.; Fura-2 acetoxymethyl ester (fura-2 AM) was from Molecular Probes, Eugene, Oregon, U.S.A.; dNTPs, oligo(dT), RNAase inhibitor and restriction enzymes were obtained from Boehringer Mannheim, Lewes, U.K.; LM1 emulsion and the Biotrak proliferation kit were from Amersham, Little Chalfont, U.K.; MCDB153 culture medium, firefly luciferase and luciferin, and all other chemicals were purchased from Sigma-Aldrich, Poole, U.K.
Results
Expression of P2Y2 receptor transcripts
cDNA derived from primary cultured human keratinocytes was subjected to 25, 30 and 35 rounds of PCR amplification using oligonucleotide primers specific for the human P2Y2 receptor. The 362 bp product was clearly visible following 30 cycles of PCR amplification demonstrating expression of P2Y2 receptor transcripts by this cell type (Figure 1A). We have previously demonstrated that human osteoclasts of giant cell tumour abundantly express P2Y2 receptor mRNA (Bowler et al., 1995). cDNA derived from a purified population of human osteoclasts was therefore included as a positive control; the 362 bp product was visible following 25 cycles of amplification (Figure 1A). The specificity of the amplified product was confirmed by sequence analysis, and amplification of GAPDH at 25 and 35 cycles confirmed the integrity of cDNAs used (Figure 1B).
Figure 1.
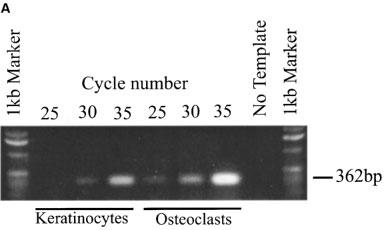
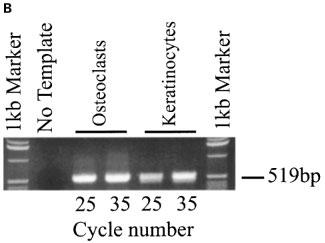
Expression of P2Y2 receptor transcripts in primary cultured human keratinocytes and osteoclasts. (A) PCR amplification of a specific 362 bp fragment of the human P2Y2 receptor using cDNA from primary cultured human keratinocytes. cDNA from a sorted human osteoclast population was included as a positive control. (B) Amplification of a specific 519 bp GAPDH product confirmed the integrity of the cDNA used. This result is typical of three analyses using keratinocytes derived from skin samples from three individual patients.
P2Y2 receptor transcripts are localized to the stratum basale
In situ hybridization revealed a striking pattern of localization of P2Y2 receptor transcripts in the basal cell layer of skin sections (Figure 2B, D and E). In control experiments with a sense RNA probe no specific granular localization was detected (Figure 2A and C). Interestingly, high levels of P2Y2 receptor mRNA were detected in both the sweat glands (Figure 2F) and the hair follicles (data not shown).
Figure 2.
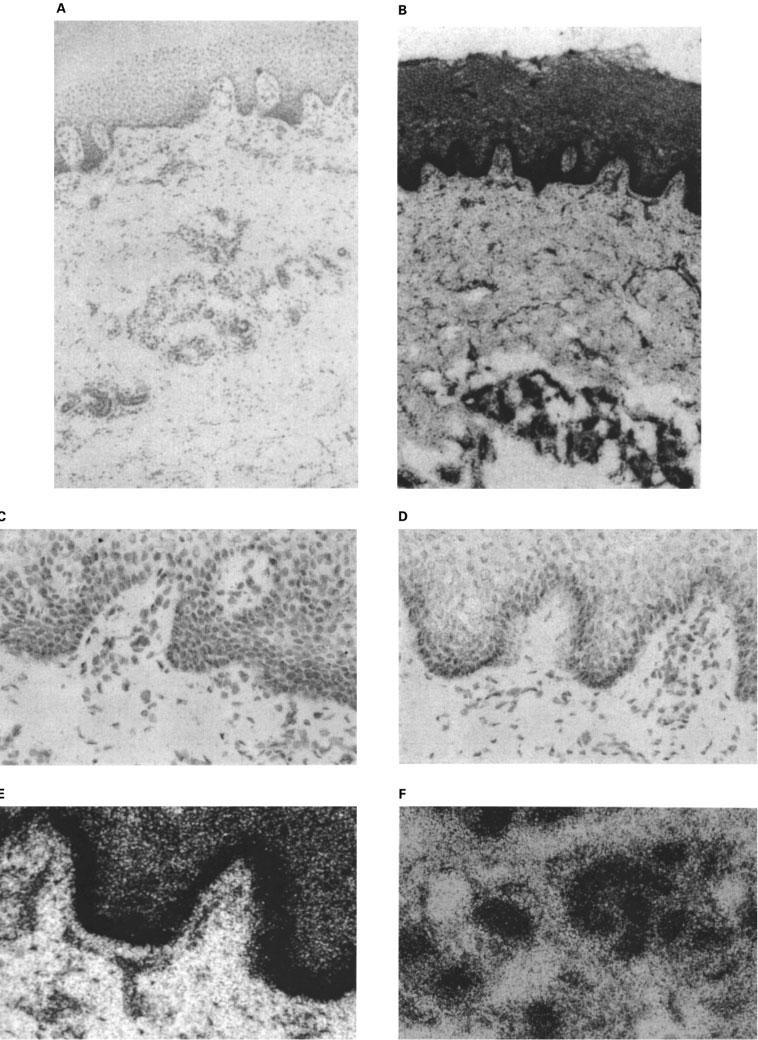
In situ localization of P2Y2 receptor transcripts in sections of human skin using 32P-dUTP-labelled probes. (A) Low power photomicrograph of hybridization with a sense probe (negative control) showing no specific granular localization. (B) Similar section hybridized with an anti-sense probe and exposed for 15 days. Note the localization over the basal (proliferative) layer of the epidermis and in the glandular structures deep in the dermis. (C) High magnification view of a skin section hybridized with a sense probe (negative control) and exposed for 5 days. Note the absence of signal. (D and E) High magnification detail of skin sections hybridized with anti-sense probe and exposed for 5 days and 15 days respectively. Note the intense specific localization of signal over the basal (proliferative) layer of the epidermis. (F) High magnification view showing specific hybridization of the anti-sense probe over sweat glands.
ATP and UTP increase [Ca2+]i in keratinocytes
Application of 10 μM ATP (n=5) or 10 μM UTP (n=5) to groups of 8–10 fura-2 loaded keratinocytes induced a rise in [Ca2+]i. Figure 3 depicts the response of a group of keratinocytes to increasing concentrations of ATP and UTP. The threshold concentrations for ATP and UTP were in the low micromolar range; 1 μM UTP evoked a response in 2/4 preparations, and 2 μM ATP was effective in 3/4 preparations (1 μM ATP did not elicit a rise in [Ca2+]i in 4/4 preparations).
Figure 3.
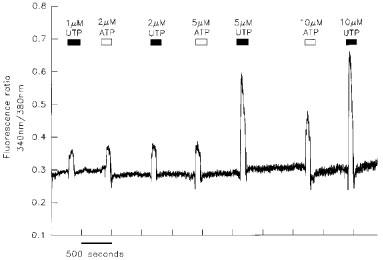
Increases in [Ca2+]i induced by ATP and UTP in cultured human keratinocytes. Cultured keratinocytes were loaded with fura-2 by incubation with 5 μM fura-2 AM. Groups of 8–10 cells were excited with light at 340 and 380 nm and the emission at 510 nm recorded. The application of ATP or UTP led to increases in [Ca2+]i as indicated by the change in the ratio of fluorescence at 340 and 380 nm.
P2Y2 agonists stimulate proliferation of human keratinocytes
Keratinocytes cultured in MCDB153, supplemented as described above, are undergoing near maximal proliferation. In order then to study the effect of nucleotide stimulation the rate of proliferation needs to be reduced. This was achieved by addition of 10% FCS, which slows keratinocyte growth as noted previously (Sharpe & Fisher, 1990). In the presence of 10% FCS, ATP (0.1–100 μM) significantly increased proliferation (Figure 4A). The poorly-hydrolyzable analogue of ATP, ATPγS, and UTP similarly stimulated keratinocyte proliferation in the presence of serum (Figure 4B).
Figure 4.
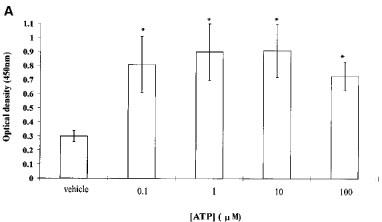
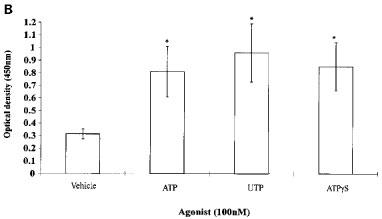
Proliferation of keratinocytes in response to (A) increasing concentrations of ATP and (B) 100 nM ATP, UTP and ATPγS was assessed using a Biotrak cell proliferation ELISA system. All data are represented as mean±s.e.mean (n=16 wells). * denotes significant difference at P<0.01. This result is typical of four separate experiments.
Keratinocytes constitutively release ATP
As depicted in Figure 5, introducing a coverslip of confluent keratinocytes into the chamber of the photon-counting detection system led to an immediate increase in the intensity of the luminescence. The signal rose from the background level of approximately 9 c.p.s. to 604±166 c.p.s. (mean±s.e.mean, n=5). This equates to a concentration of ATP in the chamber of 17±4 nM, although considerable variability in the level of ATP release from individual coverslips was seen (range: 7–30 nM). The plateau in signal was achieved when the rate of release of ATP was equal to the rate of its removal through hydrolysis and the action of extracellular enzymes. The signal remained steady at this level for the length of the recording; no change was seen even over a period of 50 min. If extreme care was not taken when introducing keratinocytes into the chamber, a large increase in signal was recorded, which could peak at 2000–3000 c.p.s., and which decayed gradually over a period of 2–3 h (data not shown). This signal may reflect lysis of some cells with concomitant release of intracellular ATP. Alternatively, it may represent the physiological response of cells to the mechanical stresses generated when a coverslip is introduced into the chamber.
Figure 5.
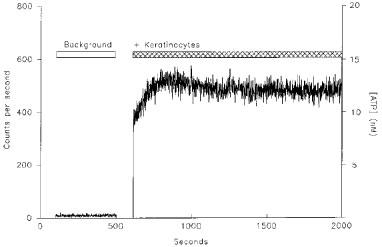
ATP release from human keratinocytes. The chamber of the photon-counting luminometer was filled with firefly luciferase (10 μg ml−1) and luciferin (10 μM) in HEPES buffer and the background signal recorded. Introduction of a coverslip of keratinocytes at confluency resulted in an increase in signal to approximately 500 c.p.s. The signal was calibrated by measuring the luciferase luminescence over a range of ATP concentrations as shown on the right hand axis. This result is typical of five coverslips although considerable variability was seen in the signals recorded from individual coverslips.
Discussion
In these studies we have described the expression of P2Y2 receptor mRNA transcripts in cultured human keratinocytes. P2Y2 receptor expression has been shown to be up-regulated in response to culture conditions (Turner et al., 1997). However, in situ hybridization studies presented here clearly demonstrate that transcripts for these receptors are present in the native tissue. These studies illustrate a striking pattern of localization of transcripts to the actively-proliferating basal cells of the epidermis. P2Y2 receptor transcripts were also detected in the outer, more differentiated layers of the epidermis, although at much lower levels. This suggests that P2Y2 mRNA receptor expression is down-regulated in a differentiation-dependent manner, and is therefore reminiscent of the P2Y2 receptor mRNA down-regulation observed during phorbol ester-induced monocytic differentiation of HL60 human promyelocytic leukocytes (Dubyak et al., 1996; Martin et al., 1997).
The most common primary effectors of G-protein-coupled P2Y receptors are the phospholipase C β enzymes; activation of receptors leads to Ins(1,4,5)P3-mediated release of intracellular Ca2+ stores. In keeping with an earlier study on human keratinocytes (Pillai & Bikle, 1992) stimulation with ATP and UTP, equipotent agonists for the P2Y2 receptor, resulted in increased [Ca2+]i. This suggests that the detected transcripts are translated into functional receptors, although a contribution from other receptors (e.g. P2Y4 and P2Y11) to the responses to ATP and UTP cannot be excluded.
The intense localization of P2Y2 mRNA transcripts to the actively-dividing basal layer suggests a role for nucleotides as regulators of epidermal proliferation. Keratinocytes cultured in MCDB153 medium, supplemented as described above, proliferate at near maximal rate and further stimulation of the growth rate will not readily be observed. In order then to study the effect of nucleotide stimulation on keratinocyte growth, the rate of proliferation must first be slowed. This was achieved by the addition of 10% FCS, which slows proliferation and promotes differentiation of cells. The inhibitory effect of serum has been noted previously and has been attributed, at least in part, to the high concentrations of TGFβ it contains (Sharpe & Fisher, 1990). Under these conditions then, stimulation with ATP and UTP led to a significant increase in proliferation, thus over-riding the inhibitory effects of factors present in serum. However, this response to ATP appears to be independent of [Ca2+]i, as proliferation was seen at concentrations lower than the threshold for [Ca2+]i mobilization. This may reflect involvement of alternative signalling pathways in mediating the ATP-stimulated proliferation. Indeed P2Y2 occupation has recently been shown to lead to activation of the p42/p44 MAPK cascade which is associated with proliferation (Boarder & Hourani, 1998). Mitogenic effects of ATP have been reported previously in a number of cell types including aortic smooth muscle cells (Wang et al., 1992; Harper et al., 1998), mesanglial cells (Schulze-Lohoff et al., 1992), Swiss 3T3 and 3T6 fibroblasts (Huang et al., 1989) and significantly in primary human keratinocytes (Pillai & Bikle, 1992).
In vivo, keratinocytes may be exposed to high levels of ATP and UTP following injury to the skin when nucleotides are released from dead or damaged tissue, and from platelets. The resulting proliferation of cells in the basal layer would promote healing of an epidermal wound by replacing damaged epidermis. Growth factors, including TGFα which is known to stimulate keratinocyte proliferation, are released along with nucleotides upon degranulation of platelets at the site of an injury. ATP has been shown to interact synergistically with growth factors to promote cell proliferation in a range of cell types including a mouse keratinocyte-derived cell line, BALB/MK (Wang et al., 1990).
In partial epidermal loss, proliferation of the basal keratinocytes re-forms the full epidermis. In deeper injury, new epidermal growth occurs from the hair follicles where preserved, or from the wound edges. The dense localization of P2Y2 mRNA transcripts to the hair follicles reported here may explain the ability of the skin to regenerate from these regions; extracellular nucleotides acting at the P2Y2 receptors would stimulate proliferation of basal cells within the inner root sheath of the follicle. High levels of P2Y2 mRNA were also detected in the sweat glands, consistent with an established role for these receptors in regulating ion conductance into sweat (Ko et al., 1997; Wilson et al., 1996).
Luciferase/luciferin experiments demonstrate that under static conditions keratinocytes are releasing ATP. The amount of ATP released into the 300 μl volume of the chamber gave rise to an ATP concentration of approximately 17 nM. Released into a restricted volume at the cell surface, ATP may reach sufficient concentrations to activate P2Y2 receptors and hence stimulate proliferation of keratinocytes. The stimulation of ATP release by extracellular ATP has been demonstrated in endothelial cells (Bodin & Burnstock, 1996), and thus the basal release recorded here may reflect ATP-induced ATP release. Interestingly, serum contains high concentrations of enzymes which hydrolyze ATP (Suter et al., 1991). If released ATP contributes to the proliferation of keratinocytes in culture, the inhibitory effects of serum on proliferation may be partly explained by the removal of ATP, and hence the proliferative stimulus.
Release of ATP has been demonstrated from a number of cell types in response to mechanical stimulation (Milner et al., 1990; Schlosser et al., 1996; Grygorczyk & Hanrahan, 1997). Indeed, the large signal observed when introducing a coverslip into the chamber without exercising extreme care may reflect the response of keratinocytes to mechanical stress. In skin, mechanical forces of pressure and shearing are transmitted to the basal layer and basement membrane (Kennedy, 1998). Repeated shearing forces acting on an area of skin results in thickening of the area. This physiological response may be mediated by ATP, released in response to shearing forces. Recently, release of UTP has been demonstrated from 1321N1 astrocytoma cells (Lazarowski et al., 1997). If UTP is similarly released from keratinocytes in response to mechanical stimulation, this nucleotide may act in concert with ATP to regulate proliferation.
It is likely that keratinocytes express other P2Y subtypes in addition to the P2Y2 receptor, possibly also in a differentiation-dependent manner. Indeed, Pillai & Bikle (1992) reported that cultured keratinocytes respond to ADP with increased [Ca2+]i; this nucleotide is not an agonist for the P2Y2 receptor, but acts at both the P2Y1 and P2Y6 subtypes (see Boarder & Hourani, 1998). Further expression and functional studies are required to determine the full complement of P2 receptors expressed by keratinocytes.
In conclusion, we have described the expression and involvement of P2Y2 receptors in the control of keratinocyte proliferation. ATP and UTP, released in the vicinity of a wound from damaged cells or as a result of the inflammatory response, may act to promote keratinocyte proliferation and inhibit differentiation through activation of P2Y2 receptors. This mechanism may be responsible for epidermal thickening due to repeated minor trauma or shearing forces if, as seems likely, keratinocytes release ATP (and possibly also UTP) in response to mechanical stimulation. Agonists for the P2Y2 receptor thus represent potential therapeutic agents for the enhancement of wound healing, whilst antagonists may be useful in the treatment of pathologies associated with hyperproliferation of keratinocytes, including psoriasis. In addition, the significant concentrations of ATP released by keratinocytes under static conditions, indicate that ATP may act as an important positive growth regulator.
Acknowledgments
We are grateful for funding from The Wellcome Trust (C.J. Dixon) and the Arthritis and Rheumatism Research Council (W.B. Bowler).
Abbreviations
- BrdU
bromodeoxyuridine
- [Ca2+]i
intracellular free calcium concentration
- cps
counts per second
- DEPC
diethyl pyrocarbonate
- GAPDH
glyceraldehyde phosphate dehydrogenase
- Ins(1,4,5)P3
inositol(1,4,5)trisphosphate
- MAPK
mitogen-activated protein kinase
References
- BARRANDON Y., GREEN H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-α and epidermal growth factor. Cell. 1984;50:1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- BIKLE D.D., RATNAM A., MAURO T., HARRIS J., PILLAI S. Changes in calcium responsiveness and handling during keratinocyte differentiation. J. Clin. Invest. 1996;97:1085–1093. doi: 10.1172/JCI118501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOARDER M.R., HOURANI S.M.O. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol. Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BODIN P., BURNSTOCK G. ATP-stimulated release of ATP by human endothelial cells. J. Cardiovasc. Pharmacol. 1996;27:872–875. doi: 10.1097/00005344-199606000-00015. [DOI] [PubMed] [Google Scholar]
- BOWLER W.B., BIRCH M.A., GALLAGHER J.A., BILBE G. Identification and cloning of human P2u purinoceptor present in osteoclastoma, bone, and osteoblasts. J. Bone Min. Res. 1995;10:1137–1145. doi: 10.1002/jbmr.5650100720. [DOI] [PubMed] [Google Scholar]
- BOWLER W.B., LITTLEWOOD-EVANS A., BILBE G., GALLAGHER J.A., DIXON C.J. P2Y2 receptors are expressed by human osteoclasts of giant cell tumour but do not mediate ATP-induced bone resorption. Bone. 1998;22:195–200. doi: 10.1016/s8756-3282(97)00280-9. [DOI] [PubMed] [Google Scholar]
- CHO J.K., BIKLE D.D. Decrease of Ca2+-ATPase activity in human keratinocytes during calcium-induced differentiation. J. Cell. Physiol. 1997;172:146–154. doi: 10.1002/(SICI)1097-4652(199708)172:2<146::AID-JCP2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- COBBOLD P.H., LEE J.A.C.Aequorin measurements of cytoplasmic free calcium Cellular Calcium: A Practical Approach 1991Oxford: I.R.L. Press; 55–81.ed. McCormack, J.G. & Cobbold, P.H pp [Google Scholar]
- COOK P.W., ASHTON N.M., PITTELKOW M.R. Adenosine and adenine-nucleotides inhibit the autonomous and epidermal growth factor-mediated proliferation of cultured human keratinocytes. J. Invest. Dermatol. 1995;104:976–981. doi: 10.1111/1523-1747.ep12606228. [DOI] [PubMed] [Google Scholar]
- DIXON C.J., BOWLER W.B., WALSH C.A., GALLAGHER J.A. Effects of extracellular nucleotides on single cells and populations of human osteoblasts: contribution of cell heterogeneity to relative potencies. Br. J. Pharmacol. 1997;120:777–780. doi: 10.1038/sj.bjp.0700961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBYAK G.R., CLIFFORD E.E., HUMPHREYS B.D., KERTESY S.B., MARTIN K.A. Expression of multiple ATP receptor subtypes during the differentiation and inflammatory activation of myeloid leukocytes. Drug Develop. Res. 1996;39:269–278. [Google Scholar]
- FINCH P.W., RUBIN J.S., MIKI T., RON D., AARONSON S.A. Human KGF is FGF-related with properties of paracrine effector of epithelial cell growth. Science. 1989;245:752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- FUCHS E. Epidermal differentiation: the bare essentials. J. Cell. Biol. 1990;111:2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTLIEB A.B., CHANG C.K., POSNETT D.N., FANELLI B., TAM J.P. Detection of transforming growth factor α in normal, malignant, and hyperproliferative human keratinocytes. J. Exp. Med. 1988;167:670–675. doi: 10.1084/jem.167.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRYGORCZYK R., HANRAHAN J.W. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am. J. Physiol. 1997;272:C1058–C1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- HARPER S., WEBB T.E., CHARLTON S.J., NG L.L., BOARDER M.R. Evidence that P2Y4 nucleotide receptors are involved in the regulation of rat aortic smooth muscle cells by UTP and ATP. Br. J. Pharmacol. 1998;124:703–710. doi: 10.1038/sj.bjp.0701895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNINGS H., MICHAEL D., CHENG C., STEINERT P., HOLBROOK K., YUSPA S.H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1990;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- HUANG N., WANG D., HEPPEL L.A. Extracellular ATP is a mitogen for 3T3, 3T6, and A431 cells and acts synergistically with other growth factors. Proc. Natl. Acad. Sci. U.S.A. 1989;86:7904–7908. doi: 10.1073/pnas.86.20.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES K.T., SHARPE G.R. Intracellular free calcium and growth changes in response to vitamin-D and 5 20-epi-analogs. Arch. Derm. Res. 1994;286:123–129. doi: 10.1007/BF00370738. [DOI] [PubMed] [Google Scholar]
- KENNEDY C.T.C.Mechanical and thermal injury Textbook of Dermatology 1998Oxford: Blackwell Science Ltd; 883–895.ed. Champion, R.H., Burton, J.L., Burns, D.A. & Breathnach, S.M. pp [Google Scholar]
- KO W.H., WILSON S.M., WONG P.Y.D. Purine and pyrimidine nucleotide receptors in the apical membranes of equine cultured epithelia. Br. J. Pharmacol. 1997;121:150–156. doi: 10.1038/sj.bjp.0701093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., HOMOLYA L., BOUCHER R.C., HARDEN T.K. Direct demonstration of mechanically-induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J. Biol. Chem. 1997;272:24348–24354. doi: 10.1074/jbc.272.39.24348. [DOI] [PubMed] [Google Scholar]
- LITTLEWOOD-EVANS A., KOKUBO T., ISHIBASHI O., INOAKA T., WLODARSKI B., GALLAGHER J.A., BILBE G. Localization of cathepsin K in human osteoclasts by in situ hybridization and immunohistochemistry. Bone. 1997;20:81–86. doi: 10.1016/s8756-3282(96)00351-1. [DOI] [PubMed] [Google Scholar]
- MARTIN K.A., KERTESY S.B., DUBYAK G.R. Down-regulation of P2U-purinergic nucleotide receptor messenger RNA expression during in vitro differentiation of human myeloid leukocytes by phorbol esters or inflammatory activators. Mol. Pharmacol. 1997;51:97–108. doi: 10.1124/mol.51.1.97. [DOI] [PubMed] [Google Scholar]
- MCGOVERN U.B., JONES K.T., SHARPE G.R. Intracellular calcium as a second messenger following growth-stimulation of human keratinocytes. Br. J. Dermatol. 1995;132:892–896. doi: 10.1111/j.1365-2133.1995.tb16944.x. [DOI] [PubMed] [Google Scholar]
- MILNER P., BODIN P., LOESCH A., BURNSTOCK G. Rapid release of endothelin and ATP from isolated aortic endothelial cells exposed to increase flow. Biochem. Biophys. Res. Commun. 1990;170:649–656. doi: 10.1016/0006-291x(90)92141-l. [DOI] [PubMed] [Google Scholar]
- PILLAI S., BIKLE D.D. Adenosine triphosphate stimulates phosphoinositide metabolism, mobilizes intracellular calcium, and inhibits terminal differentiation of human epidermal keratinocytes. J. Clin. Invest. 1992;90:42–51. doi: 10.1172/JCI115854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PILLAI S., BIKLE D.D., HINCENBERGS M., ELIAS P.M. Biochemical and morphological characterization of growth and differentiation of normal human neonatal keratinocytes in a serum-free medium. J. Cell. Physiol. 1988;134:229–237. doi: 10.1002/jcp.1041340208. [DOI] [PubMed] [Google Scholar]
- PILLAI S., BIKLE D.D., MANCIANTI M.-L., CLINE P., HINCENBERGS M. Calcium regulation and differentiation of normal human keratinocytes: modulation of differentiation competence by stages of growth and extracellular calcium. J. Cell. Physiol. 1990;143:294–302. doi: 10.1002/jcp.1041430213. [DOI] [PubMed] [Google Scholar]
- RHEINWALD J.G. Serial cultivation of normal human epidermal keratinocytes. Methods Cell Biol. 1980;21A:229–254. doi: 10.1016/s0091-679x(08)60769-4. [DOI] [PubMed] [Google Scholar]
- SCHLOSSER S.F., BURGSTAHLER A.D., NATHANSON M.H. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9948–9953. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULZE-LOHOFF E., ZANNER S., OGILVIE A., STERZEL R.B. ATP stimulates proliferation of cultured mesenglial cells via P2-purinergic receptors. Am. J. Physiol. 1992;263:F374–F383. doi: 10.1152/ajprenal.1992.263.3.F374. [DOI] [PubMed] [Google Scholar]
- SHARPE G.R., FISHER C. Time-dependent inhibition of growth of human keratinocytes and fibroblasts by cyclosporine-A – effect on keratinocytes at therapeutic blood levels. Br. J. Dermatol. 1990;123:207–213. doi: 10.1111/j.1365-2133.1990.tb01848.x. [DOI] [PubMed] [Google Scholar]
- SHARPE G.R., GILLESPIE J.I., GREENWELL J.R. An increase in intracellular free calcium is an early event during differentiation of cultured human keratinocytes. FEBS Letts. 1989;254:25–28. doi: 10.1016/0014-5793(89)81002-6. [DOI] [PubMed] [Google Scholar]
- SHIPLEY G.D., PITTLEKOW M.R., WILLE J.J., SCOTT R.E., MOSES H.L. Reversible inhibition of normal human prokeratinocyte proliferation by type β transforming growth factor–growth inhibitor in serum free medium. Cancer Res. 1986;46:2068–2071. [PubMed] [Google Scholar]
- SUTER M.M., CRAMERI F.M., SLATTERY J.P., MILLARD P.J., GONZALEZ F.A. Extracellular ATP and some of its analogs induce transient rises in cytosolic free calcium in individual canine keratinocytes. J. Invest. Dermatol. 1991;97:223–229. doi: 10.1111/1523-1747.ep12480162. [DOI] [PubMed] [Google Scholar]
- THOMAS A., DELAVILLE F.The use of fluorescent indicators for measurements of cytosolic-free calcium concentration in populations and single cells Cellular Calcium: A Practical Approach 1991Oxford: I.R.L. Press; 1–54.ed. McCormack, J.G. & Cobbold, P.H. pp [Google Scholar]
- TSAO M.C., WALTHALL B.J., HAM R.G. Clonal growth of normal human epidermal keratinocytes in a defined medium. J. Cell. Physiol. 1982;110:219–229. doi: 10.1002/jcp.1041100217. [DOI] [PubMed] [Google Scholar]
- TURNER J.T., WEISMAN G.A., CAMDEN J.M. Upregulation of P2Y2 nucleotide receptors in rat salivary gland cells during short-term culture. Am. J. Physiol. 1997;273:C1100–C1107. doi: 10.1152/ajpcell.1997.273.3.C1100. [DOI] [PubMed] [Google Scholar]
- WANG D., HUANG N.-N., HEPPEL L.A. Extracellular ATP shows synergistic enhancement of DNA synthesis when combined with agents that are active in wound healing or as neurotransmitters. Biochem. Biophys. Res. Commun. 1990;166:251–258. doi: 10.1016/0006-291x(90)91938-o. [DOI] [PubMed] [Google Scholar]
- WANG D.J., HUANG N.-N., HEPPEL L. Extracellular ATP and ADP stimulate proliferation of porcine aortic smooth muscle cells. J. Cell. Physiol. 1992;153:221–223. doi: 10.1002/jcp.1041530202. [DOI] [PubMed] [Google Scholar]
- WILSON S.M., RAKHITT S., MURDOCH R., PEDIANI J.D., ELDER H.Y., BAINES D.L., KO W.H., WONG P.Y.D. Activation of apical P2U purine receptors permits inhibition of adrenaline-evoked cyclic-AMP accumulation in cultured equine sweat gland epithelial cells. J. Exp. Biol. 1996;199:2153–2160. doi: 10.1242/jeb.199.10.2153. [DOI] [PubMed] [Google Scholar]


