Abstract
The effects of GABAB, opioid and α2 receptor activation on different subtypes of calcium channels in acutely-dissociated rat locus coeruleus (LC) neurones were investigated using whole-cell patch clamping.
Barium currents through calcium channels could be fractionated into four classes: L-type (nimodipine-sensitive), N-type (ω-conotoxin GVIA-sensitive), P/Q-type (ω-agatoxin IVA-sensitive) and R-type (remaining in the presence of all three blockers). The percentage of each was, respectively, 25±2, 34±1, 28±3 and 12±1% (mean±s.e.mean, n=4).
The GABAB receptor agonist, baclofen, and the opioid receptor agonist, enkephalin, partially inhibited the total barium current in a concentration-dependent manner with EC50 values of 2 and 0.3 μM, respectively. Maximal inhibition was 17±1% (n=38) for baclofen and 30±2% (n=20) for enkephalin. The α2-adrenoceptor agonist, UK14304 (10 μM), also inhibited barium current in these neurones (28±2%, n=11). The agonists did not shift the current-voltage relationship along the voltage axis.
Maximal baclofen inhibition of different calcium channel subtypes was 9±7% (L-type, n=4), 11±8% (N-type, n=4), 26±6% (P/Q-type, n=4), and 6±5% (R-type, n=5). The corresponding values for enkephalin inhibition were 5±9% (L-type), 30±11% (N-type), 37±9% (P/Q-type), and 17±8% (R-type).
In the presence of a saturating concentration of enkephalin, baclofen produced additional inhibition of the barium current. In contrast, in the presence of a saturating concentration of enkephalin, UK14304 produced no further inhibition of the barium current.
These results indicate that neuromodulation of calcium channels in LC neurones involves a complex pattern of overlapping and distinct second messenger pathways. Regulation of LC neuronal firing activity by the modulation of calcium channels may be important for LC-mediated behaviour such as alertness and vigilance.
Keywords: α2-Adrenoceptor, acutely dissociated, baclofen, calcium channel subtypes, calcium current, enkephalin, GABAB receptor, locus coeruleus, opioid receptor
Introduction
The locus coeruleus (LC) in the brainstem provides the most extensive noradrenergic projections in the central nervous system, with heavy contributions to the dorsal bundle and descending noradrenergic pathways. Factors that modulate the excitability of LC neurones will thus have important ramifications for the levels of noradrenaline in other parts of the brain. LC receives major excitatory afferents (glutamatergic) from nucleus paragigantocellularis and inhibitory afferents (GABAergic) from nucleus prepositus hypoglossi (Aston-Jones et al., 1991b for review). Many of the LC afferents originating from these two medullary nuclei also contain the opioid receptor agonist, enkephalin (Aston-Jones et al., 1991b; Van Bockstaele & Chan, 1997), suggesting the possibility of co-release of these neurotransmitters. Extracellular noradrenaline is also found in LC (Surprenant & Williams, 1987) where it is known to modulate potassium channels (North & Williams, 1985). Thus, at least GABA, enkephalin and noradrenaline are present in LC and so are likely to be important for the physiological regulation of the excitability of LC neurones.
This idea has been tested by examining the effects of a number of neurotransmitters on the spontaneous firing of action potentials in LC neurones. Activation of GABAB, μ-opioid, α2-adrenoceptor, somostatin, opioid-like orphan, and galanin receptors all inhibited firing by increasing an inwardly rectifying potassium conductance (Aghajanian & Wang, 1987; Connor et al., 1996; Inoue et al., 1988; North & Williams, 1985; Osmanovic & Shefner, 1988; Pieribone et al., 1995). Stimulation of most of the above postsynaptic receptors has been demonstrated to open the same population of potassium channels (Aghajanian & Wang, 1987; Connor et al., 1996; North & Williams, 1985), pointing to a convergence of the actions of different types of receptors.
We wished to ask whether another class of important ion channels, calcium channels, could also be modulated by the converging actions of the postsynaptic receptors on LC neurones. Calcium channels, like potassium channels, are involved in regulating neuronal excitability. There are a number of different subtypes of calcium channels, commonly distinguished by their sensitivity to selective blockers. Experiments done in the hippocampus and amygdala indicate that these calcium channel subtypes may be differentially modulated by a variety of neurotransmitters (Huang et al., 1998; Scholz & Miller, 1996).
Here we explore the actions of GABAB, opioid and α2-receptor stimulation on different subtypes of calcium channels in acutely-dissociated LC neurones. We show that GABAB and opioid receptor activation inhibits some of the calcium channel subtypes to varying extents. Furthermore, opioid and α2 receptor agonists inhibit calcium channels via a common pathway while opioid and GABAB receptor agonists largely act via independent pathways.
Methods
Rat pups (3–9 days old) were used to make brainstem slices (300 μm) containing the bilateral nuclei of LC. All procedures were approved by the Animal Experimentation Ethics Committee of the Australian National University. The brain was embedded in agar (1.5%) and then cut in chilled physiological saline using a vibratome. LC was micro-dissected from the slices under a stereo-microscope with a pair of fine hypodermic needles. Tissue containing LC was incubated in enzyme solution containing papain (20 units ml−1) for 20 min at room temperature. The tissue was then triturated to dissociate individual neurones which were placed in a dish and allowed to settle for at least 30 min before being used for physiological recordings. Slice preparation and dissociation were done in Earle's Balanced Salt solution supplemented with 1 mM kynurenic acid to block glutamate receptors. LC neurones were identified with an inverted microscope and whole-cell recordings were made using a patch-clamp amplifier (Axopatch 1D). Patch pipettes of resistance 3–5 MΩ were filled with intracellular solution containing (in mM): CsCl 110, CaCl2 2, EGTA 10 (ethylene glycol-bis[β-aminoethyl ether]-N,N,N′,N′-tetraacetic acid), Mg-ATP 5, Na-GTP 0.2, HEPES 10 (N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid]), pH 7.3. Acceptable access resistance was <20 MΩ, periodically monitored with repetitive 10 mV steps (20 ms duration). Series resistance compensation of 80% was used for all experiments. Voltage families of barium currents through calcium channels were evoked every 30 s. Linear leak currents and capacity transients were subtracted using a P/4 protocol. Drugs were dissolved in external solution and applied to the cell using a series of flow pipes. The external solution comprised (in mM): tetraethylammonium chloride 140, BaCl2 2, CsCl 5, HEPES 10, glucose 10, 0.05% bovine serum albumin, pH 7.3. Data was acquired using pClamp 6 and analysed with Axograph 4.0 (Axon Instruments). Sample means were statistically compared using a paired two tailed t-test with a significance level of P=0.05.
Baclofen, methionine-enkephalin, kynurenic acid and papain were from Sigma (St Louis, MO, U.S.A.); nimodipine and UK14304 were from Research Biochemicals (Natick, MA, U.S.A.); CGP56999 and CGP35348 were gifts from Novartis (Switzerland); ω-conotoxin GVIA was from Alomone Labs (Jerusalem, Israel); and ω-agatoxin IVA was a gift from Pfizer Research (Groton, CT, U.S.A.).
Results
Acutely-dissociated LC neurones were identified as relatively large cells (soma diameter ∼35 μm) with three to six processes (Ingram et al., 1997). In current clamp, the cells fired action potentials spontaneously, as has been reported in both intact animals and in vitro brain slices (e.g. Aghajanian, 1978; North & Williams, 1985; Pepper & Henderson, 1980).
Different calcium channel subtypes are present in LC neurones
Barium currents flowing through calcium channels were evoked by stepping from a prepulse to −90 mV to a range of test potentials (−60 to +60 mV; Figure 1a and b). The maximal barium currents used in this study ranged from 0.5 to 3 nA (leak current subtracted). The current was completely blocked by 100 μM Cd2+ (Figure 1a and b). The current-voltage relationship for barium currents was an inverted bell-shaped curve with a peak at −10 to 0 mV (Figure 1b). This is consistent with other studies of neuronal barium and calcium currents (e.g. Connor & Christie, 1998; Ingram et al., 1997).
Figure 1.
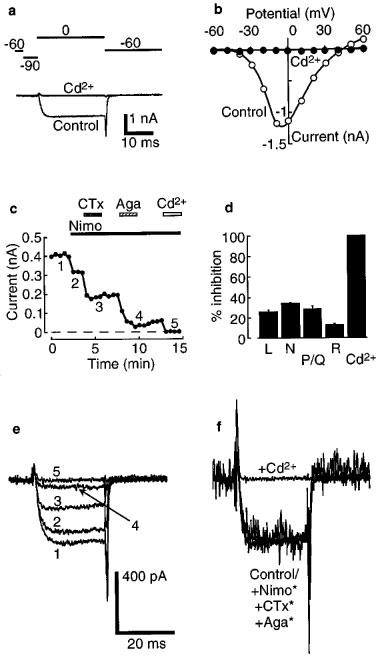
Four different subtypes of calcium channels can be distinguished in acutely-dissociated LC neurones. (a) Example of a barium current recorded during a test pulse to 0 mV from a prepulse of −90 mV (Control). This current was completely blocked by 100 μM cadmium (Cd2+). The pulse protocol is shown above. Linear leak currents and capacitance transients have been subtracted using a P/4 protocol. (b) Current-voltage plot of barium current measured in one cell (same as in a). (c) Plot of peak amplitude of barium current at 0 mV versus time during the experiment. Horizontal bars indicate periods of perfusion with nimodipine (Nimo, 3 μM), ω-conotoxin GVIA (CTx, 1 μM), ω-agatoxin IVA (Aga, 500 nM) and Cd2+ (100 μM). Numbers 1–5 identify the example traces shown in (e). (d) Summary of the results of a series of four experiments as in (c), showing the fraction of current mediated by each of L-, N-, P/Q- and R-types of calcium channels. Error bars are ±s.e.mean. (e) Example currents at the indicated time points in the experiment shown in (c). (f) Same traces as in (e); asterisks indicate that the traces are normalized to show the similarity of time courses.
The types of calcium channels present in the LC neurones were investigated using the subtype selective blockers, nimodipine (Nimo; 3 μM) for L-type channels, ω-conotoxin GVIA (CTx; 1 μM) for N-type channels and ω-agatoxin IVA (Aga; 500 nM) for P/Q-type channels (Figure 1c). The percentage of each was 25±2% (L-type), 34±1% (N-type) and 28±3% (P/Q-type), leaving a residual (R-type) current of 12±1% (mean±s.e.mean, n=4, Figure 1d). Superfusion of Cd2+ (100 μM) abolished the R-type current. Thus there was an approximately equal contribution of L-, N- and P/Q-types of calcium channels to the barium currents in these neurones.
Addition of the selective blockers did not alter the activation kinetics of the currents. All traces overlaid when they were scaled to the same amplitude (Figure 1e and f).
GABAB, opioid and α2 receptor agonists inhibited barium current
Baclofen reversibly reduced the amplitude of barium currents in a concentration-dependent manner (Figure 2) without shifting the current-voltage relationship along the voltage axis (Figure 2b). The activation kinetics were slowed in the presence of high concentrations of agonist (Figure 2a). This inhibition was abolished in the presence of GABAB-receptor antagonists, CGP56999 (100 nM) or CGP35348 (100 μM) (n=5), suggesting a GABAB-receptor mediated action by baclofen (Figure 2c). The threshold for baclofen inhibition was about 100 nM and approached a maximum at 10 μM, with an EC50 of 2 μM (Figure 2e). This estimated EC50 value is the same as that for activation of potassium channels by baclofen in LC neurones (Osmanovic & Shefner, 1988). At a saturating concentration of baclofen (100 μM), barium current was inhibited by 17±1% (n=38).
Figure 2.
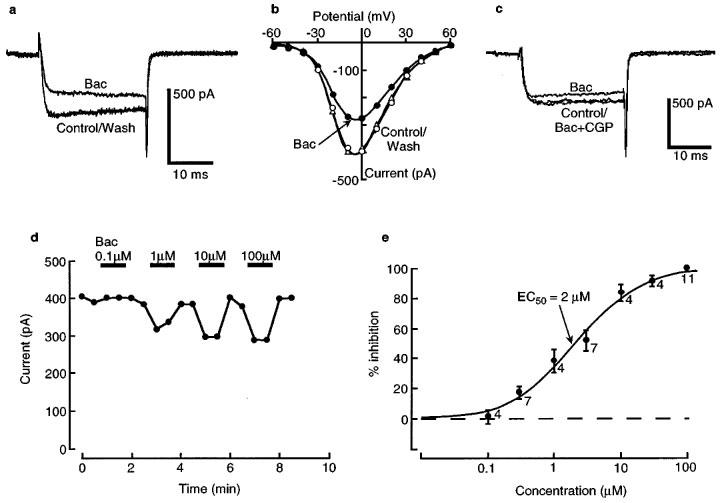
Baclofen (Bac) acts via GABAB receptors to inhibit barium current in a concentration-dependent manner. (a) A typical barium current measured in control solution and after adding a saturating concentration of baclofen (100 μM). (b) Current-voltage (I-V) plot for the same cell as in (a), showing that baclofen does not cause a shift of the I-V plot along the voltage axis. (c) The inhibitory effect of baclofen (10 μM) is blocked by addition of a GABAB receptor antagonist, CGP56999 (CGP, 100 nM). (d) Time course plot for peak barium current at 0 mV, in the indicated concentrations of baclofen. (e) Summary concentration-response for a series of experiments as in (d). The ordinate is expressed as percentage of maximal inhibition. The smooth curve is a fit of a logistic function, giving an EC50 of 2 μM. The numbers indicate n.
Like baclofen, the opioid receptor agonist, enkephalin, and the α2-adrenoceptor agonist, UK14304, reversibly inhibited barium current and slowed the activation kinetics, without shifting the current-voltage relationships (Figure 3). The mean inhibition of barium current by saturating concentrations of enkephalin (100 μM) and UK14304 (10 μM) were 30±2% (n=20) and 28±2% (n=11), respectively. From the enkephalin concentration-response curve, the EC50 was measured to be approximately 0.3 μM (c.f. ∼0.5 μM for the effect of enkephalin on the potassium conductance in LC; North & Williams, 1985) (Figure 3d).
Figure 3.
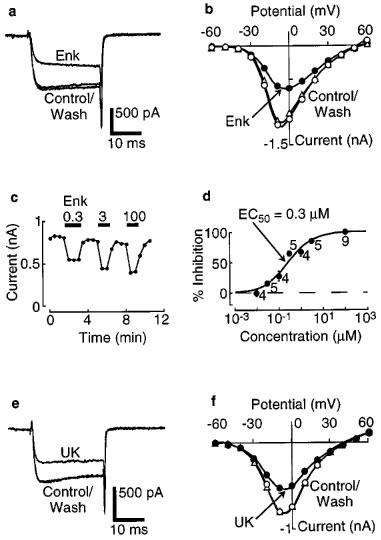
Enkephalin (Enk) acts via opioid receptors to inhibit barium current in a concentration-dependent manner. (a) Barium current measured in control solution and after adding a saturating concentration of enkephalin (100 μM). (b) Current-voltage plot for the same cell as in (a), showing the lack of shift along the voltage axis following addition of enkephalin. (c) Time course plot for peak barium current at 0 mV, in the indicated concentrations of enkephalin. (d) Summary concentration-response plot for a series of experiments as in (c). The ordinate is expressed as percentage of maximal inhibition. The smooth curve is a fit of a logistic function, giving an EC50 of 0.3 μM. The numbers indicate n. (e) Barium current measured in control solution and after adding a saturating concentration of UK14304 (UK, 10 μM). (f) Current-voltage plot for the same cell as in (e).
GABAB and opioid receptor agonists inhibited different subtypes of calcium channels to different extents
We next asked whether baclofen- and enkephalin-activated receptors were coupled to different subtypes of calcium channels. R-type channels were isolated by superfusing a combination of Nimo (3 μM), CTx (1 μM) and Aga (500 nM). Baclofen (100 μM) inhibited the residual (R-type) current by 6±5% (n=5) (Figure 4a, top right; Figure 4b). In the presence of CTx and Aga (L- plus R-type currents remaining), 100 μM baclofen inhibited by 15±5% (n=4). Thus the inhibition by baclofen of L-type current alone, obtained by subtracting the R-type inhibition, was estimated at 9±7%. Similar experiments were done to isolate the effect of 100 μM baclofen on N- and P/Q-type currents by using the appropriate combinations of blockers. The measured inhibition was 17±7% in Nimo plus Aga (N- plus R-types remaining; n=4) and 32±3% in Nimo plus CTx (P/Q- plus R-types remaining; n=4). After subtracting the measured inhibition of pure R-type current, inhibition of N-type alone was estimated at 11±8% and of P/Q-type alone at 26±6%.
Figure 4.
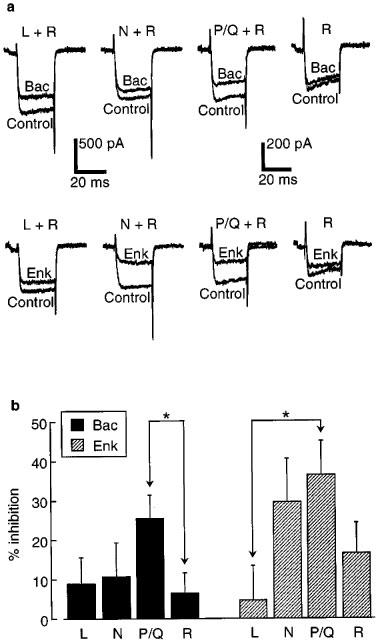
Baclofen (Bac) and enkephalin (Enk) inhibited different calcium channel subtypes to varying extents. (a) Example traces for the inhibitory effect of baclofen (100 μM, top panels) or enkephalin (100 μM, bottom panels) on the pharmacologically isolated subtypes of calcium channels. (b) Summary of the results of experiments as in (a). Inhibition of pure L-type currents was estimated by subtracting the measured inhibition of R-type currents (Nimo, CTx and Aga present) from that measured for L-type plus R-type currents (CTx and Aga present). A similar method was used to estimate the inhibition of pure N- and P/Q-type currents. A disadvantage of this subtraction procedure is that the error bars (±s.e.mean) are increased. Statistically significant differences in inhibition are indicated by the asterisks (P<0.05). n=5 for R-type measurements, n=4 for all others.
The same series of experiments was done with 100 μM enkephalin. The measured inhibition was 17±8% (R; n=5), 21±5% (L plus R; n=4), 46±9% (N plus R; n=4) and 53±4% (P/Q plus R; n=4). Following subtraction of the inhibition of pure R-type current (17±8%), the estimated inhibition of each subtype alone was 5±9% (L), 30±11% (N), and 37±9% (P/Q). All of the subtracted results are summarized in Figure 4b. Baclofen inhibits P/Q-type channels significantly more than R-type channels (P<0.05), and enkephalin inhibits P/Q-type channels more than L-type channels (P<0.05). No other differences were found to be significant.
Effects of activation of opioid and α2 receptors, but not opioid and GABAB receptors, occluded one another
We next examined whether different receptor agonists had occlusive or additive effects on calcium channel inhibition. In the presence of baclofen (100 μM), enkephalin (100 μM) significantly inhibited the remaining barium current by a further 16%, to a total of 33±4% for both drugs (n=4, Figure 5a and c). In the same cells, in the presence of enkephalin, baclofen consistently inhibited the remaining current by a further 10%, giving a total of 34±1% for both drugs. In contrast, co-application of enkephalin (100 μM) and UK14304 (10 μM) produced little further inhibition beyond that produced by each drug alone in the same cells (25±2% for enkephalin alone; 29±3% for enkephalin plus UK14304; 29±2% for UK14304 alone; 32±2% for UK14304 plus enkephalin; n=4; Figure 5b and d). This suggests that some receptor types (e.g. opioid and α2) activate converging pathways while others (e.g. opioid and GABAB) do not.
Figure 5.
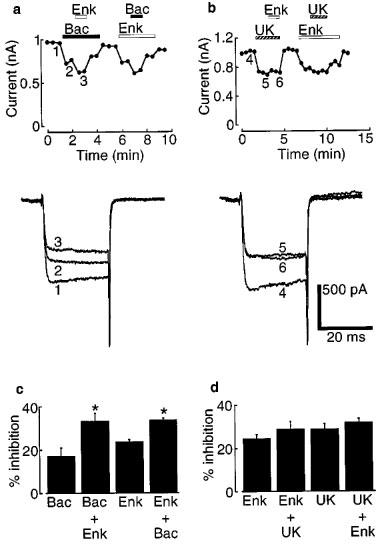
Saturating concentrations of baclofen (Bac) and enkephalin (Enk) produced additive inhibition of barium currents, whereas enkephalin and UK14304 (UK) produced mutually occlusive inhibition. (a and b) Upper panels. Time-course plots for peak barium currents at 0 mV showing the additive (left) and occlusive (right) effects of the indicated agonists. Lower panels. Example currents at the indicated time points on the time course plots. (c and d) Summary of the results of four experiments as in (a and b). The effects of baclofen and enkephalin were partially additive, whereas those of enkephalin and UK14304 were fully occlusive. Asterisks represent a significant difference (P<0.05) between baclofen alone versus baclofen plus enkephalin, and enkephalin alone versus enkephalin plus baclofen.
Discussion
In this paper we have shown that activation of GABAB, opioid and α2 receptors inhibits barium current flowing through calcium channels in acutely-dissociated LC neurones (Figures 2 and 3). This inhibition occurred without shifting the current-voltage relationship along the voltage axis. GABAB and opioid receptor activation inhibited some of the calcium channel subtypes to differing extents (Figure 4). Finally, the inhibitory effects of activation of GABAB and opioid receptors were additive, whilst those of opioid and α2 receptors were mutually occlusive (Figure 5).
At least four different classes of high voltage-activated (HVA) calcium channels are present in these cells, L, N, P/Q (about 30% of each) and R (10%) (Figure 1d). Low voltage-activated T-type calcium channels are not apparent in LC neurones; test pulses to −30 mV from a strongly hyperpolarizing prepulse did not reveal a transient conductance (not illustrated). The activation kinetics of the different classes of HVA calcium currents were similar, since their normalized time courses were identical (Figure 1f). The duration of our test pulses was too short to allow comparison of inactivation time courses, which have been reported to differ between calcium channel subtypes (Catterall, 1995).
Receptor-mediated modulation of voltage-gated calcium channels has been intensively studied in a wide range of cell types (Hille, 1994; Zamponi & Snutch, 1998). The mechanism usually involves activation of intracellular G-proteins as second messengers. Although our experiments were not designed to directly address whether G-proteins are involved in the inhibition reported here, several features of the inhibition are typical of those involving G-proteins. The activation kinetics of barium currents were slowed following receptor activation (Figures 2a, 3a and e; Connor & Christie, 1998; Ingram et al., 1997) and the inhibition could be removed by strong depolarization (Ingram et al., 1997). Moreover, the pertussis toxin-sensitive G proteins, Gi and Go, are known to be present in LC neurones, where they have been shown to mediate an inhibition of adenylate cyclase and an increase in inwardly rectifying potassium conductance (Christie, 1991).
A further characteristic of many G-protein mediated processes is the convergence of second messenger cascades, leading to occlusion between the effects of activation of different receptors (Simon et al., 1991). Such occlusion was observed for the potassium conductance in LC neurones (Agahajanian & Wang, 1987; Connor et al., 1996; North & Williams, 1985), and prompted us to look for similar effects on the calcium conductance in these cells. In one series of experiments, we studied the effects of GABAB and opioid receptor activation on different calcium channel subtypes (Figure 4). The finding that these receptors inhibited some calcium channel subtypes to differing extents (Figure 4b) suggests a divergence in the actions of some second messenger cascades. This divergence is further highlighted by the finding that the inhibitory effects of activation of GABAB and opioid receptors were additive (Figure 5a and c).
The results in Figure 5c can be predicted from those in Figures 1d and 4b if one assumes complete divergence of the actions of baclofen and enkephalin. Define FL as the fraction of L-type calcium channels in LC neurones, obtained from Figure 1d. Similar terms can be defined for N-, P/Q- and R-types. Define IL as the percentage inhibition of L-type calcium channels by baclofen, obtained from Figure 4b, and similarly for N-, P/Q- and R-types. The predicted baclofen inhibition of the total barium current is then FLIL+FNIN+FPIP+FRIR=14%, which is close to the experimental value of 17% (Figure 5c). A similar argument for enkephalin predicts inhibition of 24%, which is the same as the experimental value (Figure 5c). Assuming perfect independence of the actions of baclofen and enkephalin, the predicted co-inhibition by baclofen and enkephalin together is 14%+24%=38% which is comparable with the observed value of 33% (Figure 5c). This analysis strongly indicates a lack of cross-talk between the actions of these two agonists. This contrasts with opioid and α2-receptor-mediated inhibition, which is mutually occlusive (Figure 5b and d), suggesting that activation of these receptors can lead to a convergence of second messenger cascades.
What mechanisms might explain divergence between the pathways activated by some combinations of receptor activation? Divergence could arise in one of three ways: (i) agonists activate distinct second messengers that differ in their concentration or effectiveness at coupling to calcium channels; (ii) agonists activate the same second messengers but these are kept separate and couple to distinct populations of calcium channels; or (iii) agonists activate some combination of these two kinds of mechanism. In order to distinguish between these possibilities, future work would need to examine the identities and properties of the second messenger pathways involved in agonist-activated inhibition of calcium channels in LC neurones.
An important physiological role for LC neurones is to release noradrenaline from their extensive projections. This appears to underlie the functional behaviours involving the LC, such as arousal, attentiveness and antinociception (Aston-Jones et al., 1991a; Foote et al., 1991; Jones, 1991). Here we have studied the modulation of calcium channels found on the somas and proximal dendrites of acutely-dissociated LC neurones. These channels will contribute to the excitability of LC neurones, both directly, by their participation in calcium action potentials, and indirectly, through their role in synaptic integration (Yuste & Tank, 1996). Our results indicate that an array of endogenous agonists, including GABA, enkephalin and noradrenaline, can suppress the excitability of LC neurones through their actions on calcium channels. If it is assumed that calcium channels located on the presynpatic terminals of LC neurones function in a similar way to somatic channels, our results indicate that the above agonists will directly inhibit the release of noradrenaline from LC terminals.
In conclusion, we have shown that three classes of agonists that are endogenous to LC, GABA, enkephalin and noradrenaline, exert a powerful inhibitory effect on calcium channels in acutely-dissociated LC neurones. Different agonists inhibit different calcium channel subtypes to varying extents, and the actions of some of the agonists are mutually occlusive. These properties will help to determine the role of LC neurones in normal brain processing.
Acknowledgments
We thank Dr Pankaj Sah for discussions and comments on the manuscript. B. Chieng is the recipient of a C.J. Martin Fellowship of the National Health and Medical Research Council of Australia. The donations of the CGP compounds by Novartis and ω-agatoxin IVA by Pfizer Research are gratefully acknowledged.
Abbreviations
- α2 receptor
alpha-2 adrenoceptor
- Aga
ω-agatoxin IVA
- Bac
baclofen
- CTx
ω-conotoxin GVIA
- Enk
methionine-enkephalin
- FL
fraction of calcium current in LC neurones mediated by L-type channels
- GABAB receptor
type B γ-aminobutyric acid receptor
- IL
per cent inhibition by baclofen of L-type calcium current
- LC
locus coeruleus
- L-type
L subtype of calcium channel
- Nimo
nimodipine
- N-type
N subtype of calcium channel
- P/Q-type
P and Q subtypes of calcium channels
- R-type
R subtype of calcium channel
- UK
UK14304
References
- AGHAJANIAN G.K. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276:186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- AGHAJANIAN G.K., WANG Y.Y. Common alpha 2- and opiate effector mechanisms in the locus coeruleus: intracellular studies in brain slices. Neuropharmacology. 1987;26:793–799. doi: 10.1016/0028-3908(87)90054-2. [DOI] [PubMed] [Google Scholar]
- ASTON-JONES G., CHIANG C., ALEXINSKY T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog. Brain Res. 1991a;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- ASTON-JONES G., SHIPLEY M.T., CHOUVET G., ENNIS M., VAN BOCKSTAELE E., PIERIBONE V., SHIEKHATTAR R., AKAOKA H., DROLET G., ASTIER B., CHARLÉTY P., VALENTINO R.J., WILLIAMS J.T. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog. Brain Res. 1991b;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- CATTERALL W.A. Structure and function of voltage-gated ion channels. Annu. Rev. Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- CHRISTIE M.J. Mechanisms of opioid actions on neurons of the locus coeruleus. Prog. Brain Res. 1991;88:197–205. doi: 10.1016/s0079-6123(08)63809-1. [DOI] [PubMed] [Google Scholar]
- CONNOR M., CHRISTIE M.J. Modulation of Ca2+ channel currents of acutely dissociated rat periaqueductal grey neurons. J. Physiol. 1998;509:47–58. doi: 10.1111/j.1469-7793.1998.047bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOR M., VAUGHAN C.W., CHIENG B., CHRISTIE M.J. Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurones in vitro. Br. J. Pharmacol. 1996;119:1614–1618. doi: 10.1111/j.1476-5381.1996.tb16080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOOTE S.L., BERRIDGE C.W., ADAMS L.M., PINEDA J.A. Electrophysiological evidence for the involvement of the locus coeruleus in alerting, orienting, and attending. Prog. Brain Res. 1991;88:521–532. doi: 10.1016/s0079-6123(08)63831-5. [DOI] [PubMed] [Google Scholar]
- HILLE B. Modulation of ion-channel function by G-protein-coupled receptors. Trends in Neurosciences. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- HUANG C.C., WANG S.J., GEAN P.W. Selective enhancement of P-type calcium currents by isoproterenol in the rat amygdala. J. Neurosci. 1998;18:2276–2282. doi: 10.1523/JNEUROSCI.18-06-02276.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAM S., WILDING T.J., MCCLESKEY E.W., WILLIAMS J.T. Efficacy and kinetics of opioid action on acutely dissociated neurons. Mol. Pharmacol. 1997;52:136–143. doi: 10.1124/mol.52.1.136. [DOI] [PubMed] [Google Scholar]
- INOUE M., NAKAJIMA S., NAKAJIMA Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J. Physiol. 1988;407:177–198. doi: 10.1113/jphysiol.1988.sp017409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES S.L. Descending noradrenergic influences on pain. Prog. Brain Res. 1991;88:381–394. doi: 10.1016/s0079-6123(08)63824-8. [DOI] [PubMed] [Google Scholar]
- NORTH R.A., WILLIAMS J.T. On the potassium conductance increased by opioids in rat locus coeruleus neurones. J. Physiol. 1985;364:265–280. doi: 10.1113/jphysiol.1985.sp015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSMANOVIC S.S., SHEFNER S.A. Baclofen increases the potassium conductance of rat locus coeruleus neurons recorded in brain slices. Brain Res. 1988;438:124–136. doi: 10.1016/0006-8993(88)91331-5. [DOI] [PubMed] [Google Scholar]
- PEPPER C.M., HENDERSON G. Opiates and opioid peptides hyperpolarize locus coeruleus neurons in vitro. Science. 1980;209:394–395. doi: 10.1126/science.7384811. [DOI] [PubMed] [Google Scholar]
- PIERIBONE V.A., XU Z.Q., ZHANG X., GRILLNER S., BARTFAI T., HOKFELT T. Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience. 1995;64:861–874. doi: 10.1016/0306-4522(94)00450-j. [DOI] [PubMed] [Google Scholar]
- SCHOLZ K.P., MILLER R.J. Presynaptic inhibition at excitatory hippocampal synpases: development and role of presynaptic Ca2+ channels. J. Neurophysiol. 1996;76:39–46. doi: 10.1152/jn.1996.76.1.39. [DOI] [PubMed] [Google Scholar]
- SIMON M.I., STRATHMANN M.P., GAUTAM N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- SURPRENANT A., WILLIAMS J.T. Inhibitory synaptic potentials recorded from mammalian neurones prolonged by blockade of noradrenaline uptake. J. Physiol. 1987;382:87–103. doi: 10.1113/jphysiol.1987.sp016357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN BOCKSTAELE E.J., CHAN J. Electron microscopic evidence for coexistence of leucine5-enkephalin and gamma-aminobutyric acid in a subpopulation of axon terminals in the rat locus coeruleus region. Brain Res. 1997;746:171–182. doi: 10.1016/s0006-8993(96)01194-8. [DOI] [PubMed] [Google Scholar]
- YUSTE R., TANK D.W. Dendritic integration in mammalian neurons, a century after Cajal. Neuron. 1996;16:701–716. doi: 10.1016/s0896-6273(00)80091-4. [DOI] [PubMed] [Google Scholar]
- ZAMPONI G.W., SNUTCH T.P. Modulation of voltage-dependent calcium channels by G proteins. Curr. Opin. Neurobiol. 1998;8:351–356. doi: 10.1016/s0959-4388(98)80060-3. [DOI] [PubMed] [Google Scholar]


