Abstract
We aimed to determine whether there are any changes in responsiveness of the mesenteric arterial beds to phenylephrine (Phe) and KCl in exercise-trained rats, and whether vascular endothelium and/or vascular smooth muscle play a role in these changes.
Adult male rats were subjected to a swimming schedule every day for 28–33 days. Studies were performed in vitro using Krebs perfused mesenteric arterial beds.
Maximum perfusion pressure responses to KCl and Phe of the mesenteric arterial beds from exercise-trained rats were significantly lower than those from sedentary controls. However, these differences disappeared after blocking the nitric oxide synthase by NG-nitro-L-arginine (L-NOARG).
3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulphonate (CHAPS, 3 mg ml−1, 2 min infusion) caused a significant increase in maximum perfusion pressure responses to KCl to the same extent in both exercise-trained and sedentary control rats. CHAPS caused about a 4.5 fold leftward shift of the curve with no change in maximum response to Phe for the mesenteric arterial beds from sedentary control rats, but not for those obtained from exercise-trained rats. However, these differences were abolished in the presence of L-NOARG.
Indomethacin did not alter the dose-response curves to KCl or Phe in either swimming or control groups.
These results suggest that there was a lower vascular responsiveness to KCl and Phe in exercise-trained rats at rest. The decrease in reactivities to KCl or decrease in sensitivity to Phe after having endothelium impairment by CHAPS of the mesenteric arterial beds of exercise-trained rats were due to an increase in both spontaneous release and upregulation of phenylephrine-stimulated release of nitric oxide from both the vascular endothelium and the vascular smooth muscle cells, and may not be a consequence of an increase in vasodilator prostaglandins by the vascular bed.
Keywords: Endothelium, exercise, mesenteric artery, phenylephrine, swimming, vascular smooth muscle
Introduction
Several weeks of voluntary exercise produces training adaptation of the cardiovascular control mechanisms, especially, a lowered resting blood pressure, decreased systemic vascular resistance and decreased heart rate both in laboratory animals (Lutgemeier et al., 1987; Musch et al., 1987; Noma et al., 1987; Overton et al., 1988) and in man (Meredith et al., 1991; Seals & Reiling, 1991). However, the mechanisms responsible for these changes are not yet understood.
There has been growing evidence that endothelium derived relaxing factor (EDRF) which is defined as nitric oxide, NO (Palmer et al., 1987) plays an important role in regulation of vascular smooth muscle tone. It is produced locally in the vessel wall from L-arginine by the action of nitric oxide synthase (ecNOS) (Palmer et al., 1988). It is released from the endothelial cells under basal conditions as well as during stimulation with some agonists such as acetylcholine (Busse et al., 1993; Griffith et al., 1984; Rubanyi et al., 1985), serotonin and noradrenaline (Cocks & Angus, 1983). Physical stimuli, such as elevated shear stress due to experimentally increased blood flow, or during regular physical exercise can also induce NO release and promote vasodilatation (Gerova et al., 1983; Sessa et al., 1994; Smiesko et al., 1985; Wang et al., 1993). In addition, previous studies have found that strenuous and long-term exercise training by swimming, caused an increase in both spontaneous and stimulated release of NO from vascular endothelium to attenuate the vasoconstrictor responses to phenylephrine and KCl of the rat thoracic aorta (Jansakul, 1995). However, evidence that NO plays a role in control of regional blood flow in exercise training is controversial. Katz et al. (1997) measured forearm blood flow in response to brachial arterial administration of acetylcholine and nitroglycerine of patients with chronic heart failure before and after 8 weeks of daily handgrip exercise, and found vasodilator response to acetylcholine was significantly increased from pretraining values, whereas the vasodilator response to nitroglycerine was not changed. Kingwell et al. (1997) demonstrated that men, after 4 weeks of cycling training, had significantly increased basal release of nitric oxide by the forearm, and decreased intrabrachial blood pressure, and NG-monomethyl-L-arginine caused a greater vasoconstriction after the training. However, McAllister et al. (1996) found no differences in contractile responses to noradrenaline or KCl or relaxing responses to sodium nitroprusside of femoral arteries, brachial arteries and mesenteric arteries from long-term treadmill exercise compared to those of sedentary control miniature swine.
The mesenteric circulation of the rat receives approximately one-fifth of the cardiac output (Nichols et al., 1985). Therefore, regulation of this bed may make significant contributions towards systemic blood pressure. However, there are no reports on reactivity, vascular resistance or blood flow of the mesenteric arterial bed at rest after exercise training. Thus, it is of interest to examine whether there are any changes in responsiveness of the mesenteric vascular bed in exercise training by swimming in the rat, the condition which was found to increase both spontaneous and stimulated release of nitric oxide from vascular endothelium to attenuate the vasoconstrictor response to phenylephrine and KCl of thoracic aortae (Jansakul, 1995). The knowledge obtained may contribute to an understanding of the regional vascular control mechanism in an exercise-trained rat. Therefore, the present study was designed to determine: (1) whether there are any changes in responsiveness of the mesenteric arterial bed to phenylephrine and KCl in exercise training, and (2) whether the vascular endothelium and/or vascular smooth muscle play a role in these changes. Studies were performed in vitro using the mesenteric arterial beds obtained from adult male rats, which were subjected to a swimming schedule every day for 28–33 days. Dose-response relationship to phenylephrine, and depolarizing concentrations of KCl, were studied in the beds with healthy endothelium and with endothelium functionally impaired. The effects of perfusion flow rate on the perfusion pressure responses to KCl, and the effects of indomethacin and NG-nitro-L-arginine on the perfusion pressure responses to KCl and to phenylephrine were also investigated.
Methods
Adult male Wistar rats, initial weight 350–420 g, were used in the study. The animals were housed at 25°C on a 10 h dark and 14 h light cycle. All rats were allowed access to food and drinking water ad libitum. Animal weights were recorded on the first and the last day of swimming schedule.
The animals were exercised for 28–33 consecutive days until used for experiment on the following morning. Exercise schedule of the rats followed our previous study (Jansakul, 1995) which adopted the protocol of Ohkubo et al. (1992). Ten rats at a time were exercised by swimming in a round glass fibre tank (100 cm in diameter and 70 cm height) containing tap water approximately 45 cm deep and maintained at room temperature (28–29°C). The swimming schedule was as follows: the swimming time on the 1st day was 10 min, and then increased by 10 min increments every day. At 100 min per day, swimming was carried out in two routines of 50 min each. The two daily routines were further increased, in steps of 10 min each day, up to a maximum swimming time of 90 min each. Thereafter rats were allowed to swim at this maximum swimming time every day for another 19 days. Animals were used in the experiment to study vascular reactivity changes after 28–33 days of swimming. During exercise of the swimming group, the sedentary control animals were placed adjacent to the swimming tanks.
Animal preparation
Animals were killed by decapitation with a guillotine, and the heart removed. Twelve animals of each group were selected randomly and their body weight and atrial and ventricular weights recorded. In this case, both left and right atria were separated from the ventricles and placed in a Petri dish containing warm oxygenated Krebs solution (37°C) and allowed to beat freely for a few seconds in order to expel the blood inside the atrial and ventricular chambers. The atrial and ventricular weights were recorded after removal of excess fluid by filter paper.
Isolated perfused mesenteric arterial bed
Immediately after removal of the heart, the superior mesenteric artery was immediately canulated with polyethylene tubing (PE50) and slowly flooded with 1 ml saline. The mesenteric and associated vascular bed was removed from the rat according to the method described by McGregor (1965), placed in warm Krebs Heinseleit solution and carefully dissected away from the gastrointestinal tract and placed in a 100 ml organ bath. The vascular bed was perfused with oxygenated Krebs solution (37°C) via a canula inserted in the superior mesenteric artery by means of a peristaltic pump (Gilson) at 2 ml min−1. Perfusion pressure (mmHg) was monitored continuously through a pressure transducer connected to a side arm of the perfusion canula of the mesenteric arterial bed, which was connected to a Grass polygraph (7DWU). The vascular bed was equilibrated for 40 min. Dose-response (D-R) curve to phenylephrine or to depolarizing concentrations of KCl was studied by perfusion of each concentration of the drug in Krebs solution up to the highest response obtained at each concentration. Different preparations were used for the KCl (four doses) and Phe (six doses) dose-response curves. A total of 92 animals were used. The following protocol was undertaken with exercise-trained and control rats using 4–6 animals per group to obtain dose-response curves for KCl and Phe: (1) Dose-response curve to KCl or Phe → incubated with L-NOARG → Dose-response curve to KCl or Phe in the presence of L-NOARG; (2) Dose-response curve to KCl or Phe → incubated with IDM → Dose-response curve to KCl or Phe in the presence of IDM; (3) Dose-response curve to KCl → infusion CHAPS 2 min → Dose-response curve to KCl; (4) Infused CHAPS 2 min, equilibrated 40 min → Dose-response curve to KCl or Phe → incubated with L-NOARG → Dose-response curve to KCl or Phe in the presence of L-NOARG.
Effect of exercise training, perfusion flow rate and NG-nitro-L-arginine (L-NOARG) on perfusion pressure responses to KCl
After 40 min equilibration, a discrete D-R relationship to KCl (20–120 mM) was obtained, allowing 5–10 min interval between each concentration. The mesenteric arterial beds were re-equilibrated by perfusion with Krebs for about 20 min. The vascular bed was then perfused with Krebs in the presence of L-NOARG (300 mM) for 40 min. The second D-R curve to KCl was obtained in the presence of L-NOARG.
Similarly, in two other sets of experiments, the mesenteric arterial beds from both the control and exercise-trained rats, were perfused with Krebs at the flow rate of 5 ml min−1. Then the D-R curves to KCl were obtained in the absence and presence of L-NOARG under the same protocol as above.
The maximum perfusion pressure responses to KCl of perfusion flow rate of 2 ml min−1 elicited significantly lower responses than those of 5 ml min−1 for the mesenteric arterial beds obtained from exercise swimming rats. However, such differences were not found after the arterial bed was blocked by L-NOARG. Thus, the flow rate of 2 ml min−1 was used for the rest of the studies.
Effect of exercise training and indomethacin on perfusion pressure responses to KCl
Following the same protocol as above, other sets of experiments were undertaken to obtain the D-R curves to KCl in the absence and presence of 1 μM indomethacin (IDM) of the arterial beds from both sedentary control and exercise-trained rats.
Effect of vascular endothelial removal by CHAPS on perfusion pressure response to KCl
In order to obtain a suitable dose of 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulphonate (CHAPS) to impair the functional vascular endothelium with a minimal effect on functional vascular smooth muscle of the mesenteric arterial bed, different concentrations of CHAPS were used. The discrete D-R curve to KCl of the mesenteric arterial bed obtained from sedentary control rats was obtained as described previously. The mesenteric arterial bed was re-equilibrated for 20 min, and was then perfused with Krebs in the presence of CHAPS at concentrations of 3, 4 or 5 mg ml−1 (modified from Bhardwaj & Moore, 1988; Parsons et al., 1994) for 2 min. Another 40 min was allowed for re-equilibration with Krebs solution, and then the second D-R curve to KCl was obtained.
Effect of CHAPS and L-NOARG on perfusion pressure response to KCl
After 30 min equilibration, the mesenteric arterial beds were perfused with Krebs in the presence of CHAPS (3 mg ml−1) for 2 min. The vascular bed was subjected to another 40 min re-equilibration, and then the D-R curve to KCl was obtained. After the perfusion pressure returned to baseline for about 15 min, the mesenteric arterial bed was perfused with Krebs in the presence of 300 mM L-NOARG for 40 min. And then the second D-R curve to KCl was obtained in the presence of L-NOARG.
Effect of exercise training, indomethacin, CHAPS and L-NOARG on perfusion pressure responses to phenylephrine
Using the same protocol as those for KCl, dose-response relationship to Phe was studied before and after treatment with IDM, CHAPS, or L-NOARG.
Drugs
All drug solutions were prepared daily and kept on ice until used. (−)- Phenylephrine HCl (Sigma, U.S.A.) was dissolved in a solution containing NaCl 9.0 g l−1, NaH2PO4 0.19 g l−1 and ascorbic acid 0.03 g l−1. Acetylcholine chloride (Sigma, U.S.A.), NG-nitro-L-arginine (Sigma, U.S.A.), and 3-[(3-cholamidopropyl) -dimethylammonio] - 1 - propanesulphonate, CHAPS (Sigma, U.S.A.) were dissolved in distilled water. Indomethacin was dissolved in 0.1% sodium carbonate (Na2CO3) solution.
Statistical analysis
Absolute perfusion pressure developed was measured throughout so that comparison could be made of both sensitivity and the maximal responsiveness of the endothelium-intact and the chemical destruction of the functional endothelium of the arterial beds of both sedentary control and exercise-trained rats. The drug concentrations which produced 50% of the maximal response for the drug (EC50) were derived from regression analysis over the linear portion of the dose-response curve (Diem & Leutner, 1970). Other data are expressed as mean±s.e.mean of 4–11 experiments (n=4–11) and tests of significance made with ANOVA and Fisher PLSD or Student's paired or unpaired t-test. In all cases, a P value of 0.05 or less was considered statistically significant.
Results
Animal body weights and the weight of atria, ventricle and mesenteric arterial bed which were recorded from 12 randomly selected animals of each group are shown in Table 1. At the beginning of the studies, there was no difference in the animal body weight of the swimming and sedentary control rats. Swimming rats lost body weight during the studies, while the sedentary control rats increased their body weight. The atrial and ventricular weight-to-body weight ratios of swimming animals were higher than those of sedentary control rats, whereas the mesenteric arterial bed weight-to-body weight ratios of the swimming and sedentary control rats were not significantly different. However, the mesentery of swimming rats was visually clear of fat unlike that of sedentary controls.
Table 1.
Body weight, and atrial, ventricular and mesenteric arterial bed weight of swimming and sedentary control rats

Basal perfusion pressure of the swimming and sedentary control rats were not different whether using perfusion flow rate 2 ml min−1 (swimming 9.5±1.2 mmHg, n=12; control, 10±1.3 mmHg, n=12) or 5 ml min−1 (swimming, 18.3±1.2 mmHg, n=6; control, 19.0±2.1 mmHg, n=6).
Effects of perfusion flow rate and L-NOARG on perfusion pressure responses to KCl are shown in Figure 1. The maximum perfusion pressure responses to KCl of the mesenteric arterial beds obtained from swimming rats are significantly lower than those of sedentary control either using the perfusion flow rates 2 or 5 ml min−1. In addition, when using perfusion flow rate 5 ml min−1, the maximum perfusion pressure responses to KCl of the beds from swimming rats were significantly higher than those using flow rate 2 ml min−1 (Table 2). However, these differences were abolished by blocking the nitric oxide synthase by L-NOARG.
Figure 1.
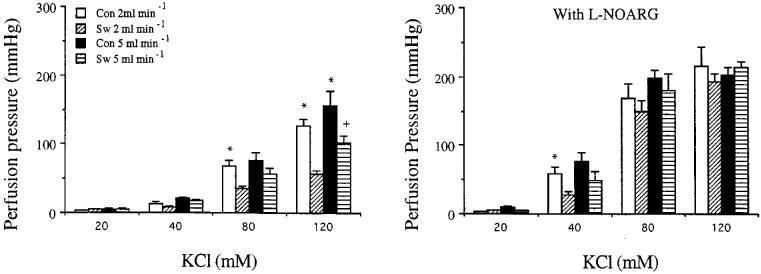
Effects of perfusion flow rate on the increase in perfusion pressure responses to KCl in the absence (left) or presence (right) of NG-nitro-L-arginine (L-NOARG, 300 mM) of the mesenteric arterial beds obtained from sedentary control (Con) and exercise-trained (Sw) rats. Each bar represents the mean±s.e.mean of 6–11 experiments. *Significantly higher than those of exercise-trained group of the same perfusion flow rate and the same concentration of KCl. +Significantly higher than those of exercise-trained group when using perfusion flow rate of 2 ml min−1 of the same concentration of KCl.
Table 2.
EC50 and maximum increase in perfusion pressure responses to Phenylephrine (Phe) and KCl of mesenteric arterial beds obtained from swimming and sedentary control rats using perfusion flow rate 2 or 5 ml min−1

Figure 2 shows the effects of different concentration of CHAPS on perfusion pressure responses to KCl of mesenteric arterial bed obtained from sedentary control rats. CHAPS, 3 mg ml−1, caused a significant shift of the D-R curve of KCl to the left with increase in maximum responses. A concentration of CHAPS of 4 mg ml−1, caused a slight increase in mean perfusion pressure responses to KCl, while the concentration of 5 mg ml−1, caused a significant decrease in mean perfusion pressure to the higher concentrations of KCl compared to their own control curves. Therefore, the concentration of 3 mg ml−1 of CHAPS was chosen for the rest of the studies.
Figure 2.
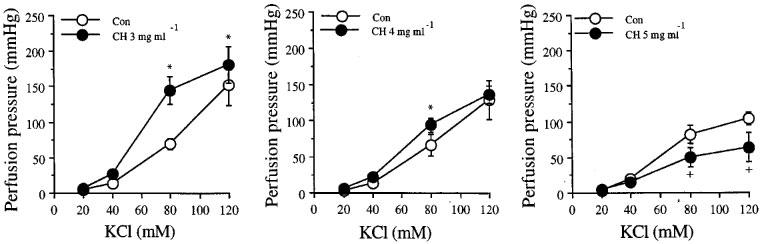
Effects of different concentrations of CHAPS, ch, 3 mg min−1 (left), 4 mg ml−1 (middle), and 5 mg ml−1 (right), on the increase in perfusion pressure responses to KCl of the mesenteric arterial bed obtained from sedentary control rats. Each point represents mean±s.e.mean of four experiments. *Significantly higher than those of control. +Significantly lower than those of control.
Effects of L-NOARG or IDM on perfusion pressure responses to KCl are shown in Figure 3 and Table 2. The dose-response curves to KCl obtained before studying the effects of L-NOARG (n=6) and the effects of IDM (n=5) were combined (n=11) for both swimming and for sedentary control groups. The maximum perfusion pressure responses to KCl of the arterial beds obtained from exercise-trained rats were lower than those of sedentary control rats, and this difference was abolished by pre-perfusion of the arterial beds with L-NOARG. Indomethacin, 1 μM, did not alter the perfusion pressure responses to KCl of either the arterial beds obtained from sedentary control or exercise-trained rats. Destruction of the vascular endothelium by CHAPS, caused a significant upward shift of the D-R curves to KCl to the same extent in both groups of rats, therefore the differences persisted. However, the differences were again abolished by L-NOARG (Figure 4).
Figure 3.
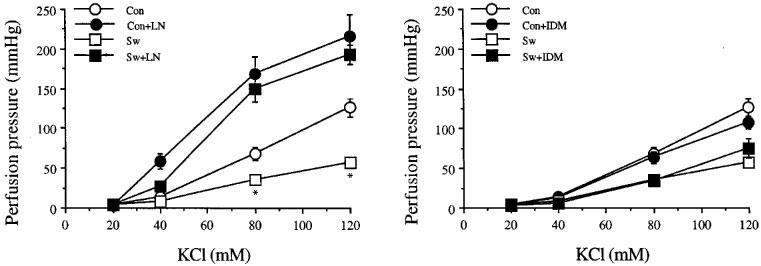
Effects of NG-nitro-L-arginine, LN, 300 mM (left) and indomethacin, IDM, 1 μM (right) on the increase in perfusion pressure responses to KCl of mesenteric arterial bed of sedentary control (con) and exercise-trained (sw) rats. Each point represents mean±s.e.mean of 5–11 experiments. *Significantly lower than those of sedentary control.
Figure 4.
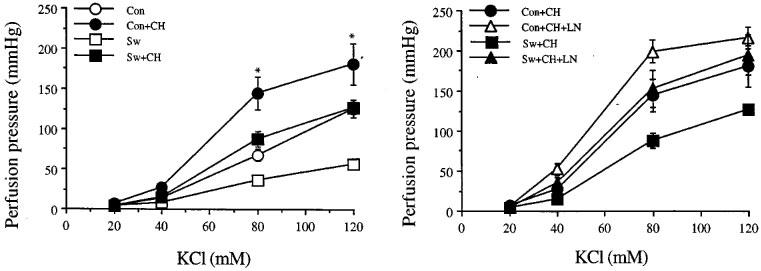
Effects of CHAPS, ch, 3 mg ml−1 (left) and CHAPS with NG-nitro-L-arginine, LN, 300 mM (right) on the increase in perfusion pressure responses to KCl of the mesenteric arterial bed obtained from sedentary control (con) and exercise-trained (sw) rats. Each point represents mean±s.e.mean of 6–11 experiments. *Significantly higher than those of exercise-trained rats after CHAPS.
Figure 5 shows the effects of L-NOARG or IDM on perfusion pressure responses to Phe of mesenteric arterial beds obtained from sedentary control and exercise-trained rats. Similar to those of the KCl, the dose-response curves to Phe obtained before studying the effects of L-NOARG (n=6) and of IDM (n=5) were combined (n=11) for both swimming and sedentary control groups. The maximum perfusion pressure responses to Phe of the arterial beds obtained from exercise swimming rats were slightly lower than those of sedentary control rats. However, these differences were abolished by L-NOARG. IDM did not modify the perfusion pressure responses to Phe in either group studied.
Figure 5.
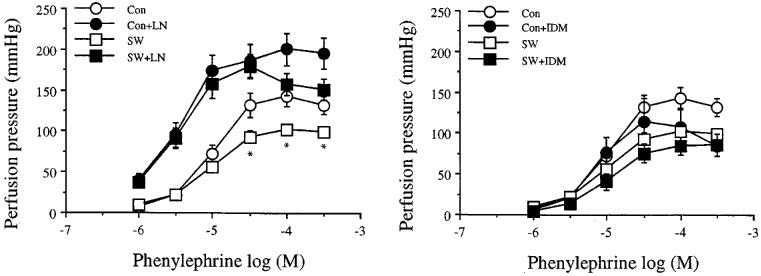
Effects of NG-nitro-L-arginine, LN, 300 mM (left) and indomethacin, IDM, 1 μM (right) on the increase in perfusion pressure responses to phenylephrine of the mesenteric arterial beds of sedentary control (con) and exercise-trained (sw) rats. Each point represents mean±s.e.mean of 5–11 experiments. *Significantly lower than those of sedentary control.
Destruction of the functional vascular endothelium of the mesenteric vascular beds by CHAPS, did not alter the D-R curve to Phe of the vascular bed obtained from the exercise-trained rats. For the sedentary control rats, however, removal of functional endothelium by CHAPS, significantly shifted the D-R curve to Phe to the left with a decrease in EC50 values of about 4.5 fold with no changes in the maximum perfusion pressure responses. Therefore, in the case of the mesenteric arterial beds with impaired functional endothelium, the responsiveness to Phe was increased in sensitivity for the mesenteric arterial beds obtained from sedentary control versus exercise-trained rats. However, these differences were abolished by blocking the nitric oxide synthase by L-NOARG (Figure 6 and Table 2).
Figure 6.
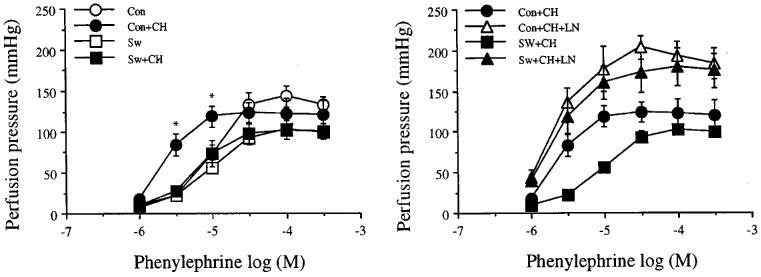
Effects of CHAPS, ch, 3 mg ml−1 (left) and CHAPS with NG-nitro-L-arginine, LN, 300 mM (right) on the increase in perfusion pressure responses to phenylphrine of the mesenteric arterial beds obtained from sedentary control (con) and exercise-trained (sw) rats. Each point represents mean±s.e.mean of 6–11 experiments. *Significantly higher than those of exercise-trained rats after CHAPS.
Discussion
The present study demonstrates that there is a decrease in reactivity to KCl and phenylephrine, with no changes in voltage- or receptor-operated channel-mediated responses of the vascular smooth muscle of the mesenteric arterial bed obtained from exercise-trained rats compared to that of controls. These results are slightly different from my previous report using thoracic aorta, which found an increase in voltage-operated induced responses to endothelium-denuded vascular smooth muscle responses to KCl of exercise-trained rats. Therefore, even though there is an increase in spontaneous release of NO from the vascular endothelial cells, the D-R curves to KCl of the endothelium-intact thoracic aortae of the exercised-trained rats were restored to the same level as those of sedentary control rats (Jansakul, 1995). The mesenteric arterial bed is a resistance vessel, while thoracic aorta is a conducting vessel. This suggests that the effect of strenuous and long-term exercise training by swimming causes different changes in responsiveness for different types of blood vessels. A similar finding was reported by McAllister et al. (1996), who found no changes in responses of the femoral, brachial, mesenteric and hepatic arteries to KCl or noradrenaline between sedentary control and 16–20 weeks of treadmill exercise-trained miniature swine, while the renal arterial rings from exercise trained-swine exhibited lower contractile response to noradrenaline than those from sedentary controls. However, the present study found smaller increase in maximal contractile responses to KCl and Phe of the mesenteric arterial beds obtained from exercise training compared to those of sedentary control rats. The reason for this may be the difference in animal species and/or type and duration of the exercise training.
Changes in vascular responsiveness of the mesenteric vascular beds of exercise-trained rats may be due to structural adaptation of that particular vasculature and/or stimulated release of some vasodilator substance(s) from the vascular tissues. The smaller mesenteric bed of the trained animal if anything would lead to an underestimation of the changes seen with training, that is, one would expect the perfusion pressure to be greater for a smaller bed at the same flow. On the other hand, training has been reported to increase basal NO in nontrained vascular beds, and the mesenteric vascular beds are analogous to non-exercised forearm muscle of cycling training (Kingwell et al., 1997). In order to prove these possibilities, we perfused the mesenteric arterial beds with flow rate of 2 ml min−1 (Dubois-Aubecq et al., 1996; Hendriks et al., 1992; Randall et al., 1988) versus 5 ml min−1 (Kamata & Makino, 1997; Parsons et al., 1994; Ralevic & Burnstock, 1996), and the D-R curves to KCl were obtained in the absence or presence of L-NOARG for the arterial beds from both exercise-trained and sedentary control rats. It would be expected that if there had been a structural adaptation, the basal perfusion pressure and the responses to KCl of those from exercise-trained animals would be greater than those of control. As shown in the results section, the mean basal perfusion pressures of arterial beds were not different between swimming and sedentary control rats at flow rates of either 2 or 5 ml min−1. In addition, the maximum perfusion pressure responses to KCl were higher for the arterial beds obtained from sedentary controls than those from swimming rats, whether using perfusion flow rate of 2 or 5 ml min−1, and these differences were abolished by L-NOARG. These results suggest that the lower maximum perfusion pressure response to KCl of the exercise-trained rats is unlikely to be due to structural adaptation of the mesenteric arterial beds during exercise training. These results also agree with the indexed mesenteric arterial bed weight per animal body weight, which did not significantly change after exercise. The lowering of the mesenteric weight of the exercise-trained rats may be due to lipolysis at the mesentary. The finding that L-NOARG abolished the difference of maximum contractile response to KCl between swimming and sedentary control groups, suggests that NO may be involved in these changes.
There has been growing evidence that NO production is increased during dynamic exercise and after exercise training both in humans (Jungersten et al., 1997; Maroun et al., 1995) and in animals (Bernstein et al., 1996; Jansakul, 1995; Sessa et al., 1994; Woodman et al., 1997; Zhou et al., 1996). Thus, it is possible that the decrease in reactivity to KCl and Phe of the superior mesenteric arterial beds of the exercise-trained rats may be attenuated by the increase in NO production after exercise training. In order to investigate this possibility, the mesenteric arterial beds were pre-perfused with Krebs in the presence of L-NOARG for 40 min before obtaining the D-R curve to KCl or Phe. L-NOARG caused a significant shift of the curves of both groups of animals to the left with an increase in maximum perfusion pressure responses to the same level, and then both curves were no longer significantly different in response either to KCl or to Phe. This suggests that there is an increase in spontaneous and stimulated release of NO from the mesenteric arterial beds of exercise-trained rats to attenuate the perfusion pressure response to KCl and Phe.
Recently, it has become apparent that NO can be produced in large amount from both the vascular endothelial cells as well as from the vascular smooth muscle in response to some stimuli, such as cytokine and endotoxin (Gross et al., 1991; Knowles et al., 1990; Marczin et al., 1993; Schini-Kerth et al., 1994). In the case of a physical stimulus, such as exercise, Sessa et al. (1994) and Zhou et al. (1996) found an increase in ecNOS gene expression from endothelial cells of thoracic aorta and arterioles. Nichols et al. (1994) using NO synthase histochemistry and endothelial cell immunohistochemistry, demonstrated that NO is synthesized both in endothelial and smooth muscle cells of submucosal blood vessels in the rat and human intestine. Thus, it is possible that the increase in spontaneous and stimulated release of nitric oxide from the superior mesenteric vascular beds of the exercise-trained rat in the present study, may be from both the endothelium and the vascular smooth muscle. To investigate this possibility, the functional endothelium of the mesenteric arterial beds were destroyed by perfusion of the tissues with CHAPS (Bhardwaj & Moore, 1988; Parsons et al., 1994). CHAPS (3 mg ml−1) caused significant increase in perfusion pressure responses to KCl of both groups of animals to the same extent. However, these differences disappeared after pre-perfusion of the mesenteric arterial beds with L-NOARG. This suggests that there is an increase in spontaneous release of NO both from vascular endothelial layer as well as from the vascular smooth muscle layers, since KCl does not stimulate NO release (Cocks & Angus, 1983). In the case of Phe which also stimulates the NO release, however, CHAPS caused a significant shift of the curve to the left with a decrease in EC50 for the mesenteric arterial bed from sedentary control, but not for those obtained from exercise swimming rats. Therefore, the mesenteric arterial beds obtained from sedentary control with impaired functional endothelium have a greater increase in sensitivity to Phe compared to those obtained from exercise-trained rats. However, these differences were also abolished by L-NOARG. This suggests that exercise training caused an increase in stimulated release of the NO from vascular smooth muscle layers to attenuate the perfusion pressure response to Phe.
In swimming exercise rats, Ohkubo et al. (1992) found an increase in prostacyclin production at the thoracic aortae, and Watanabe et al. (1991) reported an increase in plasma vasodilator prostaglandin E2 after acute exercise by swimming. Thus, an increased production of prostaglandins from the vascular tissue may play a role in the reduction in reactivity of the mesenteric arterial beds to KCl and Phe. We investigated this possibility by studying the D-R curves to KCl or Phe in the presence of indomethacin, and found that indomethacin did not modify the D-R curves to KCl or Phe in either exercise-trained or sedentary control groups. These results are consistent with my previous study (Jansakul, 1995) on the thoracic aortae of exercise-trained rats, even though the exercise schedule followed Ohkubo's protocol, except that the water temperature used in the present study was 28–29°C instead of 34–35°C. The reasons for the discrepancy between our studies and those of Ohkubo et al. (1992) and Watanabe et al. (1991) may be (1) the difference in water temperature used for animals swimming, and/or (2) the difference between trained and untrained animals. Ohkubo et al. (1992) found that the decrease in linoleic acid content at the thoracic aorta was less pronounced in SHR rats, when swimming was performed at 27–28°C compared to 34–35°C, while Watanabe et al. (1991) found the increase in the plasma level of prostaglandin E2 after acute exercise was significantly smaller in the chronically exercised (trained) than in control (untrained) groups.
In conclusion, the present study demonstrates that there is an increase in the spontaneous release of NO both from vascular endothelial layer as well as from the vascular smooth muscle layers, and upregulation of phenylephrine-stimulated release of the NO from the vascular smooth muscle layer of the mesenteric arterial beds of strenuous and long-term exercise training by swimming in the rats. The increase in NO production from the mesenteric arterial beds may cause a decrease in the vascular resistance of the beds, which may contribute to the decrease in peripheral vascular resistance in exercise-trained rats at rest.
Acknowledgments
Some parts of this work have been presented at the Second International Conference of Biochemistry and Molecular Biology of Nitric Oxide, 13–17th July 1996, UCLA-Sunset Village, Los Angeles, California, U.S.A. This research was funded by grant from the Graduate School, Prince of Songkla University, Thailand. The authors would like to thank Mr M. Naulprup, an animal technician for his generous help in animal swimming management and Dr Alan Geater and Mr Richard Metcher, Prince of Songkla University, Thailand, for critically reviewing the manuscript.
Abbreviations
- CHAPS
3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulphonate
- IDM
indomethacin
- L-NOARG
NG-nitro-L-arginine
- Phe
phenylephrine
References
- BERNSTEIN R.D., OCHOA F.Y., XU X., FORFIA P., SHEN W., THOMPSON C.I., HINTZE T.H. Function and production of nitric oxide in the coronary circulation of the conscious dog during exercise. Circ. Res. 1996;79:840–848. doi: 10.1161/01.res.79.4.840. [DOI] [PubMed] [Google Scholar]
- BHARDWAJ R., MOORE P.K. Endothelium-derived relaxing factor and the effects of acetylcholine and histamine on resistance blood vessels. Br. J. Pharmacol. 1988;95:835–843. doi: 10.1111/j.1476-5381.1988.tb11712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSSE R., MULSCH A., FLEMING I., HECKER M. Mechanisms of nitric oxide release from the vascular endothelium. Circulation. 1993;87 Suppl V:V18–V25. [Google Scholar]
- COCKS T.M., ANGUS J.A. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983;305:627–630. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- DIEM K., LEUTNER C. Documenta Geigy Scientific Tables 1970Basle: J.R. Geigy; 7th edition [Google Scholar]
- DUBOIS-AUBECQ V., DAVY M., MIDOL-MONNET M., COHEN Y. cGMP release in rat mesenteric arterioles and in conduit mesenteric artery. J. Auton. Pharmacol. 1996;16:7–11. doi: 10.1111/j.1474-8673.1996.tb00350.x. [DOI] [PubMed] [Google Scholar]
- GEROVA M., SMIESKO V., GERO J., BARTA E. Dilatation of conduit coronary artery induced by high blood flow. Physiol. Bohemoslov. 1983;32:55–63. [PubMed] [Google Scholar]
- GRIFFITH T.M., HENDERSON A.H., EDWARDS D.H., LEWIS M.J. Isolated perfused rabbit coronary artery and aortic strip preparations: the role of endothelium-derived relaxing factor. J. Physiol. 1984;351:13–24. doi: 10.1113/jphysiol.1984.sp015228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS S.S., JAFFE E.A., LEVI R., KILBOURN R.G. Cytokine-activated endothelial cells express an isotype of nitric oxide synthase which is tetrahydrobiopterin-dependent, calmodulin-independent and inhibited by arginine analogs with a rank-order of potency characteristic of activated macrophages. Biochem. Biophys. Res. Comm. 1991;178:823–829. doi: 10.1016/0006-291x(91)90965-a. [DOI] [PubMed] [Google Scholar]
- HENDRIKS M.G.C., PFAFFENDORF M., VAN ZWIETEN P.A. Characterization of the muscarinic receptor subtype mediating vasodilation in the rat perfused mesenteric vascular bed preparation. J. Auton. Pharmacol. 1992;12:411–420. doi: 10.1111/j.1474-8673.1992.tb00389.x. [DOI] [PubMed] [Google Scholar]
- JANSAKUL C. Effect of swimming on vascular reactivity to phenylephrine and KCl in male rats. Br. J. Pharmacol. 1995;115:587–594. doi: 10.1111/j.1476-5381.1995.tb14972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNGERSTEN L., AMBRING A., WALL B., WENNMALM A. Both physical fitness and acute exercise regulate nitric oxide formation in healthy humans. J. Appl. Physiol. 1997;82:760–764. doi: 10.1152/jappl.1997.82.3.760. [DOI] [PubMed] [Google Scholar]
- KAMATA K., MAKINO A. A comparative study on the rat aorta and mesenteric arterial bed of the possible role of nitric oxide in the desensitization of the vasoconstrictor response to an α1-adrenoceptor agonist. Br. J. Pharmacol. 1997;120:1221–1228. doi: 10.1038/sj.bjp.0701031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ S.D., YUEN J., BIJOU R., LE JEMTEL T.H. Training improves endothelium-dependent vasodilation in resistance vessels of patients with heart failure. J. Appl. Physiol. 1997;82:1488–1492. doi: 10.1152/jappl.1997.82.5.1488. [DOI] [PubMed] [Google Scholar]
- KINGWELL B.A., SHERRARD B., JENNINGS G.L., DART A.M. Four weeks of cycle training increases basal production of nitric oxide from the forearm. Am. J. Physiol. 1997;272:H1070–H1077. doi: 10.1152/ajpheart.1997.272.3.H1070. [DOI] [PubMed] [Google Scholar]
- KNOWLES R.G., SALTER M., BROOKS S.L., MONCADA S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem. Biophys. Res. Comm. 1990;172:1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- LUTGEMEIER I., LUFT F.C., UNGER T., GANTEN U., LANG R.E., GLESS K.H., GANTEN D. Blood pressure, electrolyte and adrenal responses in swim-trained hypertensive rats. J. Hypertens. 1987;5:241–247. doi: 10.1097/00004872-198704000-00017. [DOI] [PubMed] [Google Scholar]
- MARCZIN N., PAPAPETROPOULOS A., CATRAVAS J.D. Tyrosine kinase inhibitors suppress endotoxin- and IL-1b-induced NO synthesis in aortic smooth muscle cells. Am. J. Physiol. 1993;265:H1014–H1018. doi: 10.1152/ajpheart.1993.265.3.H1014. [DOI] [PubMed] [Google Scholar]
- MAROUN M.J., MEHTA S., TURCOTTE R., COSIO M.G., HUSSAIN S.N.A. Effects of physical conditioning on endogenous nitric oxide output during exercise. J. Appl. Physiol. 1995;79:1219–1225. doi: 10.1152/jappl.1995.79.4.1219. [DOI] [PubMed] [Google Scholar]
- MCALLISTER R.M., KIMANI J.K., WEBSTER J.L., PARKER J.L., LAUGHLIN M.H. Effects of exercise training on responses of peripheral and visceral arteries in swine. J. Appl. Physiol. 1996;80:216–225. doi: 10.1152/jappl.1996.80.1.216. [DOI] [PubMed] [Google Scholar]
- MCGREGOR D.D. The effect of sympathetic nerve stimulation on vasoconstrictor responses in perfused mesenteric blood vessels of the rat. J. Physiol. 1965;177:21–30. doi: 10.1113/jphysiol.1965.sp007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEREDITH I.T., FRIBERG P., JENNINGS G.L., DEWAR E.M., FAZIO V.A., LAMBERT G.W., ESLER M.D. Exercise training lowers resting renal but not cardiac sympathetic activity in humans. Hypertension. 1991;18:575–582. doi: 10.1161/01.hyp.18.5.575. [DOI] [PubMed] [Google Scholar]
- MUSCH T.I., HAIDET G.C., ORDWAY G.A., LONGHURST J.C., MITCHELL J.H. Training effects on regional blood flow response to maximal exercise in foxhounds. J. Appl. Physiol. 1987;62:1724–1732. doi: 10.1152/jappl.1987.62.4.1724. [DOI] [PubMed] [Google Scholar]
- NICHOLS A.J., WILSON A.C., HILEY C.R. Effects of sympathectomy with 6-hydroxydopamine on cardiac output and its distribution in the rat. Eur. J. Pharmacol. 1985;109:263–268. doi: 10.1016/0014-2999(85)90428-5. [DOI] [PubMed] [Google Scholar]
- NICHOLS K., STAINES W., RUBIN S., KRANTIS A. Distribution of nitric oxide synthase activity in arterioles and venules of rat and human intestine. Am. J. Physiol. 1994;267:G270–G275. doi: 10.1152/ajpgi.1994.267.2.G270. [DOI] [PubMed] [Google Scholar]
- NOMA K., RUPP H., JACOB R. Subacute and long term effect of swimming training on blood pressure in young and old spontaneously hypertensive rats. Cardiovasc. Res. 1987;21:871–877. doi: 10.1093/cvr/21.12.871. [DOI] [PubMed] [Google Scholar]
- OHKUBO T., JACOB R., RUPP H. Swimming changes vascular fatty acid composition and prostanoid generation of rats. Am. J. Physiol. 1992;262:R464–R471. doi: 10.1152/ajpregu.1992.262.3.R464. [DOI] [PubMed] [Google Scholar]
- OVERTON J.M., JOYNER M.J., TIPTON C.M. Reductions in blood pressure after acute exercise by hypertensive rats. J. Appl. Physiol. 1988;64:748–752. doi: 10.1152/jappl.1988.64.2.748. [DOI] [PubMed] [Google Scholar]
- PALMER R.M.J., ASHTON D.S., MONCADA S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature (Lond.) 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- PALMER R.M.J., FERRIGE A.G., MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PARSONS S.J.W., HILL A., WALDRON G.J., PLANE F., GARLAND C.J. The relative importance of nitric oxide and nitric oxide-independent mechanisms in acetylcholine-evoked dilatation of the rat mesenteric bed. Br. J. Pharmacol. 1994;113:1275–1280. doi: 10.1111/j.1476-5381.1994.tb17136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Mesenteric arterial function in the rat in pregnancy: role of sympathetic and sensory-motor perivascular nerves, endothelium, smooth muscle, nitric oxide and prostaglandins. Br. J. Pharmacol. 1996;117:1463–1470. doi: 10.1111/j.1476-5381.1996.tb15307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL M.D., KAY A.P., HILEY C.R. Endothelium-dependent modulation of the pressor activity of arginine vasopressin in the isolated superior mesenteric arterial bed of the rat. Br. J. Pharmacol. 1988;95:646–652. doi: 10.1111/j.1476-5381.1988.tb11687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBANYI G.M., LORENZ R.R., VANHOUTTE P.M. Bioassay of endothelium-derived relaxing factor(s): inactivation by catecholamines. Am. J. Physiol. 1985;249:H95–H101. doi: 10.1152/ajpheart.1985.249.1.H95. [DOI] [PubMed] [Google Scholar]
- SCHINI-KERTH V.B., FISSLTHALER B., BUSSE R. CGRP enhances induction of NO synthase in vascular smooth muscle cells via a cAMP-dependent mechanism. Am. J. Physiol. 1994;267:H2483–H2490. doi: 10.1152/ajpheart.1994.267.6.H2483. [DOI] [PubMed] [Google Scholar]
- SEALS D.R., REILING M.J. Effect of regular exercise on 24 h arterial pressure in older hypertensive human. Hypertension. 1991;18:583–592. doi: 10.1161/01.hyp.18.5.583. [DOI] [PubMed] [Google Scholar]
- SESSA W.C., PRITCHARD K., SEYEDI N., WANG J., HINTZE T.H. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ. Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- SMIESKO V., KOZIK J., DOLEZEL S. Role of endothelium in the control of arterial diameter by blood flow. Blood Vessels. 1985;22:247–251. [PubMed] [Google Scholar]
- WANG J., WOLIN M.S., HINTZE T.H. Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ. Res. 1993;73:829–838. doi: 10.1161/01.res.73.5.829. [DOI] [PubMed] [Google Scholar]
- WATANABE T., MORIMOTO S., SAKATA Y., LONG N.C., MURAKAMI N. Prostaglandin E2 is involved in adrenocorticotrophic hormone release during swimming exercise in rats. J. Physiol. 1991;433:719–725. doi: 10.1113/jphysiol.1991.sp018452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODMAN C.R., MULLER J.M., LAUGHLIN M.H., PRICE E.M. Induction of nitric oxide synthase mRNA in coronary resistance arteries isolated from exercise-trained pigs. Am. J. Physiol. 1997;273:H2575–H2579. doi: 10.1152/ajpheart.1997.273.6.H2575. [DOI] [PubMed] [Google Scholar]
- ZHOU J., SUN D., KALEY G., KUMAR A. Endothelial nitric oxide synthase gene expression is upregulated by chronic exercise in rat microvessels. FASEB J. 1996;10:A39. [Google Scholar]


