Abstract
Intrathecal (i.t.) injection of nociceptin at small doses (fmol order) elicited a behavioural response consisting of scratching, biting and licking in conscious mice. Here we have examined the involvement of substance P-containing neurons by using i.t. injection of tachykinin neurokinin (NK)1 receptor antagonists and substance P (SP) antiserum.
Nociceptin-induced behavioural response was evoked significantly 5–10 min after i.t. injection and reached a maximum at 10–15 min. Dose-dependency of the induced response showed a bell-shaped pattern from 0.375–30.0 fmol, and the maximum effect was observed at 3.0 fmol.
The behavioural response elicited by nociceptin (3.0 fmol) was dose-dependently inhibited by intraperitoneal (i.p.) administration of morphine.
The NK1 receptor antagonists, CP-96,345, CP-99,994 and sendide, inhibited nociceptin-induced behavioural response in a dose-dependent manner. A significant antagonistic effect of [D-Phe7, D-His9]SP (6–11), a selective antagonist for SP receptors, was observed against nociceptin-induced response. The NK2 receptor antagonist, MEN-10376, had no effect on the response elicited by nociceptin.
Pretreatment with SP antiserum resulted in a significant reduction of the response to nociceptin. No significant reduction of nociceptin-induced response was detected in mice pretreated with NKA antiserum.
The N-methyl-D-aspartate (NMDA) receptor antagonists, dizocilpine (MK-801) and D(−)-2-amino-5-phosphonovaleric acid (APV) (D-APV), and L-NG-nitro arginine methyl ester (L-NAME), a nitric oxide (NO) synthase inhibitor, failed to inhibit nociceptin-induced behavioural response.
The present results suggest that SP-containing neurons in the mouse spinal cord may be involved in elicitation of scratching, biting and licking behaviour following i.t. injection of nociceptin.
Keywords: Nociceptin/orphanin FQ; scratching, biting and licking; intrathecal injection; spinal cord; substance P; mouse
Introduction
Nociceptin (Meunier et al., 1995), or orphanin FQ (Reinscheid et al., 1995), the newly discovered natural agonist of opioid receptor-like (ORL1) receptor, is a putative endogenous ligand for the ORL1 receptor. Nociceptin/orphanin FQ is 17 amino acids long and has some homology with the dynorphin family of peptides, but structurally it lacks the N-terminal tyrosine essential for peptides to be active at μ, δ and κ opioid receptors (Chavkin & Goldstein, 1981). Accordingly, nociceptin has negligible affinity to μ, δ and κ opioid receptors, although this peptide exhibits high affinity to the ORL1 receptor (Reinscheid et al., 1995). The ORL immunoreactivity and ORL1 receptors are widely distributed in the central nervous system (Bunzow et al., 1994; Mollereau et al., 1994; Riedl et al., 1996; Wick et al., 1994). The ORL1 receptor belongs to the seven transmembrane receptor family, which is negatively coupled to adenylate cyclase activity via G proteins (Meunier et al., 1995; Reinscheid et al., 1995; Sim et al., 1996). The mRNA for the ORL1 receptors (Mollereau et al., 1994; Fukuda et al., 1994; Chen et al., 1994; Bunzow et al., 1994; Lachowicz et al., 1995; Wick et al., 1995) and for the precursor protein of orphanin FQ (Houtani et al., 1996) is expressed in high concentrations in the spinal dorsal horn where primary afferent nociceptors terminate. The localization of the receptors to the dorsal horn of the spinal cord indicates that nociceptin/orphanin FQ could play a possible role in modulating nociceptive transmission.
Nociceptin (55 pmol), administered acutely to the unanaesthetized mouse by intracerebroventricular (i.c.v.) injection, produces an initial hyperalgesic response followed by analgesia as measured by the hot-plate test (Meunier et al., 1995). Hyperalgesia induced by i.c.v. injection of nociceptin (10 nmol) has been shown in mice in the tail-flick test (Reinscheid et al., 1995). When injected intrathecally (i.t.) into mice, nociceptin produced no antinociceptive response in the hot-plate test in mice (Reinscheid et al., 1995). It has also been found that i.t. injection of nociceptin induces hyperalgesia and allodynia in mice (Okuda-Ashitaka et al., 1996; Hara et al., 1997).
There is ample evidence that i.t. injections of SP, NKA, NKB and their related compounds induce reciprocal hindlimb scratching, biting and licking behaviour in unanaesthetized mice and rats similar to that induced by peripheral irritation (Hylden & Wilcox, 1981; Seybold et al., 1982; Takahashi et al., 1987). On the other hand, excitatory amino acids (EAA) such as glutamate are involved in central nociceptive transmission. Behavioural studies provide evidence that i.t. injection of EAA agonists can induce scratching, biting and licking behaviour, and hyperalgesic effects (Aanonsen & Wilcox, 1986; Sakurada et al., 1990; Murray et al., 1991; Yamamoto & Yaksh, 1992). N-methyl-D-aspartate (NMDA)-induced scratching, biting and licking response was reduced by both competitive and non-competitive NMDA antagonists (Aanonsen & Wilcox, 1986; Sakurada et al., 1990, 1991a).
In the present study, we have found that low doses (fmol order) of nociceptin, injected i.t., can elicit a characteristic behavioural response of scratching, biting and licking similar to that seen after i.t. injection of SP. The involvement of SP-containing neurons in effects observed was examined by determining the ability of neurokinin (NK)1 receptor antagonists and of SP antiserum to modify the response. The purpose of this study was to ascertain whether SP-containing afferent systems in the spinal cord are involved in nociceptin-induced behaviour in mice.
Methods
Injection procedure
Male ddY mice (Japan SLC, Hamamatsu, Japan) weighing 22–26 g were used in these experiments. The animals were housed under conditions of a 12 h light-dark cycle, a constant temperature of 23°C and 50–60% relative humidity. The i.t. injection procedure was adapted from the method of Hylden & Wilcox (1980). A 28-gauge stainless-steel needle attached to a 50 μl Hamilton microsyringe was inserted between lumbar 5 and lumbar 6 in unanaesthetized mice, and drugs were given slowly in a volume of 5 μl. In combined experiments, nociceptin was co-administered with various drugs in a total volume of 5 μl. In the experiment with antisera against SP and NKA, mice received two separate i.t. injections, each volume of 5 μl. A slight flick of the tail was used as an indication that the needle had penetrated the dura.
Behavioural observation
One hour prior to i.t. injection, animals were adapted to an individual plastic cage (22.0×15.0×12.5 cm) which also served as the observation chamber. Immediately following i.t. injection of nociceptin, each mouse was placed into the transparent cage and behavioural testing was begun. The mice were observed for 20 min beginning immediately after i.t. injection of nociceptin. The total response time(s) of behaviours was measured in 5-min intervals for 20 min after nociceptin as described above. These behaviours included caudally directed biting and licking along with reciprocal hindlimb scratching. All these different behaviours were pooled as a single value for each animal.
For antagonist studies, the substances were tested for their ability to inhibit the behavioural response produced by i.t. injection of nociceptin. All tachykinin NK1 antagonists were co-administered i.t. with nociceptin (3.0 fmol). MEN-10376, a tachykinin NK2 receptor antagonist, EAA receptor antagonists and L-NG-nitro arginine methyl ester (L-NAME) in combination with nociceptin (0.75, 1.5 and 3.0 fmol) were also co-administered. Morphine was administered intraperitoneally (i.p.) 15 min prior to i.t. injection of nociceptin (3.0 fmol). Antisera against SP and NKA were diluted in artificial cerebrospinal fluid (CSF) and injected i.t. 5 min prior to i.t. nociceptin (3.0 fmol). Control values for nociceptin (0.75, 1.5 and 3.0 fmol) were determined separately in each of the co-administration studies.
Studies on the behavioural experiments were performed with the approval of the Ethics Committee of Animal Experiment in Tohoku Pharmaceutical University.
Chemicals
Nociceptin, sendide [Tyr6, D-Phe7, D-His9]SP (6–11) and [D-Phe7, D-His9]SP (6–11) were synthesized by solid-phase peptide methodology. The commercial drugs used were: morphine hydrochloride (Sankyo, Tokyo, Japan), Asp-Tyr-D-Trp-Val-D-Trp-D-Trp-Lys-NH2 (MEN-10,376) (Peninsula Laboratories, CA, U.S.A.), D-(−)-2-amino-5-phosphonovaleric acid (D-APV) (Cambridge Research Biochemicals, Cambridge, U.K.), (5R, 10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine (MK-801) hydrogen maleate (Research Biochemical Incorporated, Natick, MA, U.S.A.) and L-NAME (Wako Pure Chemical Industries, LTD, Japan). CP-96,345 [(2S,3S) - cis-2 - (diphenyl-methyl)- N-[ 2 - methoxy - phenyl) - methyl] - 1-azabicyclo [2.2.2] octan-3-amine], CP-99,994 (+)-[(2S,3S)-3-(2-methoxy-benzyl-amino)-2-phenylpiperidine] and CP-100,263 (−)-[2R,3R)-3-(2-methoxybenzyl-amino)-2-phenylpiperidine] were obtained through courtesy of Pfizer Pharmaceuticals. Morphine was dissolved in saline when injected i.p. For i.t. injections, these compounds were dissolved in sterile artificial CSF containing (mM): NaCl 126.6, KCl 2.5, MgCl2 2.0 and CaCl2 1.3. Only MEN-10,376 was dissolved in 20% dimethyl sulfoxide prepared in CSF. Substance P antiserum for i.t. injection was obtained from rabbits by repeated intradermal injection of SP coupled to bovine serum albumin by glutaraldehyde. The SP antiserum was diluted in CSF and injected i.t. The KD value of SP antiserum (titer 1 : 100,000) was 1×10−10 M. The cross-reaction was 10% for eledoisin, 9.0% for physalaemin, 8.0% for NKB, 6.0% for SP (6–11), and 4.0% for NKA. Substance P (1–7), Met-enkephalin, Leu-enkephalin, and β-endorphin showed less than 0.1% cross-reaction. NKA antiserum was purchased from Austral Biologicals (San Ramon, CA, U.S.A.).
Analyses of data
Results are presented as the mean values±standard error of the mean (s.e.m.). ED50 values with 95% confidence limits were determined for reduction in nociceptin-induced behavioural response by the method of Litchfield & Wilcoxon (1949). Statistical evaluations were performed using the Dunnett's test for multiple comparisons, after analyses of variance (ANOVA). In other comparisons, where only paired comparisons were made, the Tukey's test was used. A probability level less than 0.05 was accepted as significant.
Results
Behavioural response induced by intrathecally administered nociceptin
The i.t. administration of nociceptin (3.0 fmol) resulted in a characteristic behavioural response consisting of vigorous scratching, biting and licking, which peaked at 10–15 min and had disappeared at 20–25 min post-injection (Figure 1a). As seen in Figure 1b, a dose-dependent increase in the total time of scratching, biting and licking was observed following i.t. administration of nociceptin in doses ranging from 0.375–3.0 fmol. The behavioural response was evoked most effectively by 3.0 fmol of nociceptin. No further increase in scratching, biting and licking behaviour was produced by injections of 6.0–30.0 fmol of nociceptin. Relative to the most effective dose (3.0 fmol) of nociceptin, 12.0 and 30.0 fmol of nociceptin were less potent in inducing the behavioural response (Figure 1b). In further experiments, 3.0 fmol of nociceptin was therefore used in combination with various drugs to test their inhibitory actions. I.t. injection of artificial CSF (5 μl) had no apparent effect on the behaviour of animals.
Figure 1.
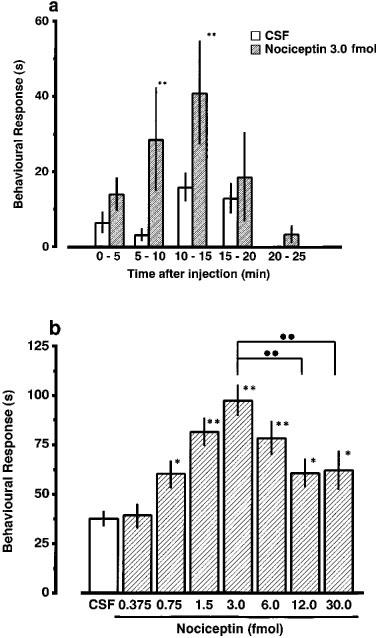
Time courses of nociceptin-induced scratching, biting and licking response (a), and the effect of varying doses of nociceptin in the mouse (b). (a) Mice were injected i.t. with 3.0 fmol. (b) The duration of scratching, biting and licking induced by nociceptin (0.3–30.0 fmol) was determined over a 20 min period starting immediately after injection. Each value represents the mean±s.e.mean of ten mice in each group. *P<0.05, **P<0.01 when compared with CSF-controls by the Dunnett's test. ••P<0.01 when compared with nociceptin (3.0 fmol)-treated groups by the Tukey's test.
Inhibition of nociceptin-induced behavioural response by morphine, tachykinin receptor antagonists, and antisera against SP and NKA
As shown in Figure 2, morphine (0.1–0.8 mg kg−1), injected i.p. before nociceptin (3.0 fmol), produced a dose-related inhibition of nociceptin-induced scratching, biting and licking response. When co-administered with nociceptin (3.0 fmol), CP-96,345 (1.0–16.0 nmol) and CP-99,994 (0.1–1.6 nmol) also produced a dose-related inhibition of the induced behavioural response (Figure 3a and b). In contrast, treatment with CP-100,263, the enantiomer of CP-99,994, did not prevent the induction of the behavioural response by nociceptin. A significant antagonistic effect of sendide (0.71 and 1.0 pmol) and [D-Phe7, D-His9]SP (6–11) (2.0 nmol), a selective antagonist for SP, was observed against the nociceptin-induced behavioural response (Figure 3c and d). The ED50 values for morphine, CP-96,345, CP-99,994, sendide and [D-Phe7, D-His9]SP (6–11) were 0.35 mg kg−1, 7.2 nmol, 0.70 nmol, 0.67 pmol and 1.75 nmol, respectively. Antiserum against SP, injected i.t. 5 min prior to nociceptin, reduced nociceptin-induced behavioural response in a dilution-related manner (Figure 4a). The behavioural response evoked by nociceptin was not affected by a 1/16 dilution of antiserum against NKA (Figure 4b). The i.t. injection of nociceptin into mice pretreated i.t. with CSF produced the behavioural response to almost the same degree as a single injection of nociceptin. I.t. injection of antiserum against SP elicited no observable behavioural response.
Figure 2.
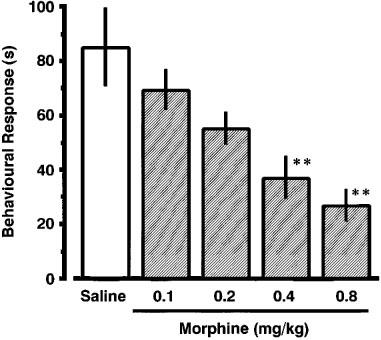
Effect of morphine on nociceptin-induced scratching, biting and licking response in mice. Morphine was given i.p. 15 min before i.t. administration of nociceptin (3.0 fmol). The duration of scratching, biting and licking induced by nociceptin was determined over a 20 min period starting immediately after injection. Each value represents the mean±s.e.mean of ten mice in each group. **P<0.01 when compared with saline-controls (84.8±14.2 s).
Figure 3.
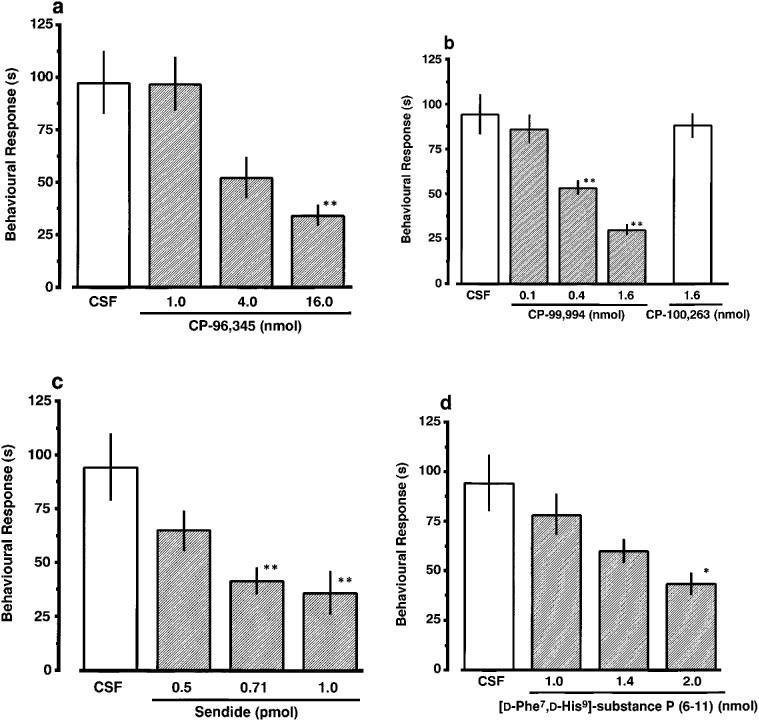
Effect of CP-96,345 (a), CP-99,994 (b), sendide (c) and [D-Phe7, D-His9]SP (6–11) (d) on nociceptin-induced scratching, biting and licking response in mice. Each antagonist was co-administered i.t. with nociceptin (3.0 fmol) in a total volume of 5 μl. The duration of scratching, biting and licking induced by nociceptin was determined over a 20 min period starting immediately after injection. Each value represents the mean±s.e.mean of ten mice in each group. *P<0.05, **P<0.01 when compared with corresponding CSF-controls ((a) 97.2±14.9 s; (b) 94.1±11.0 s; (c) 94.0±15.5 s; (d) 94.0±14.0 s).
Figure 4.
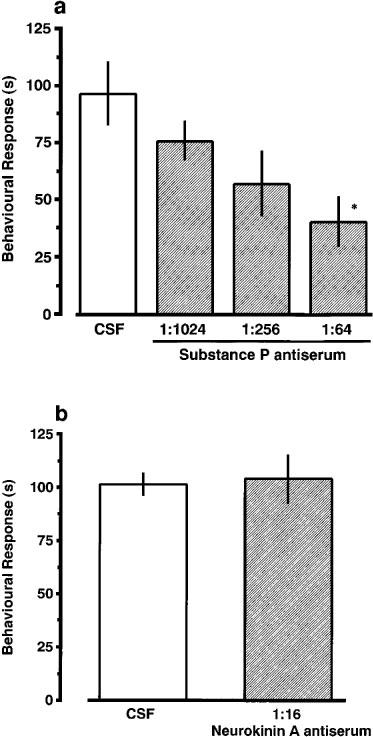
Effect of pretreatment with SP (a) and NKA (b) antisera on nociceptin-induced scratching, biting and licking response in mice. The antisera against SP and NKA was preinjected i.t. 5 min prior to i.t. injection of nociceptin (3.0 fmol). First and second injections were done in a 5 μl volume, separately. The duration of scratching, biting and licking induced by nociceptin was determined over a 20 min period starting immediately after the second injection. Each value represents the mean±s.e.mean of ten mice in each group. *P<0.05 when compared with CSF-controls ((a) 97.6±13.8 s; (b) 101.5±4.9 s).
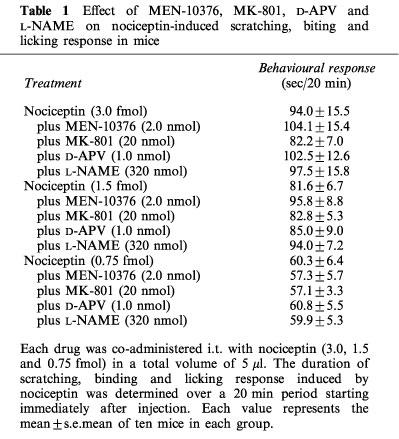
The i.t. administration of MEN-10,376 (2.0 nmol), a tachykinin NK2 receptor antagonist, produced no significant effect on the behavioural response elicited by nociceptin (3.0 fmol) (Table 1). The behavioural response to nociceptin (3.0 fmol) was not changed by D-APV (1.0 nmol), a competitive NMDA receptor antagonist and MK-801 (20 nmol), a non-competitive NMDA receptor antagonist. The nitric oxide (NO) synthase inhibitor L-NAME (320 nmol), co-injected i.t. with nociceptin, was ineffective on the response elicited by nociceptin (3.0 fmol). MEN-10,376, D-APV, MK-801 or L-NAME was also ineffective against a submaximal response to nociceptin (1.5 and 0.75 fmol).
Table 1.
Effect of MEN-10376, MK-801, D-APV and L-NAME on nociceptin-induced scratching, biting and licking response in mice
Discussion
The present data clearly show that nociceptin, when injected i.t. into conscious mice in fmol amounts, can elicit a behavioural syndrome indicative of a nociceptive behavioural response such as scratching, biting and licking. The behavioural response evoked by nociceptin was dose-dependently reduced by quite small doses of morphine (0.l–0.8 mg kg−1, i.p.). Interestingly, these results resemble those reported in mice following i.t. injection of SP (Takahashi et al., 1987). In this study, it was found that i.p. injection of morphine produced a marked inhibition of SP-induced scratching, biting and licking, with ED50 of 0.02 mg kg−1. We have previously observed that the doses of morphine tested in the present study are ineffective in producing an antinociceptive effect as measured by the conventionally employed antinociceptive assays in mice; the ED50 values for morphine were 2.8 mg kg−1 in the tail-flick test, and 6.9 and 3.9 mg kg−1 in the first and second phases of formalin nociceptive response, respectively (unpublished data). Inhibition of the nociceptin-induced behavioural response by i.p. morphine with ED50 of 0.35 mg kg−1 suggests that nociceptin, injected i.t. into mice, can evoke a behavioural response indicative of painful sensation. It is noteworthy that the peak time effect of nociceptin was much later than that of SP-induced behavioural response, which peaked at 0–5 min following i.t. injection (Takahashi et al., 1987). It is, therefore, speculated by this phenomenon that nociceptin-induced scratching, biting and licking response may be elicited indirectly, possibly through the release of excitatory neurotransmitter in the dorsal spinal cord. In addition, this behavioural response was similar to that induced by i.t. injections of SP and NMDA (Hylden & Wilcox, 1980; Aanonsen & Wilcox, 1986; Sakurada et al., 1989). Accordingly, it can be proposed that nociceptin-induced behavioural response may be mediated by an interaction with tachykinin receptors in the spinal cord. In the present study, dose-dependency of the nociceptin-induced response showed a bell-shaped pattern. It has been demonstrated that i.c.v. injection of nociceptin elicits hyperalgesia in the tail-flick test, whereas N-terminal fragments of nociceptin, nociceptin (1–7) and (1–11), are antinociceptive (Rossi et al., 1997). Possible explanations for the decreased effect of nociceptin in increasing doses (6.0, 12.0 and 30.0 fmol) in the present experiment could be that N-terminal fragments of nociceptin formed by enzymatic conversion (Montiel et al., 1997) may have an opposing effect on nociceptin-induced response.
Recently, we have found that sendide, a peptidic NK1 antagonist and CP-96,345, a non-peptidic NK1 antagonist, at some small dose ranges, are selective for NK1 receptors as assayed by the spinally-mediated behavioural response (Sakurada et al., 1994). The two NK1 antagonists, sendide at 8.0 pmol and CP-96,345 at 8.0 nmol, are able to inhibit the SP-induced responses without affecting the behavioural responses produced by NK2 (NKA and D-septide) and NK3 (NKB and eledoisin) agonists (Sakurada et al., 1994). In the present study, both peptidic and non-peptidic antagonists inhibited the scratching, biting and licking response induced by i.t. injection of nociceptin (3.0 fmol). Inferring from the ED50 values of sendide and CP-96,345, our data suggest a modulatory role of spinal tachykinin NK1 receptors in mediating excitatory behavioural effects of i.t. nociceptin in mice. Consistent with this notion is the observation that CP-99,994, but not its stereoisomer, CP-100,263, inhibited the response to i.t. injected nociceptin.
As reported in our previous studies (Sakurada et al., 1991b) analysing the effects of [D-Phe7, D-His9]SP (6–11), a novel antagonist of SP, on various tachykinin NK receptor agonists, this peptidic antagonist could selectively inhibit the SP-induced behavioural response without affecting the other tachykinin NK1 receptor agonists (physalaemin and septide), NK2 and/or NK3 agonists (NKA, NKB and eledoisin) and other substances (such as somatostatin, bombesin and NMDA) (Sakurada et al., 1989; 1990; 1991a; 1994). It was speculated by the use of this hexapeptide antagonist that two different subtypes of the NK1 receptor may exist in the mouse spinal cord (Sakurada et al., 1991b), since this antagonist has an ability to discriminate SP- and the other NK1 receptor-mediated actions. In the present study, we also observed that [D-Phe7, D-His9]SP (6–11), co-administered i.t. with nociceptin, was effective in inhibiting the nociceptin-induced behavioural response. It is, therefore, reasonable to presume that the scratching, biting and licking response elicited by nociceptin may be partially due to stimulation of SP receptors indirectly, possibly through activation of the function of neurons containing SP in the spinal cord. This interpretation is supported by an additional data that pretreatment with the antiserum against SP (titer; 1 : 64) resulted in a significant reduction of the nociceptin-induced behavioural response. Similar observations have been obtained by using SP antiserum in spinally mediated behavioural response elicited by i.t. injection of pilocarpine (Sakurada et al., 1993) and i.t. high-dose morphine (Sakurada et al., 1996b); the behavioural responses induced by pilocarpine and morphine can be reduced significantly by pretreatment with SP antiserum (titer; 1 : 256 for morphine and 1 : 32 for pilocarpine).
It appears that many of the NMDA actions are mediated via activation of NO synthase with subsequent release of NO. NO stimulates guanylyl cyclase and promotes the formation of cyclic GMP (Bredt & Snyder, 1992). An i.t. injection of NMDA produces a dose-dependent thermal hyperalgesia that is mediated by NO and cyclic GMP (Meller et al., 1992). Pretreatment with L-NAME, a NO synthase inhibitor, reversibly blocks NMDA-induced hyperalgesia (Kitto et al., 1992; Meller et al., 1992) and NMDA-induced scratching, biting and licking response (Sakurada et al., 1996a). In the present study, relatively high doses of D-APV (1.0 nmol), a competitive NMDA receptor antagonist, and MK-801 (20 nmol), a non-competitive NMDA antagonist, had little effect upon the behavioural response induced by maximal (3.0 fmol) and submaximal (1.5 and 0.75 fmol) doses of nociceptin. Indeed, 0.25 nmol of D-APV and 0.25 nmol of MK-801 have been reported to inhibit NMDA-induced scratching, biting and licking response (Sakurada et al., 1991a; Delander & Wahl, 1989). In addition, co-administration of L-NAME (80 nmol), which could inhibit NDMA-induced behavioural response (Sakurada et al., 1996a), did not modify the behavioural response to nociceptin. Taken together, these observations suggest that the behavioural response induced by i.t. injection of nociceptin may not be mediated by the glutamate receptor-NO system in the spinal cord.
There is increasing functional evidence to support a role of tachykinin NK2 receptors in spinal nociception. The i.t. administration of MEN-10207, an NK2 receptor antagonist, in very small doses, specifically reverses the facilitatory action on a nociceptive reflex produced by NKA, without affecting the response to SP (Xu et al., 1991). Another NK2 antagonist, MEN-10376 (2.0 nmol), can reduce markedly the scratching, biting and licking response elicited by i.t. co-administration of NKA (400 pmol), and NK2 receptor agonist (our unpublished results). Failure of MEN-10376 to prevent the nociceptin-induced behavioural response suggests that nociceptin may not interact with tachykinin NK2 receptors in the spinal cord. This concept is supported by the data from the NKA antiserum study that the response to nociceptin was unaffected by pretreatment with antiserum against NKA.
In conclusion, we have presented evidence that nociceptin in fmol amounts could elicit a characteristic behavioural response consisting of scratching, biting and licking after i.t. injection. NK1 receptor antagonists, CP-96,345, CP-99,994, sendide and [D-Phe7,D-His9]SP, and SP antiserum could reduce the behavioural response to nociceptin. The data presented here suggest that SP-containing neurons may play a significant role in mechanisms of the behavioural responses to nociceptin, probably through the release of SP, but not NKA and glutamate.
Acknowledgments
The authors wish to thank Dr L. Terenius, Department of Clinical Neuroscience, Drug Dependence Research Section, Karolinska Institute, Stockholm S-171 76, Sweden, for the gift of substance P antiserum. We also thank Miss Miho Nakayama for her help in preparing the manuscript.
Abbreviations
- CP-96,345
[(2S,3S) - cis-2-(diphenyl - methyl) - N - [2-methoxy - phenyl)- methyl]-1-azabicyclo[2.2.2]octan-3-amine]
- CP-99,994
[(±)-(2S,3S)-3-(2-methoxybenzyl-amino)-2-phenylpiperizine]
- CP-100,263
[(−)-(2R,3R)-3-(2-methoxybenzyl-amino)-2-phenylpiperizine]
- D-APV
D(−)-2-amino-5-phosphonovaleric acid
- EAA
excitatory amino acid
- ED50
effective dose, median
- KD
dissociation constant
- L-NAME
L-NG-nitro arginine methyl ester
- MEN-10,376
Asp-Tyr-D-Trp-Val-D-Trp-D-Trp-Lys-NH2
- MK-801
(5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine
- mRNA
messenger ribonucleic acid
- NK
neurokinin
- NMDA
N-methyl-D-aspartate
- ORL1
opioid receptor-like
- SP
substance P
References
- AANONSEN L.M., WILCOX G.L. Phencyclidine selectively blocks a spinal action of N-methyl-D-aspartate in mice. Neurosci. Lett. 1986;67:191–197. doi: 10.1016/0304-3940(86)90396-4. [DOI] [PubMed] [Google Scholar]
- BREDT D.S., SNYDER S.H. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- BUNZOW J.R., SAEZ C., MORTRUD M., BOUVIER C., WILLIAMS J.T., LOW M., GRANGY D.K. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a μ, δ or κ opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- CHEN Y., FAN Y., LIU J., MESTEK A., TIAN M., KOZAK C.A., YU L. Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 1994;347:279–283. doi: 10.1016/0014-5793(94)00560-5. [DOI] [PubMed] [Google Scholar]
- CHAVKIN C., GOLDSTEIN A. Specific receptor for the opioid peptide dynorphin: structure-activity relationships. Proc. Natl. Acad. Sci. U.S.A. 1981;78:6543–6547. doi: 10.1073/pnas.78.10.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELANDER G.E., WAHL J.J. In vivo characterization of phencyclidine/σ agonist-mediated inhibition of nociception. Eur. J. Pharmacol. 1989;159:149–156. doi: 10.1016/0014-2999(89)90699-7. [DOI] [PubMed] [Google Scholar]
- FUKUDA K., KATO S., MORI K., NISHI M., TAKESHITA H., IWABE N., MIYATA T., HOUTANI T., SUGIMOTO T. cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett. 1994;343:42–46. doi: 10.1016/0014-5793(94)80603-9. [DOI] [PubMed] [Google Scholar]
- HARA N., MINAMI T., OKUDA-ASHITAKA E., SUGIMOTO T., SAKAI M., ONAKA M., MORI H., IMANISHI T., SHINGU K., ITO S. Characterization of nociceptin hyperalgesia and allodynia in conscious mice. Br. J. Pharmacol. 1997;121:401–408. doi: 10.1038/sj.bjp.0701146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUTANI T., NISHI M., TAKESHITA H., NUKADA T., SUGIMOTO T. Structure and regional distribution of nociceptin/orphanin FQ precursor. Biochem. Biophys. Res. Commun. 1996;219:714–719. doi: 10.1006/bbrc.1996.0300. [DOI] [PubMed] [Google Scholar]
- HYLDEN J.L.K &, WILCOX G.L. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- HYLDEN J.L.K., WILCOX G.L. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 1981;217:212–215. doi: 10.1016/0006-8993(81)90203-1. [DOI] [PubMed] [Google Scholar]
- KITTO K.F., HALEY J.E., WILCOX G.L. Involvement of nitric oxide in spinally mediated hyperalgesia in the mouse. Neurosci. Lett. 1992;148:1–5. doi: 10.1016/0304-3940(92)90790-e. [DOI] [PubMed] [Google Scholar]
- LACHOWICZ J.E., SHEN Y., MONSMA F.J., JR, SIBLEY D.R. Molecular cloning of a novel G protein-coupled receptor related to the opiate receptor family. J. Neurochem. 1995;64:34–40. doi: 10.1046/j.1471-4159.1995.64010034.x. [DOI] [PubMed] [Google Scholar]
- LITCHFIELD J.T., WILCOXON F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- MELLER S.T., DYKSTRA C., GEBHART G.F. Production of endogenous nitric oxide and activation of soluble guanyulate cyclase are required for N-methyl-D-aspartate-produced facilitation of the nociceptive tail-flick reflex. Eur. J. Pharmacol. 1992;214:93–96. doi: 10.1016/0014-2999(92)90102-a. [DOI] [PubMed] [Google Scholar]
- MEUNIER J.-C., MOLLEREAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.-L., GUILLEMOT J.-C., FERRARA P., MONSARRAT B., MAZARGUIL H., VASSART G., PARMENTIER M., COSTENTIN J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MOLLEREAU C., PARMENTIER M., MAILLEUX P., BUTOIR J.-L., MOISAND C., CHALON P., CAPUT D., VASSART G., MEUNIER J.-C. ORL1, a novel member of the opioid family. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- MONTIEL J.-L., CORNILLE F., ROQUES B.P., FLORENCE N. Nociceptin/orphanin FQ metabolism: role of aminopeptidase and endopeptidase 24.15. J. Neurochem. 1997;68:354–361. doi: 10.1046/j.1471-4159.1997.68010354.x. [DOI] [PubMed] [Google Scholar]
- MURRAY C.W., COWAN A., LARSON A.A. Neurokinin and NMDA antagonists (but not a kainic acid antagonist) are antinociceptive in the mouse formalin model. Pain. 1991;44:179–185. doi: 10.1016/0304-3959(91)90135-K. [DOI] [PubMed] [Google Scholar]
- OKUDA-ASHITAKA E., TACHIBANA S., HOUTANI T., MINAMI T., MASU Y., NISHI M., TAKESHIMA H., SUGIMOTO T., ITO S. Identification and characterization of an endogenous ligand for opioid receptor homologue ROR-C: its involvement in allodynic response to innocuous stimulus. Mol. Brain Res. 1996;43:96–104. doi: 10.1016/s0169-328x(96)00165-9. [DOI] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.-P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J., CIVELLI O. Orphanin FQ: a neuropeptide that activates an opioid like G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- RIEDL M., SHUSTER S., VULCHANOVA L., WANG J., LOH H.H., ELDE R. Orphanin FQ/nociceptin-immunoreactive nerve fibers parallel those containing endogenous opioids in rat spinal cord. NeuroReport. 1996;7:1369–1372. doi: 10.1097/00001756-199605310-00007. [DOI] [PubMed] [Google Scholar]
- ROSSI G.C., LEVENTHAL L., BOLAN E., PASTERNAK G.W. Pharmacological characterization of orphanin FQ/nociceptin and its fragments. J. Pharmacol. Exp. Ther. 1997;282:858–865. [PubMed] [Google Scholar]
- SAKURADA T., MANOME Y., KATSUMATA K., TAN-NO K., SAKURADA S., OHBA M., KISARA K. Comparison of antagonistic effects of sendide and CP-96,345 on a spinally mediated behavioural response in mice. Eur. J. Pharmacol. 1994;261:85–90. doi: 10.1016/0014-2999(94)90304-2. [DOI] [PubMed] [Google Scholar]
- SAKURADA T., MANOME Y., TAN-NO K., MATSUNAGA Y., SAKURADA S., KISARA K. Possible involvement of the spinal substance P system in pilocarpine-induced scratching in mice. Pharmacol. Biochem. Behav. 1993;44:439–445. doi: 10.1016/0091-3057(93)90488-f. [DOI] [PubMed] [Google Scholar]
- SAKURADA T., MANOME Y., TAN-NO K., SAKURADA S., KISARA K. The effects of substance P analogues on the scratching, biting and licking response induced by intrathecal injection of N-methyl-D-aspartate in mice. Br. J. Pharmacol. 1990;101:307–310. doi: 10.1111/j.1476-5381.1990.tb12706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKURADA T., SUGIYAMA A., SAKURADA C., TAN-NO K., SAKURADA S., KISARA K., HARA A., ABIKO Y. Involvement of nitric oxide in spinally mediated capsaicin- and glutamate-induced behavioural responses in the mouse. Neurochem. Int. 1996a;29:271–278. doi: 10.1016/0197-0186(96)00004-6. [DOI] [PubMed] [Google Scholar]
- SAKURADA T., TAN-NO K., MANOME Y., SAKURADA S., KISARA K.Aversive responses produced by intrathecal injection of NMDA in mice: Effects of substance P and 5-HT antagonists NMDA receptor related agents: biochemistry, pharmacology and behavior 1991aAnn Arbor, MI: NPP Books; 219–225.eds. Kameyama, T., Nabeshima, T. & Domino, E.F. pp [Google Scholar]
- SAKURADA T., WAKO K., SAKURADA C., MANOME Y., TAN-NO K., SAKURADA S., KISARA K. Spinally-mediated behavioural responses evoked by intrathecal high-dose morphine: possible involvement of substance P in the mouse spinal cord. Brain Res. 1996b;724:213–221. doi: 10.1016/0006-8993(96)00319-8. [DOI] [PubMed] [Google Scholar]
- SAKURADA T., YAMADA T., SAKURADA S., KISARA K., OHBA M. Substance P analogues containing D-histidine antagonize the behavioural effects of intrathecally co-administered substance P in mice. Eur. J. Pharmacol. 1989;174:153–160. doi: 10.1016/0014-2999(89)90307-5. [DOI] [PubMed] [Google Scholar]
- SAKURADA T., YAMADA T., TAN-NO K., MANOME Y., SAKURADA S., KISARA K., OHBA M. Differential effects of substance P analogs on neurokinin 1 receptor agonists in the mouse spinal cord. J. Pharmacol. Exp. Ther. 1991b;259:205–210. [PubMed] [Google Scholar]
- SEYBOLD V.S., HYLDEN J.L.K., WILCOX G.L. Intrathecal substance P and somatostatin in rats: behavior indicative of sensation. Peptides. 1982;3:49–54. doi: 10.1016/0196-9781(82)90141-3. [DOI] [PubMed] [Google Scholar]
- SIM L.J., XIAO R., CHILDERS S.R. Identification of opioid receptor-like (ORL1) peptide-stimulated [35S]GTPγS binding in the rat brain. NeuroReport. 1996;7:729–733. doi: 10.1097/00001756-199602290-00012. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI K., SAKURADA T., SAKURADA S., KUWAHARA H., YONEZAWA A., ANDO R., KISARA K. Behavioural characterization of substance P-induced nociceptive response in mice. Neuropharmacology. 1987;26:1289–1293. doi: 10.1016/0028-3908(87)90089-x. [DOI] [PubMed] [Google Scholar]
- WICK M.J., MINNERATH S.R., LIN X., ELDE R., LAW P.-Y., LOH H.H. Isolation of a novel cDNA encoding a putative membrane receptor with high homolgoy to the cloned μ, δ and κ opioid receptors. Mol. Brain Res. 1994;27:37–44. doi: 10.1016/0169-328x(94)90181-3. [DOI] [PubMed] [Google Scholar]
- WICK M.J., MINNERATH S.R., ROY S., RAMAKRISHNAN S., LOH H.H. Expression of alternate forms of brain opioid ‘orphan' receptor mRNA in activated human peripheral blood lymphocytes and lymphocytic cell lines. Mol. Brain Res. 1995;32:342–347. doi: 10.1016/0169-328x(95)00096-b. [DOI] [PubMed] [Google Scholar]
- XU X.J., MAGGI C.A., WIESENFELD-HALLIN Z. On the role of NK-2 tachykinin receptors in the mediation of spinal reflex excitability in the rat. Neuroscience. 1991;44:483–490. doi: 10.1016/0306-4522(91)90071-u. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., YAKSH T.L. Spinal pharmacology of thermal hyperesthesia induced by constriction injury of sciatic nerve. Excitatory amino acid antagonists. Pain. 1992;49:121–128. doi: 10.1016/0304-3959(92)90198-K. [DOI] [PubMed] [Google Scholar]


