Abstract
Selective phosphodiesterase 4 (PDE4) inhibitors are of potential interest in the treatment of asthma. We examined the effects of the alkaloid S-(+)-glaucine, a PDE4 inhibitor, on human isolated bronchus and granulocyte function.
Glaucine selectively inhibited PDE4 from human bronchus and polymorphonuclear leukocytes (PMN) in a non-competitive manner (Ki=3.4 μM). Glaucine displaced [3H]-rolipram from its high-affinity binding sites in rat brain cortex membranes (IC50∼100 μM).
Glaucine inhibited the spontaneous and histamine-induced tone in human isolated bronchus (pD2∼4.5). Glaucine (10 μM) did not potentiate the isoprenaline-induced relaxation but augmented cyclic AMP accumulation by isoprenaline. The glaucine-induced relaxation was resistant to H-89, a protein kinase A inhibitor. Glaucine depressed the contractile responses to Ca2+ (pD'2∼3.62) and reduced the sustained rise of [Ca2+]i produced by histamine in cultured human airway smooth muscle cells (−log IC50∼4.3).
Glaucine augmented cyclic AMP levels in human polymorphonuclear leukocytes challenged with N-formyl-Met-Leu-Phe (FMLP) or isoprenaline, and inhibited FMLP-induced superoxide generation, elastase release, leukotriene B4 production, [Ca2+]i signal and platelet aggregation as well as opsonized zymosan-, phorbol myristate acetate-, and A23187-induced superoxide release. The inhibitory effect of glaucine on superoxide generation by FMLP was reduced by H-89.
In conclusion, Ca2+ channel antagonism by glaucine appears mainly responsible for the relaxant effect of glaucine in human isolated bronchus while PDE4 inhibition contributes to the inhibitory effects of glaucine in human granulocytes. The very low PDE4/binding site ratio found for glaucine makes this compound attractive for further structure-activity studies.
Keywords: Glaucine, human airway smooth muscle, human polymorphonuclear leukocytes, human eosinophils
Introduction
Asthma is a disease characterized by reversible bronchial obstruction, airway hyperreactivity and inflammation. Much interest is currently being directed at the inflammatory component of asthma. With respect to treating asthmatic inflammation, the cyclic nucleotide phosphodiesterase (PDE) isoenzymes have been identified as viable targets amenable to therapeutic intervention with selective or mixed inhibitors (Torphy, 1998). The possibility that some of these selective compounds combine effective bronchodilator and anti-inflammatory properties is particularly attractive for the treatment of asthma with the aim of improving the clinical benefit established for non-selective PDE inhibitors like theophylline (Sullivan et al., 1994).
Glaucine [(S)-(+)-1,2,9,10-tetramethoxyaporphine] is an alkaloid isolated from the plant Glaucium flavum Crantz (Papaveraceae) that has been used for years as a remedy for cough and other illnesses (Constant et al., 1983). Glaucine is a tetrahydroisoquinoline derivative, structurally related to papaverine. Different authors (Kukovetz & Pöch, 1970; Van Inwegen et al., 1979) postulated that the mechanism of action of many isoquinoline derivatives including papaverine, involves inhibition of PDE. Papaverine is a non-selective inhibitor of PDE isoenzymes but interestingly glaucine was found to be a relatively potent and selective inhibitor of soluble PDE4 isolated from bovine aortic muscle (Ivorra et al., 1992). Additional studies on the in vitro pharmacological profile of different isoquinoline alkaloids demonstrated that glaucine is also a non-selective α-adrenoceptor antagonist and a Ca2+ entry blocker in rat aorta and vas deferens (Ivorra et al., 1992; Orallo et al., 1993).
There are few natural products described as selective inhibitors of PDE isoenzymes. Besides selective inhibition of PDE4, the other activities reported for glaucine would not be detrimental in asthma. Calcium channel blockers received attention as potential anti-asthma drugs (Barnes, 1985) and also α-adrenoceptor antagonists (Barnes et al., 1981; Black & Armour, 1986). Glaucine relaxes guinea-pig isolated trachea in a concentration-related fashion, and inhibits acetylcholine- and histamine-induced contraction of guinea-pig airways in vitro and in vivo (Kasé et al., 1983). Glaucine is orally active in humans (Dierckx et al., 1981) and shows a trend towards increase of airways specific conductance in man (Constant et al., 1983). In view of these findings we decided to investigate the bronchodilator and anti-inflammatory effects of glaucine in vitro.
The first aim of the present study was to examine whether glaucine is a selective inhibitor of PDE4 isolated from human bronchus and human polymorphonuclear leukocytes, two preparations in which PDE4 activity is relevant to modulate their functional responses (Torphy, 1998). The potency of glaucine to displace rolipram from its high-affinity binding sites in rat brain cortex was also investigated. Second, the bronchodilatation by glaucine was examined in human isolated bronchus with additional experiments carried out to assess the calcium antagonist properties of glaucine and whether it potentiates the isoprenaline-induced relaxation and cyclic AMP accumulation. The effect of glaucine on the intracellular calcium changes in response to histamine in human cultured airway smooth muscle cells was also studied. Third, we examined the ability of glaucine to augment cyclic AMP levels in human polymorphonuclear leukocytes (PMNs) treated with N-formyl-L-methionyl-L-leucyl-L-phenylalanine (FMLP) or isoprenaline, and its inhibitory activity on the functional responses of PMNs and purified eosinophils to a variety of stimuli such as FMLP, the calcium ionophore A23187, serum opsonized zymosan (SOZ), and phorbol 12-myristate 13-acetate (PMA). This part of the study was aimed to assess the in vitro anti-inflammatory activity of glaucine on neutrophils and eosinophils which are cells relevant to asthma pathogenesis (Synek et al., 1996). The involvement of cyclic AMP-dependent protein kinase (PKA) in the inhibitory effects of glaucine was also investigated in human bronchus and PMNs by using the selective PKA inhibitor, H-89 (Chijiwa et al., 1990).
Methods
Human isolated bronchus
Relaxant activity
Macroscopically normal tissue was obtained from patients undergoing surgery for lung carcinoma. The protocol for obtaining human tissue was approved by the local Ethics Committee. Bronchial rings (2–4 mm inner diameter) were suspended in organ baths containing Krebs solution, gassed with 5% CO2 in O2 at 37°C (pH 7.4), for isometric recording of tension changes. An initial load of 2 g weight was applied that resulted at the end of the equilibration period (60–90 min) in an appropriate resting level of tone of ∼1.2 g weight (Watson et al., 1998). Preparations were initially challenged with acetylcholine (ACh, 1 mM) in order to determine the maximal contractile response of the tissue.
The relaxant effects of glaucine were investigated by adding cumulative concentrations of this alkaloid to preparations with either spontaneous tone or precontracted to a plateau with a near-maximal concentration of histamine (0.1 mM). Experiments were terminated by the addition of theophylline (1 mM), the effect of which was taken to represent the maximal relaxation in the tissue. In separate experiments, cumulative concentration-effect curves to CaCl2 were constructed in preparations equilibrated with K+-rich (40 mM), Ca2+-free medium (Advenier et al., 1986), in the absence (control tissues) and presence (30 min incubation) of glaucine. In additional experiments, cumulative concentration-response curves to isoprenaline or sodium nitroprusside were obtained in ACh (0.1 mM)-precontracted preparations in the absence (control tissues) and presence of glaucine (10 μM; 30 min preincubation) as outlined by Naline et al. (1996). In another group of experiments, cumulative concentration-response curves to glaucine, rolipram or forskolin were obtained in preparations with spontaneous tone, in the absence (control tissues) and presence of H-89 (5 μM, 30 min preincubation).
Changes in force were measured from isometric recordings and expressed in g weight. The maximum response (Emax) induced with each contractile or relaxant agent was expressed as a percentage of the response to ACh (1 mM) or theophylline (1 mM), respectively. The molar concentration required to produce 50% (EC50) of maximal response was calculated from concentration-response curves and transformed into −log values (i.e. pD2). To assess the inhibition by glaucine of the concentration-response curves to Ca2+, the pD'2 values were calculated according to Van Rossum (1963).
PDE activity
These experiments were carried out as previously described (Cortijo et al., 1993). Individual human bronchi were homogenized in 5 volumes of ice-cold buffer A (composition in mM: bis-Tris ([bis(2-hydroxyethyl)imino]-tris(hydroxymethyl)methane) 20, sodium acetate 50, benzamidine 2, EDTA 2, β-mercaptoethanol 5, and phenylmethylsulphonylfluoride (PMSF) 0.05; pH 6.5). The homogenate was centrifuged (15,000×g, 10 min) and the supernatant injected into a Mono-Q HR 5/5 column (Pharmacia) attached to an FPLC chromatography system. The PDEs were eluted against a sodium acetate gradient (50–1000 mM). Fractions of 0.5 ml were collected, analysed and stored as previously described (Gristwood et al., 1992). The cyclic nucleotide PDE isoenzymes were identified according to the nomenclature proposed by Beavo et al. (1994).
Cyclic nucleotide PDEs were assayed following the procedure of Thompson & Strada (1984). The standard incubation mixture contained, in a final volume of 400 μl, Tris-HCl 40 mM, MgCl2 5 mM, β-mercaptoethanol 3.75 mM, 1 μM 3H-labelled/unlabelled cyclic nucleotide (∼200,000 d.p.m.) and glaucine. Substrate was cyclic AMP or cyclic GMP as appropriate. The assay was initiated by adding 100 μl of the enzyme solution to the standard incubation mixture and the reaction was carried out at 30°C for 20 min. The cyclic AMP PDE activity was also determined in the presence of either Ca2+ (10 μM)/calmodulin (1.2 μM) or cold cyclic GMP (5 μM). PDE3 and PDE4 activities were determined in the presence of 10 μM rolipram and 10 μM SKF94120, respectively (Cortijo et al., 1996). The kinetic analysis of the inhibition of PDE4 activity exerted by glaucine was carried out at different substrate and drug concentrations by means of the Lineweaver-Burk and Dixon plots as previously described (Collado et al., 1998).
Content of cyclic AMP
Bronchial rings (∼0.5 g; 1–2 mm inner diameter) were denuded of epithelium, equilibrated in Krebs solution gassed with 5% CO2 in O2 at 37°C (pH 7.4) for 90 min, and then exposed for 20 min to glaucine (10 μM) or its vehicle followed by addition of isoprenaline (10 μM) or its vehicle for 10 min. Next, tissues were rapidly removed, blotted, snap-frozen in liquid nitrogen, and stored at −80°C. The tissues (∼0.5 g in 1 ml of cold 10% trichloroacetic acid) were homogenized (6×10 s bursts) and centrifuged (600×g for 15 min at 4°C) as outlined by Fujii et al. (1998). The soluble fraction was stored at −20°C until assay for cyclic AMP content. The residual precipitation was used for the measurement of protein content (Lowry et al., 1951). The amount of cyclic AMP was estimated by enzyme immunoassay kit (RPN 225; Amersham Life Sciences, U.K.) following the instructions of the manufacturer without acetylation.
Intracellular Ca2+ levels
Primary cultures of human trachealis muscle cells were prepared, and measurements of [Ca2+]i performed as described previously (Cortijo et al., 1997). Briefly, fluo-3/AM (2 μM) loaded cells were prepared as 106 cells ml−1. Histamine (100 μM) was added in a volume of 20 μl to 1 ml cell suspension, and the changes were monitored for 3 min in the absence and presence of glaucine (10–300 μM; 3 min incubation).
Human polymorphonuclear leukocytes
Isolation of human polymorphonuclear leukocytes (PMNs)
Human blood from healthy donors was obtained in heparin, and PMNs were separated by standard laboratory procedures (Böyum, 1968). The purity of PMNs was about 95% and the viability as measured by trypan blue exclusion was >95%.
PDE activity
The method described by Schudt et al. (1991) was followed with modifications. A suspension of 107 cells ml−1 of buffer A (composition as indicated for bronchial muscle) was homogenized by sonication (6×10 pulses), centrifuged (15,000×g, 20 min), and the supernatant was applied to a Mono-Q column attached to an FPLC system. The procedure for separation of PDE isoenzymes and assay of PDE activity was as outlined for human bronchus.
Content of cyclic AMP
The protocol outlined by Collado et al. (1998) was followed. Freshly prepared human PMNs (107 cells ml−1), resuspended in HBSS containing Ca2+ and Mg2+, were incubated with glaucine (10 μM) or its vehicle for 10 min at 37°C, followed by FMLP (1 μM), isoprenaline (10 μM) or vehicle for a further 2 min; experiments were terminated by the addition of two volumes of cold ethanol. The samples were centrifuged (2000×g, 15 min, 4°C) and the supernatant transferred to a clean tube. The samples were dried by gassing with nitrogen at 60°C and the pellet was resuspended in water. Cyclic AMP was quantified by use of an enzyme-immunoassay kit as indicated for bronchial muscle.
Superoxide anion generation
Release of superoxide from PMNs was measured as previously described (Sedgwick et al., 1988). In brief, generation of superoxide was measured as the superoxide dismutase (SOD)-inhibitable reduction of ferricytochrome c with a modified microassay. With 96-well microtiter plates and a 200 μl reaction volume, 1×105 cells were added to 100 μmol l−1 of cytochrome c in HBSS and 5 μg ml−1 of cytochalasin B. Pre-incubation with glaucine (1 μM–1 mM, 5 min, 37°C) or its vehicle was carried out; then, cells were incubated with FMLP (30 nM), PMA (3 nM), serum-opsonized zymosan (SOZ, 0.5 mg ml−1) or the calcium ionophore A23187 (1 μM). In separate experiments, the effects of glaucine and rolipram against FMLP (30 nM)-induced superoxide generation were examined in the absence and presence of H-89 (5 μM, added 5 min before the addition of each PDE4 inhibitor). Superoxide generation was expressed as nmol of cytochrome c reduced per 5×105 cells per time (min) minus SOD (20 μg ml−1; approximately 2000 units mg−1 protein) control. Glaucine-induced reduction was expressed as per cent inhibition of control response measured at 60 min for each stimulus (absorbance at 550 nm in a Microplate Autoreader EL309, Bio-Tek Instruments). Any direct interaction of glaucine (up to 1 mM) with superoxide or the detecting reaction was excluded by measuring superoxide production in a cell-free system (Gillissen et al., 1997; data not shown).
Elastase release
Release of elastase from PMNs was measured by a spectrofluorometric method as previously described (De Vries et al., 1990). Cell suspensions (2×106 ml−1) were incubated for 5 min at 37°C in the absence and presence of glaucine, then FMLP (30 nM) was added and fluorescence recorded. Glaucine (3 mM) had no direct effect on enzyme activity (data not shown).
Quantification of leukotriene B4
These experiments were carried out as described previously (Cortijo et al., 1996). Cell suspensions (107 cells ml−1) were incubated with glaucine or its vehicle for 7 min, then thimerosal (20 μM) was added for 3 min followed by addition of FMLP (30 nM) for 5 min. This protocol was derived from Hatzelmann et al. (1990) who demonstrated that the addition of thimerosal enhances the response of PMNs in vitro towards FMLP. Incubations were terminated by immersion of the tubes in ice and the addition of three volumes of ice-cold methanol. Cells were pelleted by centrifugation (1500×g, 20 min, 4°C). The methanolic supernatants (containing LTs released by cells) and extracts of cell pellets (containing LTs retained intracellularly) were evaporated to dryness in a speed vacuum concentrator, and stored at −80°C. Leukotriene B4 was quantified by enzymeimmunoassay (EIA) as described by the manufacturer of the kit (Biotrak, RPN 223, Amersham Int., U.K.).
Intracellular Ca2+-levels
Measurement of [Ca2+]i was performed as previously described (Cortijo et al., 1996). Cell suspensions (107 cells ml−1) were loaded with fluo-3/AM 2 μM for 45 min at 37°C, then washed and resuspended (107 cells ml−1) for incubation with glaucine or its vehicle (7 min, 37°C); thimerosal (20 μM) was added for 3 min followed by addition of FMLP (30 nM) for 5 min. The fluorescence intensity and intracellular Ca2+ concentration were estimated as indicated above for cultured airway smooth muscle. The initial peak and the area under the curve (AUC1–5 min) were measured.
Platelet aggregation induced by activation of human PMNs
In these experiments, blood was collected as for PMN preparation and then the protocol described by Renesto et al. (1991) was followed to obtain a PMN-platelet cooperation system. Platelet aggregation was studied with a Single Chrono-Log Aggregometer (Chrono-Log-Corp., Hevertown, PA, U.S.A.) in the absence and presence of glaucine. FMLP (0.5 μM) was then added to activate PMNs, and 3 min later, a stopping solution (composition, mM: EDTA 38.5, NaCl 68.8, formaldehyde 2.05%) was added. In separate experiments carried out with human platelets, aggregation was induced with ADP (20 μM) in the absence or presence of glaucine (1 mM). Aggregation was expressed as the percentage of change in light transmission.
Human eosinophils
Isolation of human eosinophils
PMN preparation was obtained as indicated above and eosinophils were separated by depletion of neutrophils with anti-CD16 coated magnetic microbeads using the magnetic cell separation system (MACS; Miltenyi Biotec, Bergisch-Gladbach, Germany) according to the method of Hansel et al. (1991); eosinophils of greater than 98% purity were used in all functional experiments.
Superoxide anion production
Generation of superoxide by eosinophils was measured as indicated above for human PMNs. Eosinophils (1×105 cells in 200 μl reaction volume and 96-well microtiter plates) were stimulated with SOZ (0.5 mg ml−1) in the absence and presence of glaucine.
Eosinophil peroxidase release
Release of EPO was measured as previously outlined (Munoz et al., 1994). Aliquots of 105 cells in 100 μl were loaded onto microplate wells. Pre-incubation (30 min, 37°C) with glaucine or its vehicle was carried out; then cells were activated with FMLP (1 μM, plus 5 μg ml−1 of cytochalasin B). The substrate solution (0.1 mM o-phenylenediamine dihydrochloride in 0.05 M Tris-HCl containing 0.1% Triton X-100 and 1 mM H2O2) was added to wells and the plate incubated (30 min, 37°C) before stopping the reaction (4 M sulphuric acid). The absorbance was then determined at 492 nm using a Microplate Autoreader (EL309, Bio-Tek Instruments). The EPO release was expressed in peroxidase units/106 cells as determined from comparison with a standard curve.
Binding to the [3H]-rolipram binding site from rat brain cortex
The binding of [3H]-rolipram to rat brain membranes was performed as previously outlined (Collado et al., 1998). At least six drug concentrations were assayed in duplicate to generate individual displacement curves.
Drugs and solutions; statistical analysis of results
Drug concentrations are expressed in terms of the molar concentration of the active species. Rolipram and SKF94120 were synthesized at the Department of Chemistry (Almirall-Prodesfarma, Barcelona, Spain); (+)-glaucine was from Sigma-Aldrich Química, S.A. (Madrid, Spain). H-89 N-[2- p-bromocinnamylamino)ethyl] - 5 -isoquinoline-sulphonamide) was from Calbiochem (Nottingham, U.K.). [8-3H]-adenosine 3′:5′-cyclic monophosphate and [8-3H]-guanosine 3′:5′-cyclic monophosphate were from Amersham International (U.K.). Fluo-3 acetoxymethyl ester (fluo-3/AM) was from Molecular Probes Inc. (Eugene, OR, U.S.A.). Racemic [3H]-rolipram was a special preparation made by Amersham and had a specific activity of 15.8 Ci mmol−1. All other drugs and chemicals used were from the same sources previously stated (Cortijo et al., 1993; 1996; Collado et al., 1998). Water purified on a Milli-Q (Millipore Iberica, Madrid, Spain) system was used throughout. Opsonized zymosan was prepared by incubating zymosan A for 30 min at 37°C in human serum. A stock solution of FMLP was prepared in dimethylsulphoxide. Stock solutions of SKF94120 and rolipram were prepared in 20% polyethyleneglycol 300. Ascorbic acid (1 μg ml−1) was added to the isoprenaline solutions.
Data are presented as mean±s.e.mean of n experiments. In biochemical experiments, the effect of glaucine was expressed as per cent inhibition, and IC50 values were calculated from the concentration-inhibition curves by non-linear regression analysis. Statistical analysis of results was carried out by analysis of variance (ANOVA) followed by Bonferroni test or by Student's t-test as appropriate (GraphPad Software Inc., San Diego, U.S.A.). Significance was accepted when P<0.05.
Results
Human isolated bronchus and cultured airway smooth muscle cells
Relaxant activity of glaucine in human bronchus
Glaucine (0.1 μM–1 mM) caused concentration-dependent inhibition of both the spontaneous and histamine (0.1 mM)-induced tone of human isolated bronchus as shown in Figure 1A. The initial resting tension was 1.17±0.09 g weight, and active tension generated by histamine (0.1 mM) was 1.41±0.2 g weight (n=12 preparations from five patients in each group). Maximal relaxation produced by glaucine was near to full relaxation (Emax values were 92.6±1.3% and 87.8±1.2% of theophylline 1 mM for spontaneous and histamine-induced tone, respectively; theophylline caused a smaller relaxation in resting (0.76±0.10 g weight) than in precontracted (1.66±0.17 g weight) tissues; n=12 preparations obtained from five patients in each group). The sensitivity to relaxation by glaucine did not change between resting and precontracted tissues (pD2 values were 4.58±0.04 and 4.49±0.05 for spontaneous and histamine-induced tone, respectively) although the −log concentration of glaucine causing 50% of the maximal relaxation to theophylline in resting tissues was slightly greater than the corresponding values in precontracted tissues (4.47±0.03 vs 4.31±0.04, respectively; P<0.05).
Figure 1.
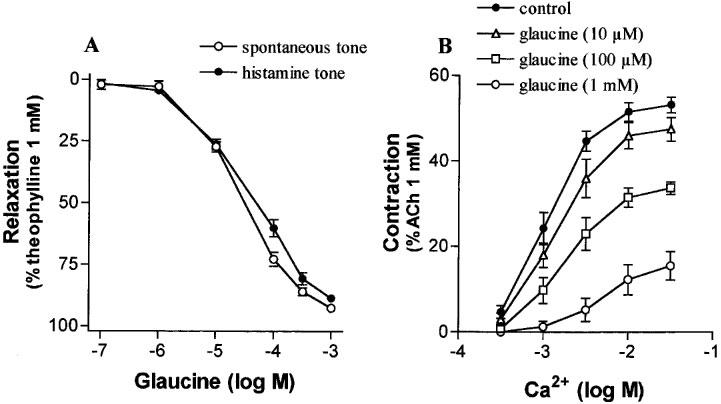
(A) Relaxant effects of glaucine in human isolated bronchi with spontaneous or histamine (0.1 mM)-induced tone. (B) The contractile response to Ca2+ in K+-depolarized human isolated bronchus, in the absence and presence of glaucine, as indicated. Points are means±s.e.mean. n values are 12 preparations from five patients (A) and four preparations from four patients (B).
Glaucine depressed in a concentration-related manner the concentration-response curve to Ca2+ in potassium-depolarized tissues (Figure 1B). The pD2 values of Ca2+ were scarcely affected (2.98±0.11, 2.89±0.16, 2.77±0.15, and 2.30±0.25 in the absence and presence of 0.01, 0.1 or 1 mM glaucine, respectively; n=4 experiments in each group from four patients) but maximal contraction was significantly inhibited (pD'2=3.62±0.06).
After incubation with glaucine (10 μM; this concentration caused 26±4% inhibition of spontaneous tone), ACh (0.1 mM) contracted bronchial rings to a plateau level (1.4±0.2 g weight) which did not significantly differ from the plateau contraction obtained in control tissues (1.3±0.3 g weight). Isoprenaline relaxed preparations with potency and maximal effect which did not significantly differ between control (pD2=7.09±0.07, Emax=78±7%; five preparations from three patients) and glaucine-treated preparations (pD2=7.12±0.06, Emax=85± 6%; five preparations from three patients). Glaucine (10 μM) did not alter the concentration-response curves to sodium nitroprusside obtained in ACh pre-contracted tissues (pD2 and Emax values were 5.48±0.07 and 58±3%, and 5.59±0.11 and 63±5%, in control and glaucine-treated tissues, respectively; 3–4 preparations from three patients).
Effects of glaucine on histamine-induced [Ca2+]i changes in cultured human airway smooth muscle cells
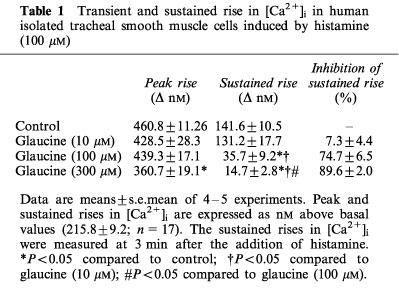
Histamine (100 μM) produced an initial rapid increase in [Ca2+]i to a peak followed by rapid decline to a sustained level above baseline values (Table 1). Glaucine scarcely affected the initial [Ca2+]i elevation but inhibited the sustained phase (−log IC50=4.31±0.07).
Table 1.
Transient and sustained rise in [Ca2+]i in human isolated tracheal smooth muscle cells induced by histamine (100 μM)
Effects of glaucine on the human bronchial PDEs and cyclic AMP content
Glaucine preferentially inhibited PDE4 and was about one log unit less active against calmodulin stimulated PDE (PDE1) and cyclic GMP stimulated PDE (PDE2) with minor inhibition of other activities like PDE3 and PDE5 (Table 2). The mechanism of inhibition by glaucine of PDE4 activity was characterized in two independent assays which produced similar results. In Figure 2, a Lineweaver-Burk plot is presented to illustrate the variation of 1/Vmax (y-axis intercept) and 1/KM (x-axis intercept) for this enzyme as a function of glaucine concentration. KM was barely affected by glaucine, whereas Vmax was concentration-dependently reduced by the drug. This indicates that glaucine acted as non-competitive inhibitor of PDE4. A value for Ki of 3.4 μM was obtained with the Dixon plot (not shown), which is in agreement with the IC50 values reported in Table 2. Under the same experimental conditions, rolipram behaved as a competitive inhibitor of PDE4 (data not shown).
Table 2.
Inhibition by glaucine of cystolic cyclic nucleotide phosphodiesterase activities isolated from human bronchus and polymorphonuclear leukocytes (PMNs)
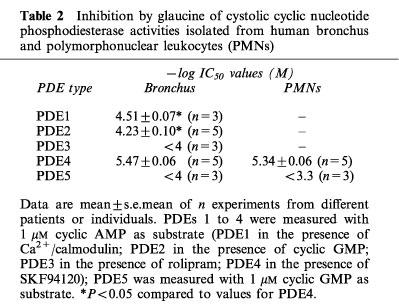
Figure 2.
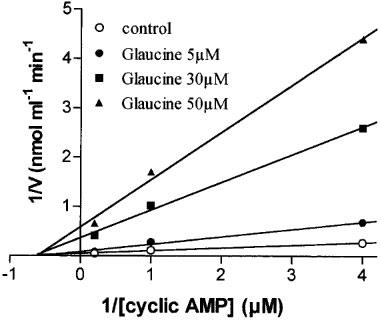
Kinetic analysis of the effect of glaucine on PDE4 cyclic AMP hydrolysis shown as Lineweaver-Burk plot. Data are the values obtained from a representative experiment run in duplicate.
Isoprenaline significantly increased cyclic AMP content in human bronchial preparations from basal values of 9.8±0.7 up to 29.7±3.1 pmol mg−1 protein (P<0.05, n=5 in each group). Glaucine (10 μM) did not significantly increase the resting levels of cyclic AMP (12.7±1.2 pmol mg−1 protein; n=5) but augmented the isoprenaline-stimulated cyclic AMP accumulation (45.2±3.9 pmol mg−1 protein; n=5, P<0.05 from values in the absence of glaucine).
Human isolated PMNs and purified eosinophils
Effects of glaucine on the PDE activity and cyclic AMP content of human PMNs
As observed for human bronchial PDEs, glaucine displayed selectivity for PDE4 with little effect on PDE5 (Table 2). Other PDE isoenzymes were not found in the preparations examined (data not shown).
Basal levels of cyclic AMP in unstimulated human PMNs were 381±11 fmol/106 cells (n=5). Separate addition of FMLP (1 μM) or glaucine (10 μM) failed to increase the cell content of cyclic AMP (432±22 and 445±33 fmol/106 cells, respectively; n=5 in each group) but their combination raised significantly the cyclic AMP levels (876±35 fmol/106; n=5). Isoprenaline (10 μM) alone increased cyclic AMP content (792±32 fmol/106 cells; n=5, P<0.05 from basal values), and glaucine (10 μM) augmented further the isoprenaline-induced accumulation of cyclic AMP (1329±88 fmol/106 cells; n=5; P<0.05 from isoprenaline control).
Influence of glaucine on FMLP-, SOZ-, PMA- and A 23187-induced superoxide release
Stimulation of PMNs with FMLP (30 nM), PMA (3 nM), SOZ (0.5 mg ml−1), and A 23187 (1 μM) produced similar levels of superoxide anion generation (7.74±1.44, 11.04±0.87, 8.42±0.53, and 7.48±0.46 nmoles cytochrome c reduction/5×105 cells, respectively; n=5–6, P>0.05). Glaucine reduced the superoxide anion generation produced by these different stimuli in a concentration-dependent manner (Figure 3). The order of potencies of glaucine as inhibitor of these stimuli was A 23187 (5.13±0.19)⩾FMLP (4.76±0.17)>PMA (3.87±0.04)⩾SOZ (3.60±0.07).
Figure 3.
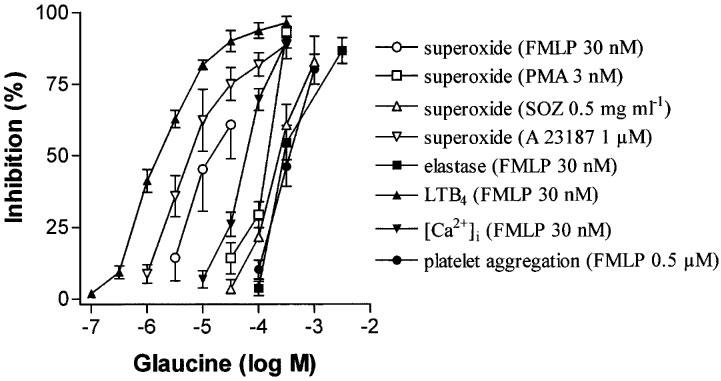
Inhibitory effects of glaucine on different functional responses of human polymorphonuclear leukocytes (PMN). Superoxide generation was obtained in cells stimulated by N-formyl-Met-Leu-Phe (FMLP), phorbol myristate acetate (PMA), serum-opsonized zymosan (SOZ), and calcium ionophore A23187. In addition, FMLP elicited elastase release, leukotriene B4 (LTB4) production, and an intracellular [Ca2+]i signal (assessed as AUC1–5 min). Platelet aggregation was produced by FMLP in a PMN-platelet system after activation with FMLP. Data are derived from 5–6 PMN preparations from different individuals and data are given as mean±s.e.mean.
Influence of glaucine on FMLP-induced elastase release
Incubation of human PMN in the presence of FMLP (30 nM; ∼EC50; Cortijo et al., 1996) led to release of elastase (53.4±6.7% of total elastase content; n=5). Glaucine reduced FMLP (30 nM)-induced elastase release in a concentration-dependent manner as shown in Figure 3 (−log IC50=3.53±0.03).
Influence of glaucine on FMLP-induced leukotriene B4 production
PMNs stimulated by FMLP (30 nM) in the presence of thimerosal (20 μM) produced an increase in LTB4 levels of 397±37 ng 107 cells−1 (n=5). The production of LTB4 promoted by FMLP plus thimerosal was sensitive to the addition of glaucine in a concentration-dependent fashion (Figure 3; −log IC50=5.85±0.07).
Influence of glaucine on FMLP-induced increase of intracellular Ca2+ levels
Baseline values of [Ca2+]i were 198±22 nM (n=5). Addition of FMLP (30 nM) resulted in a rapid initial increase in intracellular Ca2+ concentration (peak increase of [Ca2+]i above baseline was 705±30 nM; n=5) followed by a sustained oscillating elevation. The value of the initial peak of intracellular Ca2+ was not significantly (P>0.05) affected by glaucine (not shown) but the later phase of sustained elevation of intracellular Ca2+ (assessed as AUC1–5 min) was reduced in a concentration-related manner (Figure 3; −log IC50=4.22± 0.03).
Effect of glaucine on human platelet aggregation induced by activation of PMNs
Glaucine produced a concentration-dependent inhibition of platelet aggregation induced by FMLP (30 nM)-stimulated PMNs (Figure 3; −log IC50=3.43±0.05; n=5). This effect was due to inhibition of PMN function since glaucine (1 mM) had no effect on platelet aggregation promoted by ADP (20 μM) in the absence of PMNs.
Effect of glaucine on superoxide generation and eosinophil peroxidase release by human eosinophils
Purified eosinophils generate superoxide anions in response to SOZ (0.5 mg ml−1). This superoxide production was scarcely affected by glaucine (up to 3 mM) as shown in Figure 4A. Activation of purified eosinophils with FMLP (1 μM) caused an augmented release of EPO into the supernatants. Glaucine produced a concentration-related inhibition of EPO release (Figure 4B) with −log IC50 values of 3.74±0.17 (n=5).
Figure 4.
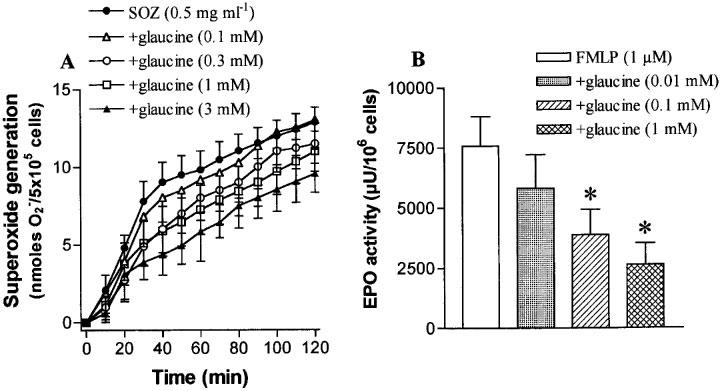
The effects of glaucine on superoxide generation by serum-opsonized zymosan (SOZ; (A)) and eosinophil peroxidase (EPO) release by N-formyl-Met-Leu-Phe (FMLP; (B)) in human purified eosinophils. Data are derived from 5–6 different PMN preparations and given as means±s.e.mean. *P<0.05 compared to control values.
Cyclic AMP-dependent protein kinase (PKA) inhibition experiments
In these experiments we used the potent, selective, and membrane permeant, PKA inhibitor, H-89 (Chijiwa et al., 1990). The concentration used of H-89 was greater than 1 μM as outlined by Linde & Quast (1995). In human isolated bronchus, inhibition of PKA by H-89 (5 μM) failed to antagonize the relaxant responses to glaucine (−log EC50 values were 4.24±0.25 and 3.92±0.23 in the absence and presence of H-89, respectively; n=5) and rolipram (EC50 values not shown) in preparations with spontaneous tone (Figure 5A). Confirmation that H-89 was blocking PKA was obtained from the results with forskolin where treatment with H-89 produced a rightward shift of the concentration-relaxation curve to this drug (Figure 5A; 6.02 fold shift of EC50). In human PMNs, H-89 (5 μM) antagonized the inhibitory effect of glaucine (−log EC50 values were 5.11±0.13 and 3.99±0.07 in the absence and presence of H-89, respectively; n=5, P<0.05) and markedly depressed that of rolipram on FMLP-induced superoxide release (Figure 5B).
Figure 5.
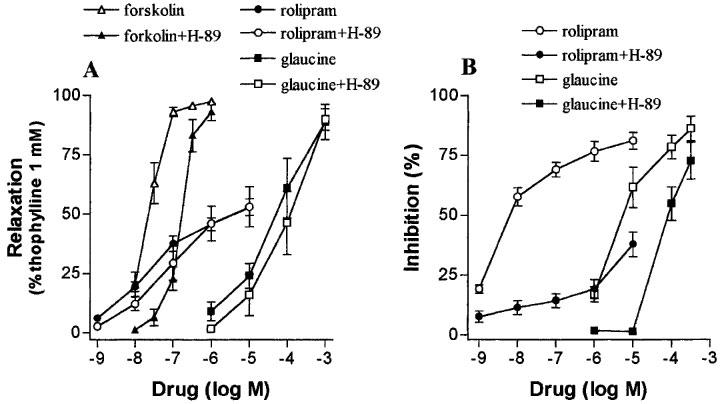
The effect of H-89 (5 μM), a selective protein kinase A inhibitor, on the functional responses to glaucine in human isolated bronchus (A) and human polymorphonuclear leukocytes (B). The effect of H-89 on the responses to rolipram and forskolin is also shown for comparison. (A) Concentration-relaxation curves for glaucine, rolipram and forskolin in preparations with spontaneous tone. H-89 produced a significant (P<0.05) rightward shift of the curve to forskolin without significantly affecting those to glaucine and rolipram. (B) Concentration response curves for rolipram and glaucine as inhibitors of the superoxide generation elicited by FMLP (30 nM). H-89 antagonized the response to these two phosphodiesterase 4 inhibitors. Data are derived from 4–5 preparations from five patients (A) and from 3–4 different PMN preparations (B) and given as means±s.e.mean.
Displacement of [3H]-rolipram from rat cortex membranes
As shown in Figure 6, glaucine displaced [3H]-rolipram from its binding site with a potency (−log IC50=4.04±0.06) lower than that shown as inhibitor of PDE4 activity (∼5.5 as shown in Table 2; PDE4/binding site ratio of ∼0.04 fold), whereas the opposite was found for rolipram (−log IC50 values were 8.41±0.04 and 6.67±0.05 for displacement of binding and for PDE4 inhibition, respectively; PDE4/binding site ratio of 55 fold).
Figure 6.
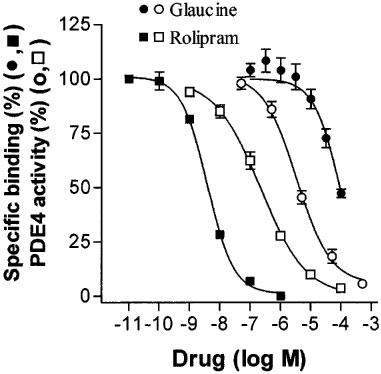
Inhibition of displacement of [3H]-rolipram binding from its high affinity binding site in rat brain by glaucine and rolipram. For comparison, the inhibition by glaucine and rolipram of PDE4 activity isolated from human PMNs is shown as indicated. Data of displacement of binding by glaucine correspond to two independent assays run in duplicate; data of enzyme inhibition by glaucine correspond to experiments of Table 2; data of rolipram correspond to a representative assay run in duplicate.
Discussion
Inhibition of PDE4 activity and displacement of [3H]-rolipram from its high-affinity binding site by glaucine
The present study demonstrated that glaucine inhibits soluble PDE4 isolated from human bronchus and human PMNs (Ki=3.4 μM) whereas its potency as inhibitor of other PDE isoenzymes, in particular PDE3 and PDE5, was much lower. Therefore, our data indicate that glaucine is a relatively selective inhibitor of PDE4 in human bronchial tissue and granulocytes. These results are consistent with previous findings in bovine aorta (Ivorra et al., 1992). In addition, we found that the kinetic mechanism of inhibition of PDE4 was of non-competitive nature. This type of enzyme inhibition has been also reported for other selective PDE4 inhibitors (Cohen et al., 1996; Collado et al., 1998).
A low ratio between the potencies at the PDE4 catalytic site and at the high-affinity [3H]-rolipram binding site in rat brain is considered of interest for new PDE4 inhibitors with anti-asthma activity (Torphy, 1998). Thus, the archetypal PDE4 inhibitor, rolipram, has a PDE4/binding site ratio of ∼50 fold (Collado et al., 1998; this study) or greater (Torphy, 1998) whilst second-generation PDE4 inhibitors (RP 73401, SB 207499) inhibit PDE4 activity and the high-affinity rolipram binding site with similar potencies (Torphy, 1998). In this study we found that glaucine is some 10 fold less potent than rolipram at the catalytic site of PDE4 but four orders of magnitude less potent than rolipram to interact with the high-affinity binding site for [3H]-rolipram. The very low PDE4/binding site ratio found for glaucine may account for the absence of vomiting in its past clinical use (Dierckx et al., 1981), and makes this aporphinoid alkaloid an interesting compound for further structure-activity studies.
Relaxation of human isolated bronchus by glaucine
Glaucine inhibited resting and histamine-evoked tension with pD2 ∼4.5 and maximal relaxation close to that of theophylline (1 mM). Since the cyclic AMP hydrolysing isoenzymes that mainly regulate human bronchial tone are PDE3 and PDE4 (Torphy, 1998), the inhibition of PDE4 by glaucine could be envisaged as the mechanism underlying its bronchorelaxing effect. However, glaucine failed to potentiate the isoprenaline-induced relaxation although it augmented cyclic AMP accumulation produced by this β-adrenoceptor agonist (this study). Furthermore, we found that H-89, a selective PKA inhibitor (Chijiwa et al., 1990), did not antagonize the bronchial relaxant response to glaucine but inhibited the responses to forskolin, an agent that activates adenylyl cyclase directly and, in contrast to β-adrenoceptor agonists, operates solely via cyclic AMP-dependent mechanisms (Torphy, 1994).
When comparing the effects of glaucine on human isolated bronchus with those of the selective PDE4 inhibitor, rolipram, we found that the relaxant response to rolipram was not inhibited either by H-89, which is consistent with results in vascular smooth muscle (Eckly-Michel et al., 1997). However, rolipram augmented basal and isoprenaline-stimulated cyclic AMP levels in human airway smooth muscle (Hall et al., 1992) and potentiates isoprenaline-induced relaxation of human isolated bronchus (Qian et al., 1993). Similar effects have been described for other selective PDE4 inhibitors (Fujii et al., 1998; Naline et al., 1996). Therefore, it seems unlikely that glaucine exerted its relaxant effects primarily or exclusively via inhibition of PDE4 activity in human bronchus but a contribution of this mechanism cannot be completely excluded. Furthermore, we found no basis for the contribution of cyclic GMP PDE inhibition to the relaxant effects of glaucine.
Glaucine is a non-selective antagonist of α-adrenoceptors (Orallo et al., 1993). However, functional responses to agonists and antagonists of α-adrenoceptors in human isolated bronchus are weak (Black & Armour, 1986), and EC50 values of glaucine for relaxing human bronchus are well above its potency values at α-adrenoceptors (Ki∼0.3 μM, Ivorra et al., 1992). Alternatively, bronchial relaxation by glaucine may be attributed to its blocking properties at the benzothiazepine site of Ca2+-channels. Airway smooth muscle cells possess voltage-operated Ca2+ channels sensitive to Ca2+ antagonists, and these blockers, including diltiazem, inhibit the spontaneous tone of this preparation (Cortijo et al., 1997). The potency values reported for this effect of glaucine in rat aorta and vas deferens are in the range of 10–100 μM (Ivorra et al., 1992; Orallo et al., 1993) which is in the same order of magnitude as its potency values as relaxant of human isolated bronchus and as antagonist of calcium-induced contraction (this study).
In cultured airway smooth muscle cells, the initial rise of [Ca2+]i to a peak in response to histamine is due to intracellular Ca2+ release but the subsequent sustained phase depends on extracellular Ca2+ influx through pathways that are not sensitive to organic Ca2+ channel antagonists (Murray & Kotlikoff, 1991). At concentrations producing effective relaxation of human bronchus, glaucine scarcely affected the peak [Ca2+]i response to histamine but markedly depressed the sustained [Ca2+]i level. This finding suggests that glaucine scarcely affects intracellular Ca2+ release but interferes with the Ca2+ entry that follows depletion of intracellular stores, which is consistent with data from rat aorta (Ivorra et al., 1992).
Taken together, the results from this part of the study indicate that Ca2+ channel antagonism appears as the main mechanism responsible for the relaxation produced by glaucine in human isolated bronchus.
Inhibitory effects of glaucine on human polymorphonuclear leukocytes
PDE4 is the major isoenzyme present in human PMNs and its inhibition leads to elevation of cyclic AMP levels and the subsequent inhibition of a number of functional responses (Schudt et al., 1991). The functional relevance of the PDE4 inhibition produced by glaucine was demonstrated in this study by the finding that glaucine (10 μM) augmented cyclic AMP levels in FMLP-activated human PMNs, and enhanced also the cyclic AMP accumulation produced by isoprenaline. Furthermore, the inhibitory effect of glaucine against superoxide generation elicited by FMLP was antagonized by H-89, a selective PKA inhibitor that also depressed the inhibitory response produced by rolipram in the same preparation.
Consistent with these results, glaucine inhibited a wide array of functional responses of human PMNs activated by FMLP. The potency values of glaucine as inhibitor of superoxide generation, elastase release, [Ca2+]i signal, and platelet aggregation were one to two orders of magnitude lower than its potency as PDE4 inhibitor. Similar differences in potencies have been reported for second-generation PDE4 inhibitors (Souness et al., 1995; Barnette et al., 1998). However, glaucine potently inhibited LTB4 production, a response mainly mediated by extracellular Ca2+ entry (Hatzelmann et al., 1990). To extend further the observations made with FMLP, we examined the responses of human PMNs to SOZ, PMA, and A23187. Glaucine was less potent as inhibitor of SOZ- and PMA-induced superoxide release than against A23187, which may reflect a greater sensitivity of Ca2+-mediated signal transduction mechanisms to cyclic AMP compared to those involved in a phagocytic stimulus or in protein kinase C-mediated pathways.
Glaucine was scarcely effective as inhibitor of SOZ-induced superoxide generation in human eosinophils (−log IC50 <2.5). Consistent with these findings, Hatzelmann et al. (1995) reported that PDE3/4 inhibitors failed to influence the formation of reactive oxygen species in eosinophils activated by different stimuli including FMLP and SOZ. We examined also the effect of glaucine on the release of EPO, a marker of granular secretion and a cytotoxic product (Munoz et al., 1994). Glaucine inhibited FMLP-induced EPO release with a potency value within the range of its inhibitory potency against other functional responses of human PMNs activated by FMLP. Munoz et al. (1994) demonstrated that salbutamol decreased EPO release from FMLP-activated eosinophils but PDE4 inhibitors were not studied. These results indicate that glaucine may have some inhibitory effects on functional responses of human eosinophils.
A role for cyclic GMP in regulating PMN function is still debated (Torphy, 1998); therefore, any contribution of cyclic GMP PDE inhibition to the effects of glaucine seems unlikely. Taken together, the results obtained in human granulocytes point to PDE4 inhibition and interference of Ca2+ entry as the main mechanisms involved in the inhibitory action exerted by glaucine. This latter action is not exerted at benzothiazepine sites since voltage-operated Ca2+ channels are not present in PMNs (Rosales & Brown, 1992). The inhibitory effect of glaucine is unrelated to blockade of α-adrenoceptors since they are not present in neutrophils (Musgrave & Seifert, 1994).
In conclusion, glaucine is a relatively selective, non-competitive, inhibitor of PDE4, with a very low potency at the high-affinity rolipram binding site. Ca2+ channel antagonism by glaucine appears mainly responsible for the relaxant effect of glaucine in human isolated bronchus while PDE4 inhibition surely contributes to the inhibitory effects of glaucine in human peripheral blood granulocytes. The very low PDE4/binding site ratio found for glaucine is of potential interest in asthma but further research will be necessary to find out the structural requirements for more potent inhibition of PDE4 with less contribution of other activities.
Acknowledgments
The present work was supported in part by grants SAF96-0200 and SAF97-0047 from CICYT (Comision Interministerial de Ciencia y Tecnología; Spanish Government) and research and formation funds from local government (Generalitat Valenciana). The authors are indebted to the teams of the Services of Thoracic Surgery and Pathology of the Hospital Universitario La Fe and Hospital Clinico Universitario of Valencia (Spain) for making the human lung tissue available to us. The advise provided by Dr F. Orallo (University of Santiago de Compostela) and the technical assistance of Pedro Santamaria and Dora Martí is also gratefully acknowledged.
Abbreviations
- ACh
acetylcholine
- ADP
adenosine diphosphate
- AUC
area under the curve
- DMEM
Dulbecco's modified Eagle medium
- EIA
enzyme immunoassay
- EPO
eosinophil peroxidase
- FCS
foetal calf serum
- FMLP
N-formyl-L-methionyl-L-leucyl-L-phenylalanine
- HBSS
Hank's balanced salt solution
- HEPES
N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid]
- LTB4
leukotriene B4
- PBS
phosphate buffered saline
- PDE
phosphodiesterase
- PKA
cyclic AMP-dependent protein kinase
- PMA
phorbol 12-myristate 13-acetate
- PMN
polymorphonuclear leukocytes
- PMSF
phenylmethylsulphonyl fluoride
- SOD
superoxide dismutase
- SOZ
serum-opsonized zymosan
References
- ADVENIER C., NALINE E., RENIER A. Effect of Bay K 8644 on contraction of human isolated bronchus and guinea-pig isolated trachea. Br. J. Pharmacol. 1986;88:33–39. doi: 10.1111/j.1476-5381.1986.tb09468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES P.J. Clinical studies with calcium antagonists in asthma. Br. J. Clin. Pharmacol. 1985;20:289S–298S. doi: 10.1111/j.1365-2125.1985.tb02815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES P.J., IND P., DOLLERY C.T. Inhaled prazosin in asthma. Thorax. 1981;36:378–381. doi: 10.1136/thx.36.5.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNETTE M.S., CHRISTENSEN S.B., ESSAYAN D.M., GROUS M., PRABHAKAR U., RUSH J.A., KAGEY-SOBOTKA A., TORPHY T.J. SB 207499 (Ariflo), a potent and selective second-generation phosphodiesterase 4 inhibitor: in vitro anti-inflammatory actions. J. Pharmacol. Exp. Ther. 1998;284:420–426. [PubMed] [Google Scholar]
- BEAVO J.A., CONTI M., HEASLIP R.J. Multiple cyclic nucleotide phosphodiesterases. Mol. Pharmacol. 1994;46:399–405. [PubMed] [Google Scholar]
- BLACK J.L., ARMOUR C.L. α-Adrenoceptor function in asthma. Trends Pharmacol. Sci. 1986;7:303–304. [Google Scholar]
- BÖYUM A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 1968;21 suppl. 97:77–89. [PubMed] [Google Scholar]
- CHIJIWA T., MISHIMA A., HAGIWARA M., SANO M., HAYASHI K., INOUE T., NAITO K., TOSSHIOKA T., HIDAKA T. Inhibition of forskolin-induced outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC 12D Pheochromocytoma cells. J. Biol. Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- COHEN V.L., SHOWELL H.J., FISHER D.A., PAZOLES C.J., WATSON J.W., TURNER C.R., CHENG J.B. In vitro pharmacology of the novel phosphodiesterase type 4 inhibitor, CP-80633. J. Pharmacol. Exp. Ther. 1996;278:1356–1361. [PubMed] [Google Scholar]
- COLLADO M.C., BELETA J., MARTINEZ E., MIRALPEIX M., DOMENECH T., PALACIOS J.M., HERNANDEZ J. Functional and biochemical evidence for diazepam as a cyclic nucleotide phosphodiesterase type 4 inhibitor. Br. J. Pharmacol. 1998;123:1047–1054. doi: 10.1038/sj.bjp.0701698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSTANT O., SLAVIN B., LEHANE J.R., JORDAN C., JONES J.G. Effect of the antitussive glaucine on bronchomotor tone in man. Thorax. 1983;38:537–542. doi: 10.1136/thx.38.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORTIJO J., BOU J., BELETA J., CARDELÚS I., LLENAS J., MORCILLO E., GRISTWOOD R.W. Investigation into the role of phosphodiesterase IV in bronchorelaxation, including studies with human bronchus. Br. J. Pharmacol. 1993;108:562–568. doi: 10.1111/j.1476-5381.1993.tb12841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORTIJO J., VILLAGRASA V., MARTÍ-CABRERA M., VILLAR V., MOREAU J., ADVENIER C., MORCILLO E.J., SMALL R.C. The spasmogenic effects of vanadate in human isolated bronchus. Br. J. Pharmacol. 1997;121:1339–1349. doi: 10.1038/sj.bjp.0701277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORTIJO J., VILLAGRASA V., NAVARRETE C., SANZ C., BERTO L., MICHEL A., BONNET P.A., MORCILLO E.J. Effects of SCA40 on human isolated bronchus and human polymorphonuclear leukocytes: comparison with rolipram, SKF94120 and levcromakalim. Br. J. Pharmacol. 1996;119:99–106. doi: 10.1111/j.1476-5381.1996.tb15682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE VRIES G.W., AMDAHL L.D., KRAMER K.D., WHEELER L.A. Inhibition by manoalide of fMLP-stimulated elastase release from human neutrophils. Biochem. Pharmacol. 1990;40:2487–2494. doi: 10.1016/0006-2952(90)90090-8. [DOI] [PubMed] [Google Scholar]
- DIERCKX P., LEBLANC G., DECOSTER A., CRISCUOLO D. Double blind study of glaucine in chronic cough. Int. J. Clin. Pharmacol. 1981;19:396–399. [PubMed] [Google Scholar]
- ECKLY-MICHEL A., MARTIN V., LUGNIER C. Involvement of cyclic nucleotide-dependent protein kinases in cyclic AMP-mediated vasorelaxation. Br. J. Pharmacol. 1997;122:158–164. doi: 10.1038/sj.bjp.0701339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJII K., KOHROGI H., IWAGOE H., HAMAMOTO J., HIRATA N., GOTO E., KAWANO O., WADA K., YAMAGATA S., ANDO M. Novel phosphodiesterase 4 inhibitor T-440 reverses and prevents human bronchial contraction induced by allergen. J. Pharmacol. Exp. Ther. 1998;284:162–169. [PubMed] [Google Scholar]
- GILLISSEN A., JAWORSKA M., SCHÄRLING B., VAN ZWOLL D., SCHULTZE-WERNINGHAUS G. Beta-2-Agonists have antioxidant function in vitro. Respiration. 1997;64:16–22. doi: 10.1159/000196637. [DOI] [PubMed] [Google Scholar]
- GRISTWOOD R.W., BELETA J., BOU J., CARDELUS I., FERNANDEZ A.G., LLENAS J., BERGA P. Studies on the cardiac actions of flosequinan in vitro. Br. J. Pharmacol. 1992;105:985–991. doi: 10.1111/j.1476-5381.1992.tb09089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL I.P., WIDDOP S., TOWNSEND P., DAYKIN K. Control of cyclic AMP levels in primary cultures of human tracheal smooth muscle cells. Br. J. Pharmacol. 1992;107:422–428. doi: 10.1111/j.1476-5381.1992.tb12762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSEL T.T., DE VRIES J.M., IFF T., RIHS S., WANDZILAK M., BETZ S., BLASER K., WALKER C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J. Immunol. Meth. 1991;145:105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- HATZELMANN A., HAURAND M., ULLRICH V. Involvement of calicum in the thimerosal-stimulated formation of leukotriene by fMLP in human polymorphonuclear leukocytes. Biochem. Pharmacol. 1990;39:559–567. doi: 10.1016/0006-2952(90)90064-r. [DOI] [PubMed] [Google Scholar]
- HATZELMANN A., TENOR H., SCHUDT C. Differential effects of non-selective phosphodiesterase inhibitors on human eosinophil functions. Br. J. Pharmacol. 1995;14:821–831. doi: 10.1111/j.1476-5381.1995.tb13278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVORRA M.D., LUGNIER C., SCHOTT C., CATRET M., NOGUERA M.A., ANSELMI E., D'OCON P. Multiple actions of glaucine on cyclic nucleotide phosphodiesterases, α1-adrenoceptor and benzothiazepine binding site at the calcium channel. Br. J. Pharmacol. 1992;106:387–394. doi: 10.1111/j.1476-5381.1992.tb14345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASÉ Y., KAWAGUCHI M., TAKAHAMA K., MIYATA T., HIROTSU I., HITOSHI T., OKANO Y. Pharmacological studies on dl-glaucine phosphate as an antitussive. Arzneim. Forsch. 1983;33:936–946. [PubMed] [Google Scholar]
- KUKOVETZ W.R., PÖCH G. Inhibition of cyclic-3′,5′-nucleotide phosphodiesterase as a possible mode of action of papaverine and similarly acting drugs. Naunyn-Schmiedeberg's Arch. Pharmacol. 1970;267:189–194. doi: 10.1007/BF00999402. [DOI] [PubMed] [Google Scholar]
- LINDE C., QUAST U. Potentiation of P1075-induced K+ channel opening by stimulation of adenylate cyclase in rat isolated aorta. Br. J. Pharmacol. 1995;115:515–521. doi: 10.1111/j.1476-5381.1995.tb16364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O.H., ROSENBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MUNOZ N.M., VITA A.J., NEELEY S.P., MCALLISTER K., SPAETHE S.M., WHITE S.R., LEFF A.R. Beta adrenergic modulation of formyl-methionine-leucine-phenylalanine-stimulated secretion of eosinophil peroxidase and leukotriene C4. J. Pharmacol. Exp. Ther. 1994;268:139–143. [PubMed] [Google Scholar]
- MURRAY R.K., KOTLIKOFF M.I. Receptor-activated calcium influx in human airway smooth muscle cells. J. Physiol. 1991;435:123–144. doi: 10.1113/jphysiol.1991.sp018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSGRAVE I.F., SEIFERT R. Human neutrophils and HL-60 cells do not possess alpha 2-adrenoceptors. Biochem. Pharmacol. 1994;47:233–239. doi: 10.1016/0006-2952(94)90011-6. [DOI] [PubMed] [Google Scholar]
- NALINE E., QIAN Y., ADVENIER C., RAEBURN D., KARLSSON J.A. Effects of RP 73401, a novel, potent and selective phosphodiesterase type 4 inhibitor, on contractility of human, isolated bronchial muscle. Br. J. Pharmacol. 1996;118:1939–1944. doi: 10.1111/j.1476-5381.1996.tb15628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORALLO F., ALZUETA A.F., LOZA M.I., VIVAS N., BADIA A., CAMPOS M., HONRUBIA M.A., CADAVID M.I. Study of the mechanism of the relaxant action of (+)-glaucine in rat vas deferens. Br. J. Pharmacol. 1993;110:943–948. doi: 10.1111/j.1476-5381.1993.tb13904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QIAN Y., NALINE E., KARLSSON J.A., RAEBURN D., ADVENIER C. Effects of rolipram and siguazodan on the human isolated bronchus and their interaction with isoprenaline and sodium nitroprusside. Br. J. Pharmacol. 1993;109:774–778. doi: 10.1111/j.1476-5381.1993.tb13641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENESTO P., BALLOY V., VARGAFTIG B.B., CHIGNARD M. Interference of anti-inflammatory and anti-asthmatic drugs with neutrophil-mediated platelet activation: singularity of azelastine. Br. J. Pharmacol. 1991;103:1435–1440. doi: 10.1111/j.1476-5381.1991.tb09807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSALES C., BROWN E.J. Calcium channel blockers nifedipine and diltiazem inhibit Ca2+ release from intracellular stores in neutrophils. J. Biol. Chem. 1992;267:1443–1448. [PubMed] [Google Scholar]
- SCHUDT C., WINDER S., FORDERKUNZ S., HATZELMANN A., ULLRICH V. Influence of selective phosphodiesterase inhibitors on human neutrophil functions and levels of cAMP and Cai. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;344:682–690. doi: 10.1007/BF00174752. [DOI] [PubMed] [Google Scholar]
- SEDGWICK J.B., VRTIS R.F., GOURLEY M.F., BUSSE W.W. Stimulus-dependent differences in superoxide anion generation by normal human eosinophils and neutrophils. J. Allergy Clin. Immunol. 1988;81:876–883. doi: 10.1016/0091-6749(88)90945-1. [DOI] [PubMed] [Google Scholar]
- SOUNESS J.E., MASLEN C., WEBBER S., FOSTER M., RAEBURN D., PALFREYMAN M.N., ASHTON M.J., KARLSSON J.A. Suppression of eosinophil function by RP 73401, a potent and selective inhibitor of cyclic AMP-specific phosphodiesterase: comparison with rolipram. Br. J. Pharmacol. 1995;115:39–46. doi: 10.1111/j.1476-5381.1995.tb16317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULLIVAN P., BEKIR S., JAFFAR Z., PAGE C., JEFFREY P., COSTELLO J.Anti-inflammatory effects of low-dose oral theophylline in atopic asthma Lancet 19943431006–1008.(Erratum: Lancet 1994, 343, 1512) [DOI] [PubMed] [Google Scholar]
- SYNEK M., BEASLEY R., FREW A.J., GOULDING D., HOLLOWAY L., LAMPE F.C., ROCHE W.R., HOLGATE S.T. Cellular infiltration of the airways in asthma of varying severity. Am. J. Respir. Crit. Care Med. 1996;154:224–230. doi: 10.1164/ajrccm.154.1.8680684. [DOI] [PubMed] [Google Scholar]
- THOMPSON W.J., STRADA S.J.Cyclic nucleotide phosphodiesterase (PDE) Methods of Enzymatic Analysis 1984Weinheim: Verlag Chemie; 127–134.ed. Bergmayer III, H.U., Vol. IV pp [Google Scholar]
- TORPHY T.J. β-Adrenoceptors, cAMP and airway smooth muscle relaxation: challenges to the dogma. Trends Pharmacol. Sci. 1994;15:370–374. doi: 10.1016/0165-6147(94)90157-0. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J. Phosphodiesterase isozymes. Molecular targets for novel antiasthma agents (State of the art) Am. J. Respir. Crit. Care Med. 1998;157:351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- VAN INWEGEN R.G., SALAMAN P., STGEORGIER V., WEINRYB I. Dihydro and tetrahydroisoquinolines as inhibitors of cyclic nucleotide phosphodiesterases from dog heart. Biochem. Pharmacol. 1979;28:1307–1312. doi: 10.1016/0006-2952(79)90430-1. [DOI] [PubMed] [Google Scholar]
- VAN ROSSUM J.M. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch. Int. Pharmacodyn. Ther. 1963;143:299–330. [PubMed] [Google Scholar]
- WATSON N., MAGNUSSEN H., RABE K.F. The relevance of resting tension to responsiveness and inherent tone of human bronchial smooth muscle. Br. J. Pharmacol. 1998;123:694–700. doi: 10.1038/sj.bjp.0701637. [DOI] [PMC free article] [PubMed] [Google Scholar]


