Abstract
In order to investigate the role of KATP channel activation and repolarization dispersion on the ‘border zone' arrhythmias induced by ischaemia-reperfusion, the effects of glibenclamide and bimakalim, agents modifying action potential (AP) duration, were studied in an in vitro model of myocardial ‘border zone'.
The electrophysiological effects of 10 μM glibenclamide and 1 μM bimakalim (n=8 each), respectively KATP channel blocker and activator, were investigated on guinea-pig ventricular strips submitted partly to normal conditions (normal zone, NZ) and partly to simulated ischaemic then reperfused conditions (altered zone, AZ).
By preventing the ischaemia-induced AP shortening (P<0.0001), glibenclamide reduced the dispersion of AP duration 90% (APD90) between NZ and AZ (P<0.0001), and concomitantly inhibited the ‘border zone' arrhythmias induced by an extrastimulus (ES), their absence being significantly related to the lessened APD90 dispersion (χ2=8.28, P<0.01).
Bimakalim, which also reduced the APD90 dispersion (P<0.005) due to differential AP shortening in normal and ischaemic tissues, decreased the incidence of myocardial conduction blocks (25% of preparations versus 83% in control, n=12, P<0.05) and favoured ‘border zone' spontaneous arrhythmias (75% of preparations versus 25% in control, P<0.05).
During reperfusion, unlike bimakalim, glibenclamide inhibited the ES-induced arrhythmias and reduced the incidence of the spontaneous ones (12% of preparations versus 92% in control, P<0.05), this latter effect being significantly related (χ2=6.13, P<0.02) to the lessened ischaemia-induced AP shortening in the presence of glibenclamide (P<0.0001).
These results suggest that KATP blockade may protect the ischaemic-reperfused myocardium from ‘border zone' arrhythmias concomitantly with a reduction of APD90 dispersion between normal and ischaemic regions. Conversely, KATP channel activation may modify the incidence of conduction blocks and exacerbate the ischaemia-induced ‘border zone' arrhythmias.
Keywords: Cardiac muscle; ischaemia; reperfusion, action potential; arrhythmia, KATP channel
Introduction
During myocardial ischaemia, the ‘border zone' located between normal and hypoxic/ischaemic tissues (Hofman, 1985) has been described as a site which may favour the occurrence of arrhythmias such as automatic activity, focal reexcitation or reentry arrhythmia (El-Sherif et al., 1981; Duo et al., 1989). This cardiac region, presenting inhomogeneous distribution of electrophysiological properties and both anatomic and biochemical changes, has been associated with injury currents possibly responsible for the emergence of arrhythmias (Janse et al., 1980; Duo et al., 1989). Evidences have been provided for a link between the dispersion of action potential (AP) duration (APD) and the occurrence of triggered activities in sheep Purkinje fibres (Kupersmith et al., 1994) or the inducibility of monomorphic ventricular tachycardia in human heart (Yuan et al., 1995).
KATP channels are involved in several types of myocardial damage induced by ischaemia and reperfusion (Coetzee, 1992), such as myocardial conduction disturbances (Sawanabori et al., 1995), arrhythmias (Wilde, 1994), contractile failure (Bril et al., 1992), and biochemical impairment resulting in alteration of the cell membrane lipid composition (Freyss-Béguin et al., 1995). On the other hand, the ischaemia-induced KATP channel activation has been postulated as an endogenous protective mechanism (Cavero et al., 1995; Hearse, 1995), since the resulting mechanical arrest might protect myocardium from further ischaemia damage (Cole et al., 1991) by preservation of intracellular high energy phosphate (McPherson et al., 1993; Docherty et al., 1997). Nevertheless, if the activation of KATP channels may exhibit protective effects on both contractile dysfunction (Auchampach et al., 1992; Djellas et al., 1993) and infarct size (Yao & Gross, 1994; Ohno et al., 1997), it remains yet by far more controversial as regards arrhythmias induced by ischaemia and reperfusion (Siegl, 1994; Haverkamp et al., 1995; Sawanabori et al., 1995), and particularly those occurring around the myocardial ‘border zone'.
Considering the ability of the KATP channel blocker glibenclamide to antagonize the ischaemia-induced AP shortening (Cole et al., 1991; Yao et al., 1993; Tanaka et al., 1996), we aimed at evaluating in vitro its effects on the APD dispersion between normal and ischaemic guinea-pig ventricular regions and at determining if it may so prevent the occurrence of ‘border zone' arrhythmias. The effects of the KATP channel activator bimakalim, able to shorten cardiac AP in both normal and ischaemic tissues (Yao & Gross, 1994), were also investigated in an in vitro model of myocardial ‘border zone' (Rouet et al., 1989; Picard et al., 1998b,1998c).
Methods
Care of the animals conformed to the recommendations of the Helsinki Declaration, and the study was performed in accordance with the regulations of the official edict of the French Ministry of Agriculture.
Material
Guinea-pigs of either sex weighing 300–400 g were sacrificed after a brief anaesthesia with ether. The hearts were quickly removed and a thin longitudinal strip of the right ventricle was pinned, the endocardial surface upward, in a special perfusion chamber (Rouet et al., 1989; Picard et al., 1998b). This chamber (5 ml) is bisected by a thin latex membrane containing a centrally located hole allowing the preparation to be passed carefully through and divided into two zones: one called Normal Zone (NZ) and the other Altered Zone (AZ), respectively. The two compartments were independently superfused at the rate of 2 ml min−1 with Tyrode's solution oxygenated with 95% O2 and 5% CO2 and maintained at 36.5±0.5°C (Polystat 5HP, Bioblock, Illkirch, France). The composition of the Tyrode's solution is (in mM): Na+ 135, K+ 4, Ca2+ 1.8, Mg2+ 1, H2PO4− 1.8, HCO3− 25, Cl− 117.8 and glucose 5.5 (pH 7.35±0.05). At the end of each experiment, absence of leakage between the two compartments was tested by a dye injection (methylene blue) in one chamber.
Data acquisition and analysis
The preparations were stimulated at a frequency of 1 Hz either in the NZ or in the AZ, via a bipolar Teflon coated steel wire electrode. Rectangular pulses of 2 ms in duration and twice the diastolic threshold intensity were delivered by a programmable stimulator SMP 310 (Biologic, France). During the protocol, stimulation was stopped whenever sustained spontaneous arrhythmias occurred. An extrastimulus (ES) was applied every four stimulations in an attempt to elicit ES-induced repetitive responses by a progressive increase in 5 ms steps of the time interval between the stimulus and the ES. Transmembrane potentials were recorded simultaneously in both myocardial regions using glass microelectrodes filled with KCl 3 M (tip resistance: 10–30 megohms) and coupled to the input stages of an home-built high impedance capacitance-neutralizing amplifier. The recordings were displayed on a memory dual beam storage oscilloscope (Gould Ins. Sys. Inc. OH, U.S.A.). The following AP characteristics were automatically stored and measured by a system of cardiac AP automatic acquisition and processing device (DATAPAC, Biologic, France): resting membrane potential (RMP), AP amplitude (APA), AP duration at 50% of repolarization (APD50), and at 90% of repolarization (APD90), maximal upstroke velocity (Vmax). Whenever it was possible the same impalement was maintained throughout the experiment; however, when it was lost readjustment was attempted. If the readjusted parameters were deviant not more than 10% from the previous ones experiments were continued otherwise they were terminated.
Experimental protocol
After a 120 min equilibration period, simulated ischaemia was induced for 30 min in one compartment (AZ) by superfusion with a modified Tyrode's solution while the other compartment remained in normal conditions (NZ). The modified Tyrode's solution differed from normal by elevated K+ concentration (from 4 to 12 mM), decreased HCO3− concentration (from 25 to 9 mM) leading to a decrease in pH (from 7.35±0.05 to 7.00±0.05), decrease in pO2 by replacement of 95% O2 and 5% CO2 with 95% N2 and 5% CO2 and withdrawal of glucose. As previously reported (Rouet et al., 1989; Bélichard et al., 1991; Schiariti et al., 1994) the present modifications combining hypoxia, hyperkalemia, acidosis and lack of substrates are similar to those reported by Morena et al. (1980), which reproduced in vitro electrophysiological abnormalities similar to those observed in vivo during ischaemia. The AZ then returned to superfusion with the normal Tyrode's solution for 30 min (reperfusion period).
During both simulated ischaemia and reperfusion, conduction disturbances (Monti et al., 1991; Picard et al., 1998b) and arrhythmias (Rouet et al., 1989; Picard et al., 1998c) were recorded: (i) conduction blocks between the AZ and the NZ, (ii) ES-induced repetitive responses defined as spontaneous extrasystoles induced by a single ES and (iii) spontaneous arrhythmias independent of the stimulation.
During the simulated ischaemia and reperfusion phases, Tyrode's solution with either 10 μM glibenclamide (n=8) previously diluted in ethanol (<0.5%) or 1 μM bimakalim (n=8), or Tyrode's solution alone (control, n=12) was randomly superfused simultaneously in both compartments (NZ and AZ). These agents were used at relatively high concentrations, as pharmacological tools to investigate the role of KATP channels around the ‘border zone' and to study the relation between repolarization dispersion and the occurrence of arrhythmias between normal and ischaemic-reperfused ventricular regions. The concentrations were thus chosen as efficient to modulate APD in normal and ischaemic tissues. Ethanol (<0.5%) alone did not modify significantly AP parameters.
Statistical analysis
All results were expressed as mean±standard error of the mean (s.e.m.). Analysis of variance (ANOVA for repeated measures) was performed to compare AP parameters to initial values (measured before initiation of the ischaemic period) and to compare variations of APD90 and APD90 NZ/APD90 AZ ratio among groups. Exact Fisher's test and χ2 with the correction of Yates were used for comparison of nonparametric categorical data. Differences were considered significant when P<0.05.
Due to loss of microelectrodes impalements, variations of AP parameters were analysed for eight preparations in Control, eight in Glibenclamide 1 μM group and seven in Bimakalim 1 μM group.
Results
Effects of glibenclamide and bimakalim on the AP parameters and the dispersion of APD90 in normoxic and simulated ischaemic/reperfused conditions
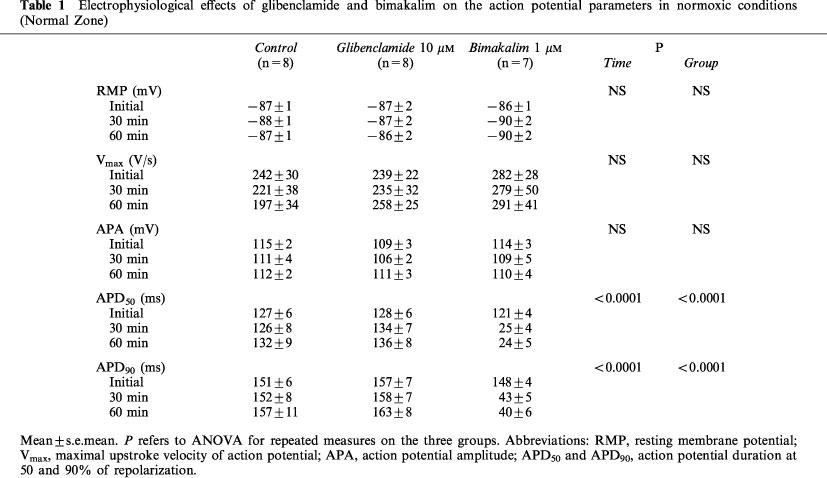
As summarized in Table 1, in normoxic conditions (Normal Zone), 10 μM glibenclamide and 1 μM bimakalim did not affect significantly RMP, Vmax and APA. Whereas glibenclamide did not significantly modify AP duration, bimakalim reduced APD50 and APD90 by 80±5% and 73±5% respectively, after 60 min (P<0.0001 for Time and Group).
Table 1.
Electrophysiological effects of glibenclamide and bimakalim on the action potential parameters in normoxic conditions (Normal Zone)
As shown in Table 2, simulated ischaemia (control) induced significant membrane depolarization and decreased Vmax, APA, APD50 and APD90, which was reversed by reperfusion (P<0.0001 for Time). The Vmax and APA variations were similar between treated and control groups (NS for Group), whereas the ischaemia-induced depolarization was lessened in the presence of bimakalim (RMP reduced by 25±3% versus 31±2% for Control after 30 min, P<0.05). The ischaemia-induced AP shortening was also significantly affected by both agents (APD50 and APD90: P<0.0001 for Group). As illustrated on Figure 1, glibenclamide significantly lessened the AP shortening in AZ (Figure 1B, P<0.0001) with no effect in NZ (Figure 1A), whereas bimakalim significantly enhanced the ischaemia-induced APD90 reduction (Figure 1B, P<0.0001) and decreased APD90 in normoxic conditions (Figure 1A, P<0.0001 versus control). During reperfusion (Table 2), APD returned close to initial values in control and glibenclamide groups and remained reduced in the presence of bimakalim (P<0.0001 versus control) by 70±6% and 62±5% for APD50 and APD90 respectively, after 30 min of reperfusion.
Table 2.
Electrophysiological effects of glibenclamide and bimakalim on the action potential parameters in ischaemic/reperfused conditions (altered zone)
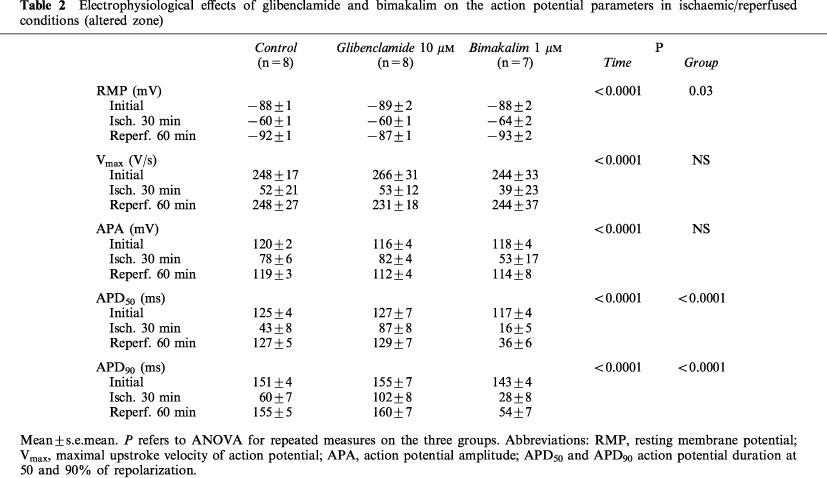
Figure 1.
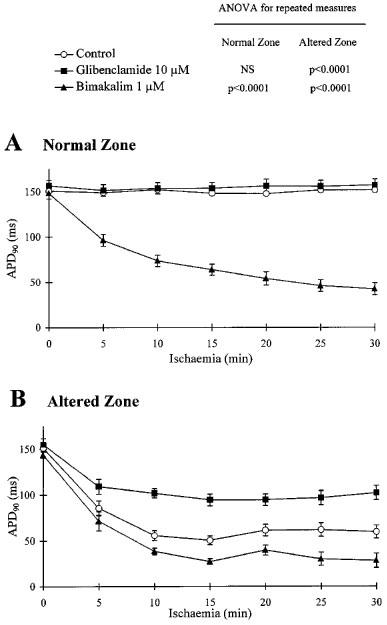
Effects of glibenclamide and bimakalim on action potential duration 90% (APD90) in normal and ischaemic conditions. Data are expressed as mean±s.e.mean. APD90 values were measured simultaneously on both normal zone (A) and altered zone (B) during 30 min of the ischaemic phase. Results of ANOVA (for repeated measures) are given for both treated groups: Glibenclamide 10 μM (n=8) and Bimakalim 1 μM (n=7), versus control (n=8). Note that glibenclamide lessened the ischaemic-induced action potential (AP) shortening (B) whereas bimakalim reduced APD90 in normoxia (A) and worsened the AP shortening induced by ischaemia (B).
As illustrated in Figure 2A, the ischaemia-induced AP shortening led to a dispersion of APD90 between both adjacent myocardial regions (P<0.0001 for Time), measured as ratio APD90 NZ/APD90 AZ. This APD90 dispersion was significantly reduced in presence of 10 μM glibenclamide (P<0.0001 versus control) or 1 μM bimakalim (P<0.005 versus control), resulting respectively from the preventive effects of glibenclamide on the ischaemic-induced APD shortening (Figure 1B) and the marked APD90 reduction induced by bimakalim in NZ (Figure 1A).
Figure 2.
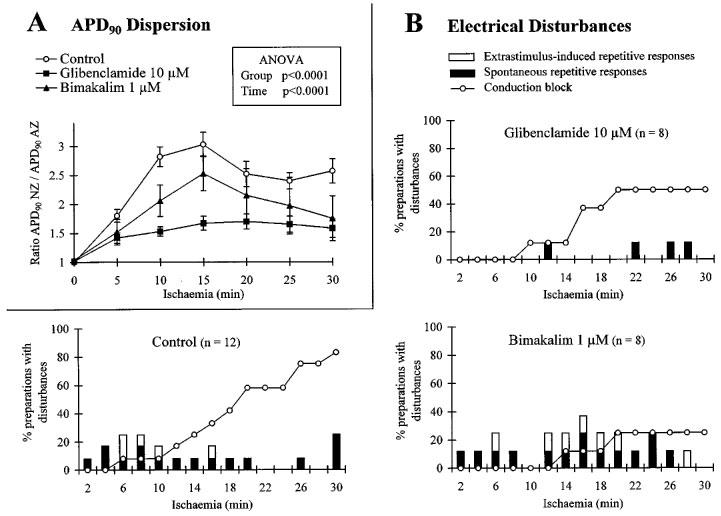
Effects of glibenclamide and bimakalim on the dispersion of action potential duration 90% (APD90) between normal and ischaemic myocardial zones (A) and on the incidence of electrical disturbances during simulated ischaemia (B). In (A) data are expressed as mean±s.e.mean and the dispersion of APD90 is represented by ratio APD90 normal zone/APD90 altered zone. Results of ANOVA (for repeated measures) are given for group and time factors. In (B), for each time interval (2 min) of the ischaemic period (30 min), values are expressed as a percentage of preparations presenting (i) conduction block between the two myocardial regions, (ii) Extrastimulus-induced repetitive responses and (iii) spontaneous arrhythmias. Patterns are shown in absence of drug (control) and in presence of 10 μM glibenclamide or 1 μM bimakalim. Note that the APD90 dispersion between AZ and NZ was effectively lessened by glibenclamide, concomitantly with a reduced occurrence of arrhythmias. Note also that the APD90 dispersion was slightly reduced in presence of 1 μM bimakalim and, concomitantly with a decreased incidence of conduction blocks, the occurrence of arrhythmias was not prevented.
Effects of glibenclamide and bimakalim on the incidence of electrical disturbances during simulated ischaemia/reperfusion
As summarized in Table 3, simulated ischaemia-induced conduction blocks in 83% of preparations: 66% with unidirectional blocks from NZ towards AZ or conversely and 17% with bidirectional blocks between both regions. ES-induced arrhythmias and spontaneous repetitive responses occurred in 25% of preparations. During the ischaemic period, the occurrence of arrhythmias decreased as conduction blocks occurred (Figure 2B, control). In presence of glibenclamide, few spontaneous repetitive responses and no ES-induced ones occurred during the ischaemic phase. The absence of ES-induced arrhythmias was significantly related to the lessened APD90 dispersion between AZ and NZ in presence of 10 μM glibenclamide (Figure 2A) as compared to control (χ2=8.28, P<0.01). Unlike glibenclamide group, the incidence of conduction blocks was significantly reduced (P<0.05, Table 3, Figure 2B) in presence of 1 μM bimakalim (in 25% of preparations: 12.5% with unidirectional blocks and 12.5% with bidirectional ones). Bimakalim enhanced significantly (P<0.05) the incidence of ES-induced spontaneous responses, and worsened the severity of arrhythmias occurring around the ‘border zone' along the ischaemic period as illustrated in Figure 3. This figure shows representative recordings obtained from the same preparation and illustrating that arrhythmia severity was progressively worsened by 1 μM bimakalim: the ES-induced extrasystole occurring during the early ischaemic phase (Figure 3A, at 6 min) became salvos of APs as the simulated ischaemia prolonged (Figure 3B, at 17 min) and led to sustained spontaneous arrhythmias independent of the stimulation during the late ischaemic phase (Figure 3C, at 28 min).
Table 3.
Effects of glibenclamide and bimakalim on the incidence of conduction blocks and arrhythmias during simulated ischaemia and reperfusion (% of preparations with disturbances)
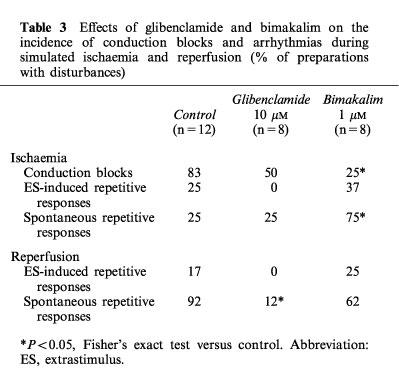
Figure 3.
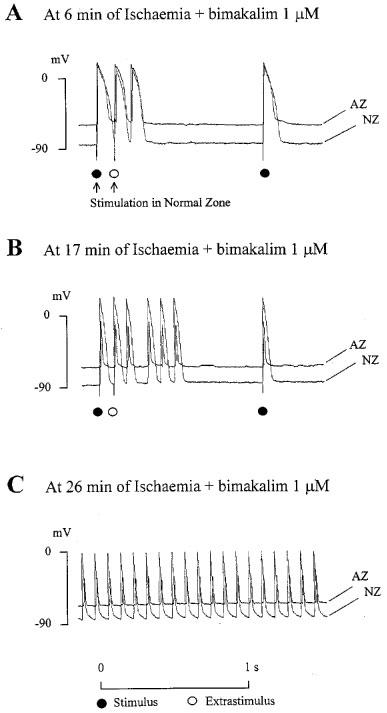
Representative action potentials (APs) recordings illustrating the arrhythmia severity progressively worsened by 1 μM bimakalim during simulated ischaemia. APs were recorded simultaneously in normal zone (NZ) and altered zone (AZ). During the early phase of ischaemia (A, at 6 min), the Extrastimulus (ES) induced a unique repetitive response, namely an extrasystole following the ES-induced AP. As the simulated ischaemia prolonged (B, at 17 min), the ES-induced repetitive response became a salvos of four extrasystoles. Finally, during the late ischaemic period (C, at 28 min), the arrhythmia was a sustained spontaneous activity (frequency ≈10 Hz) which persisted in absence of stimulation.
Reperfusion removed all conduction blocks during the first 2 min of reperfusion similarly among control and treated groups (Figure 4). During reperfusion (Table 3 and Figure 4), the incidence of both ES-induced and spontaneous arrhythmias was not significantly modified by 1 μM bimakalim whereas 10 μM glibenclamide abolished the ES-induced repetitive responses and prevented significantly (P<0.05) the occurrence of the spontaneous ones. The prevention of reperfusion-induced spontaneous arrhythmias was significantly related to a reduced AP shortening in AZ in presence of 10 μM glibenclamide at the end of the ischaemic period (Figure 1B), as compared to control (χ2=6.13, P<0.02).
Figure 4.
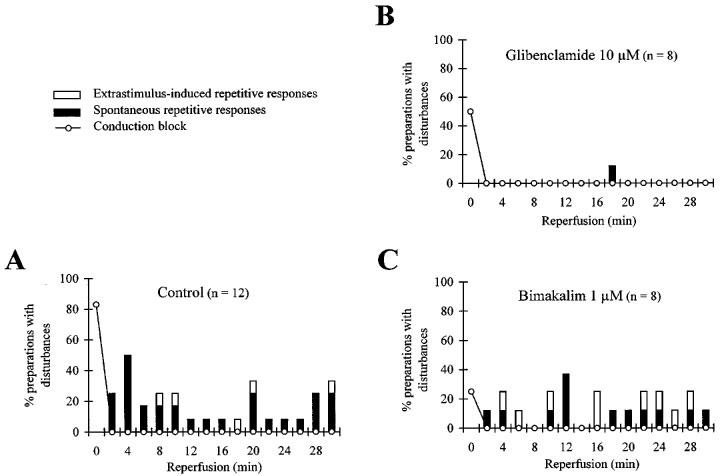
Effects of glibenclamide and bimakalim on the incidence of conduction disturbances and arrhythmias during simulated reperfusion. For each time interval (2 min) of the reperfusion period (30 min), values are expressed as a percentage of preparations presenting (i) conduction blocks between the two myocardial regions, (ii) Extrastimulus (ES)-induced repetitive responses and (iii) spontaneous arrhythmias. Patterns are shown in absence of drug (A, control) and in presence of 10 μM glibenclamide (B) or 1 μM bimakalim (C). Note that, unlike bimakalim, glibenclamide prevented the occurrence of both spontaneous and ES-induced arrhythmic events.
Discussion
The main results of this in vitro study are: (1) the prevention of the APD90 dispersion between both normal and ischaemic myocardial zones by KATP channel blockade with 10 μM glibenclamide, concomitantly with an antiarrhythmic efficacy around the simulated ‘border zone' against both ES-induced and spontaneous repetitive responses; (2) despite a reduction of the APD90 dispersion, KATP channel activation with 1 μM bimakalim led to proarrhythmic effects on ‘border zone' spontaneous arrhythmias during ischaemia, concomitantly with a decreased incidence of myocardial conduction blocks; (3) unlike bimakalim, glibenclamide was antiarrhythmic during reperfusion in this in vitro model of ‘border zone' arrhythmias.
The expected preventive effect of glibenclamide on the ischaemia-induced AP shortening confirmed data already obtained in vitro (Cole et al., 1991; McPherson et al., 1993; Tanaka et al., 1996) and in vivo during coronary artery occlusion in dogs (Yao et al., 1993). No benefit were found however on the ischaemia-induced AP shortening after 5 min of coronary occlusion in rabbits (Barrett & Walker, 1998). Although in the present study ischaemia was performed by using a modified Tyrode's solution, these ischaemic conditions have been shown to reproduce the electrical alterations on cardiac AP observed in more complex in vivo animal models during acute myocardial ischaemia (Janse et al., 1979). The different results might be merely due to the model used. Otherwise, the absence of glibenclamide effects in the normal ventricular region was expected considering the KATP channels in their closed state under normoxia, contributing to reduce the APD90 dispersion between normal and ischaemic tissues.
Concomitantly, the KATP channel blockade by glibenclamide prevented the occurrence of ischaemia-induced spontaneous arrhythmias and inhibited the ES-induced repetitive responses occurring around the simulated ‘border zone'. This antiarrhythmic efficacy on ES-induced arrhythmias might be explained by the benefit of glibenclamide on the repolarization dispersion which might contribute to impair reentry movements, likely responsible for the emergence of these arrhythmic events, as previously discussed (Rouet et al., 1989). Indeed, reentry movements require a site of unidirectional conduction block and a slow retrograde conduction throughout the cardiac tissue, similarly to the conduction disturbances observed in this ‘border zone' model. On the other hand, the ischaemia-induced spontaneous repetitive responses observed in the present model are unlikely related to early (EAD) or delayed (DAD) afterdepolarization, not seen in our experiments, but may also be attributed to reentry, explaining the protective effect of glibenclamide. Supporting this, other investigators have reported differential effects of KATP channel blockers as regards the type of arrhythmia, namely antiarrhythmicity against reentries (Pasnani & Ferrier, 1992; D'Alonzo et al., 1994a), and enhancement of arrhythmic events related to EAD or DAD (Spinelli et al., 1991; D'Alonzo et al., 1994b). The diversity of mechanisms underlying the ischaemia-induced arrhythmias and the diversity of experimental models might explain the contradictory findings of the literature as concerns pro- and antiarrhythmic effects of glibenclamide in isolated cardiac preparations (Pasnani & Ferrier, 1992), isolated hearts (Bril et al., 1992; Bernauer, 1997), and anaesthetized rabbits (Barrett & Walker, 1998).
The bimakalim-induced worsening of the AP shortening during ischaemia also confirmed data obtained with other KATP channel openers in vitro (McPherson et al., 1993; Tanaka et al., 1996) and in vivo (Yao et al., 1993; Yao & Gross, 1994). Nevertheless the extent of the AP shortening induced by these agents in ischaemic myocardial muscle as compared with normal region and their resulting action on APD dispersion in inhomogeneous cardiac tissues were not clarified. The less potent action of bimakalim in ischaemic region as compared to normal tissue is likely due to KATP channels already activated in ischaemic conditions. This differential efficacy to shorten AP led to a lessened APD90 dispersion around the simulated ‘border zone', as did glibenclamide, whereas ischaemia-induced arrhythmias were not prevented.
Otherwise, the absence of protective effect of the KATP channel activator on the ‘border zone' ES-induced arrhythmias might be considered in regard of its concomitant action on the incidence of conduction blocks. Indeed, as illustrated on Figure 3B, the ES-induced repetitive responses occurred mainly when the myocardial conduction was slowed but before the total block of the signal conduction occurred (until 16–18 min of ischaemia), since no ES-induced arrhythmias were observed after the myocardial conduction around the ‘border zone' was completely blocked. By preventing the occurrence of conduction block, bimakalim may contribute to maintain a slowed myocardial conduction and favour reentrant circuits, overriding so any potential antiarrhythmic benefit of the reduced APD90 dispersion between normal and simulated ischaemic zones. Supporting this, we recently observed in this ‘border zone' model that the slowing of the myocardial conduction induced by 1 μM bupivacaine, a local anaesthetic, was accompanied by an enhanced incidence of arrhythmias whereas neither ES-induced nor spontaneous repetitive responses occurred when the myocardial conduction was completely blocked by higher concentrations of this agent (Picard et al., 1998b). As concerns the ‘border zone' spontaneous arrhythmias, the proarrhythmicity induced by bimakalim is likely due to the dramatic AP shortening observed in the ischaemic myocardial region, leading to spike-like APs which might thus facilitate the emergence of abnormal automaticity (Figure 4C). Moreover, this proarrhythmic effect confirms that the spontaneous repetitive responses observed in our model are unlikely based on EAD or DAD, since several KATP channel openers have been shown as efficient in vitro and in vivo against arrhythmias related to these repolarization abnormalities (Fish et al., 1990; Spinelli et al., 1991; Takahashi et al., 1991).
During reperfusion also, the KATP channel blockade by glibenclamide was accompanied by antiarrhythmic efficacy around the simulated ‘border zone' in relation to the lessened AP shortening during the previous ischaemic phase. Glibenclamide might therefore indirectly prevent reperfusion-induced arrhythmias by its protective effects during ischaemia. However, it cannot be ruled out that the KATP channel blockade acts also during reperfusion, since it has been demonstrated in guinea-pig isolated ventricular walls that these channels may remain activated during the early reperfusion (Shigematsu et al., 1995). Otherwise, we recently provided evidence that glibenclamide was able to prevent the reperfusion-induced accumulation of free arachidonic acid released from the cell membrane phospholipids in guinea-pig ventricular preparations (Picard et al., 1998a). Considering the arrhythmogenic properties of free fatty acids accumulated in the cardiac muscle (Oliver & Opie, 1994), an indirect antiarrhythmic action of the KATP channel blockade, involving a protective action against the degradation of cell membrane lipids, cannot be excluded also.
Unlike the ischaemia-induced arrhythmias, the incidence of reperfusion repetitive responses around the simulated ‘border zone' was not modified by KATP channel activation. These results differ from those previously obtained in our laboratory on guinea-pig ventricular preparations with the KATP channel opener cromakalim, which favoured the occurrence of reperfusion spontaneous arrhythmias without affecting those occurred during ischaemia (Picard et al., 1998a). Interestingly, these latter findings were obtained in a model of global simulated ischaemia-reperfusion, suggesting that the mechanisms underlying the emergence of arrhythmias around the myocardial ‘border zone' are likely different from those responsible for arrhythmias observed in cardiac tissue rendered globally ischaemic. This might help to explain the controversial data of the literature supporting either profibrillatory (Tosaki et al., 1993) or antiarrhythmic effects (Tanaka et al., 1996) of KATP channel openers, since in addition to different species and drug concentrations, different experimental models of myocardial ischaemia were used.
In conclusion, this study provided evidence for an antiarrhythmic efficacy during simulated ischaemia and reperfusion of the reduction of repolarization dispersion induced by KATP channel blockade in an in vitro model of ‘border zone'. Conversely, KATP channel activation favoured the emergence of ischaemia-induced spontaneous arrhythmic events around the simulated ‘border zone' and did not confer any protection during reperfusion. Some limitations for the interpretation of our findings and relative to the in vitro model have now to be taken into account, since the demarcation between normal and ischaemic-reperfused myocardial zones is likely more narrow and regular than it might occur in vivo in diseased hearts. As previously discussed in detail (Rouet et al., 1989), this ‘border zone' model has been shown to reproduce arrhythmias similar to those seen in vivo during coronary artery occlusion and after abrupt reperfusion (Corr & Witowski, 1983) or in man during coronary transluminal angioplasty and after thrombolytic therapy (Tzivoni et al., 1983). However, it suits to keep in mind that an in vitro model using superfused cardiac tissues under simulated ischaemic-reperfused conditions is largely distant from in vivo experiments and the clinic. As pertinently discussed by Rosen (1988), an in vitro model as complex as possible cannot reproduce exactly in vivo situations and may provide interesting information on the limits of a cell ability to perform in a controlled environment simulating pathological conditions rather than its usual behaviour in healthy and diseased heart. Caution is therefore needed when confronting in vitro and in vivo data and overall for all attempts to extrapolate in vitro findings to in vivo and clinical settings. Now, the present study might help to understand the contributory role of the ischaemia-induced KATP channel activation as regards the electrical disturbances occurring during simulated ischaemia in this in vitro model of ‘border zone' and the potential benefits of KATP channel blockers in this peculiar myocardial region. These findings might also explain why controversial results have been obtained with KATP channel activators and blockers in amount of experimental studies using in vitro simulated pathophysiological conditions or in vivo coronary artery occlusion. It also supports the interest that must be placed, in the context of the anti and proarrhythmic effects of cardioactive agents, in the study of their electrophysiological effects in myocardial regions presenting inhomogeneous distribution of electrical properties and anatomic and biochemical changes, as it may occur around the ‘border zone'.
Acknowledgments
The authors thank Mr Hervé Tombette for technical assistance during the study.
Abbreviations
- ANOVA
analysis of variance
- AP
action potential
- APA
action potential amplitude
- APD
action potential duration
- APD50, 90
action potential duration measured at 50 and 90% of repolarization
- AZ
altered zone
- DAD
delayed afterdepolarizations
- EAD
early afterdepolarizations
- ES
extrastimulus
- KATP channel
adenosine triphosphate dependent potassium channel
- NZ
normal zone
- RMP
resting membrane potential
- Vmax
maximal upstroke velocity of action potential
- χ2
Chi2 test
References
- AUCHAMPACH J.A., CAVERO I., GROSS G.J. Nicorandil attenuates myocardial dysfunction associated with transient ischemia by opening ATP-dependent potassium channels. J. Cardiovasc. Pharmacol. 1992;20:765–771. [PubMed] [Google Scholar]
- BARRETT T.D., WALKER M.J.A. Glibenclamide does not prevent action potential shortening induced by ischemia in anesthetized rabbits but reduces ischemia-induced arrhythmias. J. Mol. Cell. Cardiol. 1998;30:999–1008. doi: 10.1006/jmcc.1998.0664. [DOI] [PubMed] [Google Scholar]
- BÉLICHARD P, , PRUNEAU D., ROUET R., SALZMANN J.L. Electrophysiological responses of hypertrophied rat myocardium to combined hypoxia, hyperkalemia and acidosis. J. Cardiovasc. Pharmacol. 1991;17:S141–S145. doi: 10.1097/00005344-199117002-00034. [DOI] [PubMed] [Google Scholar]
- BERNAUER W. Concerning the effect of the K+ channel blocking agent glibenclamide on ischaemic and reperfusion arrhythmias. Eur. J. Pharmacol. 1997;326:147–156. doi: 10.1016/s0014-2999(97)85409-x. [DOI] [PubMed] [Google Scholar]
- BRIL A., LAVILLE M.P., GOUT B. Effects of glibenclamide on ventricular arrhythmias and cardiac function in ischaemia and reperfusion in isolated rat heart. Cardiovasc. Res. 1992;26:1069–1076. doi: 10.1093/cvr/26.11.1069. [DOI] [PubMed] [Google Scholar]
- CAVERO I., DJELLAS Y., GUILLON J.M. Ischemic myocardial cell protection conferred by the opening of ATP-sensitive potassium channels. Cardiovasc. Drugs Ther. 1995;9:245–255. doi: 10.1007/BF00878472. [DOI] [PubMed] [Google Scholar]
- COETZEE W.A. ATP-sensitive potassium channels and myocardial ischemia: Why do they open. Cardiovasc. Drugs Ther. 1992;6:201–208. doi: 10.1007/BF00051140. [DOI] [PubMed] [Google Scholar]
- COLE W.C., MCPHERSON C.D., SONTAG D. ATP-regulated K+ channels protect the myocardium against ischaemia/reperfusion damage. Circ. Res. 1991;69:571–581. doi: 10.1161/01.res.69.3.571. [DOI] [PubMed] [Google Scholar]
- CORR P.B., WITOWSKI F.X. Potential electrophysiologic mechanisms responsible for disrhythmias associated with reperfusion of ischemic myocardium. Circulation. 1983;68:16–24. [PubMed] [Google Scholar]
- D'ALONZO A.J., DARBENZIO R.B., HESS T.A., SEWTER J.C., SLEPH P.G., GROVER G.J. Effect of potassium on the action of the K+ ATP modulators cromakalim, pinacidil, or glibenclamide on arrhythmias in isolated perfused rat heart subjected to regional ischemia. Cardiovasc. Res. 1994a;28:881–887. doi: 10.1093/cvr/28.6.881. [DOI] [PubMed] [Google Scholar]
- D'ALONZO A.J., HESS T.A., DARBENZIO R.B., SEWTER J.C. Effects of intracoronary glyburide on cesium-induced arrhythmias in the anesthetized dog. J. Cardiovasc. Pharmacol. 1994b;23:446–452. [PubMed] [Google Scholar]
- DJELLAS Y., MESTRE M., CAVERO I. Aprimakalim protection against ischemic injury occurs with an accelerated decrease in action potential duration but not in myocardial contractility. Circulation. 1993;88:I632. [Google Scholar]
- DOCHERTY J.C., GUNTER H.E., KUZIO B., SHOEMAKER L., YANG L., DESLAURIERS R. Effects of cromakalim and glibenclamide on myocardial high energy phosphates and intracellular pH during ischemia-reperfusion: 31P NMR studies. J. Mol. Cell. Cardiol. 1997;29:1665–1673. doi: 10.1006/jmcc.1997.0404. [DOI] [PubMed] [Google Scholar]
- DUO G.S., HOFF P., KUPERSMITH J. Repolarization interactions between cardiac segments of varying action potential duration. Am. Heart J. 1989;117:854–861. doi: 10.1016/0002-8703(89)90623-6. [DOI] [PubMed] [Google Scholar]
- EL-SHERIF N., MEHRA R., GOUGH W.B., ZEILER R.H. Ventricular activation patterns of spontaneous and induced ventricular rhythms in canine one-day-old myocardial infarctions: evidence for local and reentrant mechanisms. Circ. Res. 1981;51:152–166. doi: 10.1161/01.res.51.2.152. [DOI] [PubMed] [Google Scholar]
- FISH F.A., PRAKASH C., RODEN D.M. Suppression of repolarization-related arrhythmias in vitro and in vivo by low-dose potassium channel activators. Circulation. 1990;82:1362–1369. doi: 10.1161/01.cir.82.4.1362. [DOI] [PubMed] [Google Scholar]
- FREYSS-BÉGUIN M., SIMON J., DUVAL D. Effects of glibenclamide on the metabolism of fatty acids in cultures of newborn rat heart cells under normoxic and hypoxic conditions. Prostaglandins Leukot. Essential Fatty Acids. 1995;52:325–331. doi: 10.1016/0952-3278(95)90034-9. [DOI] [PubMed] [Google Scholar]
- HAVERKAMP W., BORGGREFE M., BREITHARDT G. Electrophysiologic effects of potassium channel openers. Cardiovasc. Drugs Ther. 1995;9:195–202. doi: 10.1007/BF00878466. [DOI] [PubMed] [Google Scholar]
- HEARSE D.J. Activation of ATP-sensitive potassium channels: a novel pharmacological approach to myocardial protection. Cardiovasc. Res. 1995;30:1–17. [PubMed] [Google Scholar]
- HOFMAN H. Interaction between a normoxic and a hypoxic region of guinea pig and ferret papillary muscle. Circ. Res. 1985;56:876–883. doi: 10.1161/01.res.56.6.876. [DOI] [PubMed] [Google Scholar]
- JANSE M.J., CINCA J., MORENA H., FIOLET J.W., KLEBER A.G., DE VRIES G.P., BECKER A.E., DURRER D. The ‘border zone' in myocardial ischaemia. An electrophysiological, metabolic and histochemical correlation in the pig heart. Circ. Res. 1979;44:576–588. doi: 10.1161/01.res.44.4.576. [DOI] [PubMed] [Google Scholar]
- JANSE M.J., VAN CAPELLE J.L., MORSINK H., KLEBER A.G., WILMS-SCHOPMAN F., CARDINAL R., D'ALNONCOURT C.N., DURRER D. Flow of ‘injury' current and patterns of excitation during early ventricular arrhythmias in acute myocardial ischemia in isolated porcine and canine hearts. Evidence for two different arrhythmogenic mechanisms. Circ. Res. 1980;47:151–165. doi: 10.1161/01.res.47.2.151. [DOI] [PubMed] [Google Scholar]
- KUPERSMITH J., LI Z.Y., MALDONADO C. Marked action potential prolongation as a source of injury current leading to border zone arrhythmogenesis. Am. Heart J. 1994;127:1543–1553. doi: 10.1016/0002-8703(94)90384-0. [DOI] [PubMed] [Google Scholar]
- MCPHERSON C.D., PIERCE G.N., COLE W.C. Ischemic cardioprotection by ATP-sensitive K+ channels involves high-energy phosphate preservation. Am. J. Physiol. 1993;265:H1809–H1818. doi: 10.1152/ajpheart.1993.265.5.H1809. [DOI] [PubMed] [Google Scholar]
- MONTI F., SCHIARITI M., DAWODU A.A., LANTI M., PULVIRENTI G., TERRACCIANO C.M.N., EVANGELISTA E., PUDDU P.E., MARINO B., CAMPA P.P. Prevenzione del blocco del'eccitoconduzione durante ischemia miocardica acute: esiste un ruolo per la prostaciclina o per l'istamina. Cardiologia. 1991;36:519–526. [PubMed] [Google Scholar]
- MORENA H., JANSE M.J., FIOLET J.W.T., KRIEGER W.J.G., CRIJNS H., DURRER D. Comparison of the effects of regional ischemia, hypoxia, hyperkalemia and acidosis on intracellular and extracellular potentials and metabolism in isolated porcine heart. Circ. Res. 1980;46:634–646. doi: 10.1161/01.res.46.5.634. [DOI] [PubMed] [Google Scholar]
- OHNO Y., MINATOGUCHI S., UNO Y., KARIYA T., YAMASHITA K., FUJIWARA T., FUJIWARA H. Nicorandil reduces myocardial infarct size by opening the K(ATP) channel in rabbits. Int. J. Cardiol. 1997;62:181–190. doi: 10.1016/s0167-5273(97)00270-2. [DOI] [PubMed] [Google Scholar]
- OLIVER M.F., OPIE L.H. Effects of glucose and fatty acids on myocardial ischaemia and arrhythmias. Lancet. 1994;343:155–158. doi: 10.1016/s0140-6736(94)90939-3. [DOI] [PubMed] [Google Scholar]
- PASNANI J.S., FERRIER G.R. Differential effects of glyburide on premature beats and ventricular tachycardia in an isolated tissue model of ischemia and reperfusion. J. Pharmacol. Exp. Ther. 1992;262:1076–1084. [PubMed] [Google Scholar]
- PICARD S., ROUET R., DUVAL D., CHESNAY F., GÉRARD J.L. KATP channel modulators and myocardial damages induced by ischemia-reperfusion: membrane lipids injury and arrhythmias. J. Mol. Cell. Cardiol. 1998a;30:2613–2621. doi: 10.1006/jmcc.1998.0819. [DOI] [PubMed] [Google Scholar]
- PICARD S., ROUET R., FLAIS F., DUCOURET P., BABATASI G., KHAYAT A., POTIER J.C., BRICARD H., GÉRARD J.L. Pro- and antiarrhythmic effects of bupivacaine in an in vitro model of myocardial ischemia-reperfusion. Anesthesiology. 1998b;88:1318–1329. doi: 10.1097/00000542-199805000-00024. [DOI] [PubMed] [Google Scholar]
- PICARD S., ROUET R., MONTI F., PUDDU P.E., DUCOURET P., FLAIS F., LIBERSA C., GÉRARD J.L. Proarrhythmic effects of dl- and d-sotalol on the ‘border zone' between normal and ischemic regions of isolated ventricular myocardium and antiarrhythmic effects on reperfusion. J. Cardiovasc. Pharmacol. 1998c;31:126–139. doi: 10.1097/00005344-199801000-00018. [DOI] [PubMed] [Google Scholar]
- ROSEN M.R. The links between basic and clinical cardiac electrophysiology. Circulation. 1988;77:251–263. doi: 10.1161/01.cir.77.2.251. [DOI] [PubMed] [Google Scholar]
- ROUET R., ADAMANTIDIS M., HONORÉ E., DUPUIS B. In vitro abnormal repetitive responses in guinea pig ventricular myocardium exposed to combined hypoxia, hyperkalemia and acidosis. J. Appl. Cardiol. 1989;4:19–29. [Google Scholar]
- SAWANOBORI T., ADANIYA H., YUKISADA H., HIRAOKA M. Role of ATP-sensitive K+ channel in the development of A-V block during hypoxia. J. Mol. Cell. Cardiol. 1995;27:647–657. doi: 10.1016/s0022-2828(08)80057-0. [DOI] [PubMed] [Google Scholar]
- SCHIARITI M., PUDDU P.E., ROUET R. Multivariate prediction of spontaneous repetitive responses in ventricular myocardium exposed in vitro to simulated ischemic conditions. Int. J. Cardiol. 1994;45:9–22. doi: 10.1016/0167-5273(94)90050-7. [DOI] [PubMed] [Google Scholar]
- SHIGEMATSU S., SATO T., ABE T., SAIKAWA T., SAKATA T., ARITA M. Pharmacological evidence for the persistent activation of ATP-sensitive K+ channels in early phase of reperfusion and its protective role against myocardial stunning. Circulation. 1995;92:2266–2275. doi: 10.1161/01.cir.92.8.2266. [DOI] [PubMed] [Google Scholar]
- SIEGL P. Blockers of ATP sensitive potassium current are of potential benefit in ischaemic heart disease. Cardiovasc. Res. 1994;28:31–33. doi: 10.1093/cvr/28.1.31. [DOI] [PubMed] [Google Scholar]
- SPINELLI W., SOROTA S., SIEGAL M., HOFFMAN B.F. Antiarrhythmic actions of the ATP-regulated K+ current activated by pinacidil. Circ. Res. 1991;68:1127–1137. doi: 10.1161/01.res.68.4.1127. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI N., ITO M., SAIKAWA T., ARITA M. Nicorandil suppresses early afterdepolarizations and ventricular arrhythmias induced by caesium chloride in rabbits in vivo. Cardiovasc. Res. 1991;25:445–452. doi: 10.1093/cvr/25.6.445. [DOI] [PubMed] [Google Scholar]
- TANAKA H, , OKAZAKI K., SHIGENOBU K. Cardioprotective effects of NIP-121, a novel ATP-sensitive potassium channel opener, during ischemia and reperfusion in coronary perfused guinea pig myocardium. J. Cardiovasc. Pharmacol. 1996;27:695–701. doi: 10.1097/00005344-199605000-00012. [DOI] [PubMed] [Google Scholar]
- TOSAKI A., SZERDAHELYI P., ENGELMAN R.M., DAS D.K. Potassium channel openers and blockers; do they possess proarrhythmic or antiarrhythmic activity in ischemic and reperfused rat hearts. J. Pharmacol. Exp. Ther. 1993;267:1355–1362. [PubMed] [Google Scholar]
- TZIVONI D., KEREN A., GRANOT H., GOTTLIEB S., BENHORIN J., STERN S. Ventricular fibrillation caused by myocardial reperfusion in Prinzmetal angina. Am. Heart J. 1983;105:323–325. doi: 10.1016/0002-8703(83)90534-3. [DOI] [PubMed] [Google Scholar]
- WILDE A.A. K+ATP-channel opening and arrhythmogenesis. J. Cardiovasc. Pharmacol. 1994;24:S35–S40. [PubMed] [Google Scholar]
- YAO Z., CAVERO I., GROSS G.J. Activation of cardiac KATP channels: an endogenous protective mechanism during repetitive ischemia. Am. J. Physiol. 1993;264:H495–H504. doi: 10.1152/ajpheart.1993.264.2.H495. [DOI] [PubMed] [Google Scholar]
- YAO Z., GROSS G.J. Effects of the KATP channel opener bimakalim on coronary blood flow, monophasic action potential duration, and infarct size in dogs. Circulation. 1994;89:1769–1775. doi: 10.1161/01.cir.89.4.1769. [DOI] [PubMed] [Google Scholar]
- YUAN S., WOHLFART B., OLSSON S.B., BLOMSTRÖM-LUNQVIST C. The dispersion of repolarization in patients with ventricular tachycardia. Eur. Heart J. 1995;16:68–76. doi: 10.1093/eurheartj/16.1.68. [DOI] [PubMed] [Google Scholar]


