Abstract
The aim of the present study was to determine the effects of maitotoxin on nerve growth factor production and the Ca2+ influx in clonal rat glioma cells (C6-BU-1).
Maitotoxin (1–10 ng ml−1) induced a profound increase in 45Ca2+ influx in an extracellular Ca2+-dependent manner. However, high KCl had no effect at all. These effects were supported by the results from the analysis of intracellular Ca2+ concentration using fura 2.
The maitotoxin-induced 45Ca2+ influx was inhibited by inorganic Ca2+ antagonists, such as Mg2+, Mn2+ and Co2+. The inhibitory effect of Co2+ was antagonized by increasing the extracellular Ca2+ concentrations.
Maitotoxin (3 ng ml−1) as well as A-23187 (1 μM) and dibutyryl cyclic AMP (0.5 mM) caused an acceleration of nerve growth factor (NGF) production in C6-BU-1 cells, as determined by NGF enzyme immunoassay.
Reverse transcription polymerase chain reaction (RT–PCR) analysis showed that maitotoxin (10 ng ml−1) enhanced the expression of NGF mRNA, which was abolished by the removal of extracellular Ca2+. A-23187 also accelerated its expression.
These results suggest that maitotoxin activates a voltage-insensitive Ca2+ channel and accelerates NGF production mediated through a Ca2+ signalling pathway in C6-BU-1 glioma cells.
Keywords: Maitotoxin, rat glioma cell (C6-BU-1), calcium channel, calcium influx, nerve growth factor (NGF), NGF mRNA
Introduction
It is now generally accepted that Ca2+ plays an important role in various signalling processes in cellular activity (Campbell, 1983). Calcium channels are one of the key factors in the control of the intracellular Ca2+ level, and thus are important in cellular signalling processes such as maintenance of protein synthesis (Wong et al., 1993).
Numerous toxins have been shown to be very useful pharmacological tools for studying on ion channels and drug receptors (Ohizumi, 1997). For instance, tetrodotoxin has contributed to the understanding of the Na+ channel. Maitotoxin, the most potent marine toxin obtained from toxic dinoflagellate and poisonous fishes inhabiting tropical and subtropical seas, has been reported to produce many responses in a wide variety of mammalian cells, including the stimulation of hormone and neurotransmitter secretion, contraction of cardiac and smooth muscles, and the stimulation of inositol phosphate production (Gusovsky & Daly, 1990). All these diverse actions are dependent on the stimulation of Ca2+ influx into the cells.
Neurons cannot proliferate and regenerate as they are terminally differentiated cells. Therefore, neurotrophic factors are essential to maintain and organize neurons functionally. Glial cells support neuron by releasing neurotrophic factors, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3) (Rudge et al., 1995) and glia-derived neurotrophic factor (GDNF) (Lin et al., 1993). NGF has been extensively investigated showing pleiotrophic effects such as the induction of neuronal differentiation, promoting the survival and the prevention of apoptosis in both central and peripheral nervous systems (Levi-Montalcini, 1987).
It has been reported that C6 cells, derived from rat glial tumours induced by N-nitrosomethylurea (Benda et al., 1968), synthesize/secrete NGF upon the stimulation by β-adrenergic agonists, forskolin or cyclic AMP analogues (Schwartz, 1988; Mocchetti et al., 1989; Fukumoto et al., 1994; Colangelo et al., 1996). These studies indicated that cyclic AMP increased NGF synthesis and secretion by initiating its gene expression. Furthermore, the expression of the NGF mRNA is dramatically enhanced when primary astrocytes are exposed to phorbol ester (Neveu et al., 1992). This effect is in part mimicked by diacylglycerol, and prevented by an inhibitor of protein kinase C (PKC). These data provide evidence that PKC exerts as a key enzyme in the up-regulation of NGF synthesis. Nuclear factor κB (NFκB) is known to play an important role in regulation of gene expression involved in immune and inflammatory responses in brain (Kaltschmidt et al., 1994; O'Neill & Kaltschmidt, 1997). It has recently been shown that an activation of NFκB by lipopolysaccharide, ceramide or sphingomyelinase induces NGF synthesis (Galve-Roperh et al., 1997; Heese et al., 1998). It is assumed that an induction of NGF synthesis by interleukin-1β, tumour necrosis factor-α or activated vitamin D3 depends on the accumulation of ceramide (Okazaki et al., 1994; Hannun et al., 1996). In addition, certain growth factors, cytokines, interleukins, proteases and serum have been shown to express the NGF mRNA dramatically in primary astrocytes (Carman-krzan & Wise, 1993; Jehan et al., 1995). Thus, it is thought that the expression of NGF mRNA is under the regulation of multiple signalling pathways.
In spite of many reports concerning NGF synthesis, the role of Ca2+ in the production still remains unknown. It is supposed that those pathways to induce NGF synthesis are not always independent but correlated by cross-talk (Jehan et al., 1995; Colangelo et al., 1996). Therefore, it is important to investigate the role of Ca2+, one of the representative second messengers, in the regulation of NGF gene expression. In this report, we examined the effects of maitotoxin on Ca2+ channel activation and NGF release in C6-BU-1 glioma cells.
Methods
Materials
Maitotoxin and A-23187 were obtained from Wako (Osaka, Japan). Dulbecco's modified Eagle's medium (DMEM) and horse serum were purchased from ICN Biochemicals, Inc. (Costa Mesa, CA, U.S.A.). Foetal calf serum was purchased from Cell Culture Laboratory (Cleveland, OH, U.S.A.). The following materials were obtained from the companies as indicated. 45CaCl2, Amersham (Buckinghamshire, U.K.); tetracaine, dibutyryl cyclic AMP (dbcAMP), Sigma (St. Louis, MO, U.S.A.); tetrodotoxin, Sankyo (Tokyo, Japan); fura 2 acetoxy methylester, 3-(4,5-dimethylthiazol-2-yl-) diphenyltetrazolium bromide (MTT), Dojindo (Kumamoto, Japan); NGF enzyme-linked immunosorbent assay (ELISA) kit, Boehringer Mannheim (Mannheim, Germany); RNA extraction kit, Pharmacia biotech (Piscataway, NY, U.S.A.); Reverse transcription polymerase chain reaction (RT–PCR) kit, Toyobo Co, Ltd (Osaka, Japan). All other chemicals were reagent grade.
Cell culture
Clonal rat glioma cells (C6-BU-1) were kindly supplied to us by Dr Amano (The Mitsubishi-Kagaku Institute of Life Sciences). The cells were maintained in DMEM containing 5% foetal calf serum and 5% heat-inactivated horse serum. Two days before experiments, these cells were subcultured on 35 mm plastic dishes (Becton Dickinson, Lincoln Park, NJ, U.S.A.) at a density of 2×105 cells dish−1 for C6-BU-1 cells.
Measurement of 45Ca2+ influx by maitotoxin
For assay of 45Ca2+ influx, cells cultured on 35 mm plastic dishes were washed once with a wash solution containing (mM): NaCl 130, KCl 5.4, CaCl2 1.8, MgSO4 0.8, glucose 5.5 and HEPES 50, pH 7.3 at 37°C. After aspiration, 45Ca2+ influx was inititated by adding 0.6 ml of assay solution containing (mM): sucrose 260, KCl 5.4, 45CaCl2 1.8, (0.3–0.9 Ci per dish), glucose 5.5 and HEPES 50, pH 7.3 with or without supplements of 10 ng ml−1 of maitotoxin or 46 mM KCl. After the desired incubation period at 37°C, the assay solution was removed to stop the 45Ca2+ influx and the cells were washed with the ice-cold wash solution quickly four times within 15 s. After lysis of the cells in 1 ml of 1% Triton X-100, the radioactivity was determined by scintillation counting. The protein content was measured in parallel plates. The background 45Ca2+ influx in the absence of maitotoxin and high KCl has been subtracted from each set of values.
Measurement of intracellular free Ca2+ concentration with fura 2
Intracellular free Ca2+ concentration ([Ca2+]i) was measured by the fura 2 assay as described previously (Ohkubo et al., 1996).
NGF enzyme immunoassay
NGF secretion from C6-BU-1 cells was examined by ELISA. Samples for NGF ELISA were prepared as follows. C6-BU-1 cells were seeded into 24-well multiplates and allowed to grow to confluence. The day before incubation, the medium was replaced with serum-free DMEM. After washing with serum-free DMEM, drugs in DMEM/1% BSA without the serum were added to the wells. The cells were cultivated for 24 h, and 500 μl of the conditioned medium was collected. The NGF content in the medium was measured as extracellular NGF by the sandwich ELISA, according to the instructions of the NGF ELISA kit.
RT–PCR analysis
NGF mRNA expression was examined by using a RT–PCR technique as described previously (Honma et al., 1998). The sense primer (5′-CTT CAG CAT TCC CTT GAC AC-3′, 316–335 of rat NGF cDNA) and the antisense primer (5′-AGC CTT CCT GCT GAG CAC ACA-3′, 889–909) were conserved regions of the cDNA from rat NGF (Amand et al., 1996). The NGF cDNA fragment was amplified 35 cycles (94°C for 60 s, 57°C for 60 s, 72°C for 10 s). Glyceraldehyde 3-phosphate dehydrogenase (G3PDH) transcripts were used as a positive control. PCR products, which had been migrated by electrophoresis on 2% agarose gels and stained by ethidium bromide, were analysed by an image scanner (Foto/Eclipse, Fotodyne Inc., WI, U.S.A.).
MTT assay
C6-BU-1 cells were seeded on 96-well plates (200 μl well−1) at a density of 5×105 cells ml−1. At the end of the experiment, MTT (0.1 mg) was added to each well and the plates were incubated for 4 h at 37°C. After centrifugation at 350×g for 5 min, the medium was replaced with dimethyl sulphoxide. The absorbance of reduced MTT at 595 nm was measured with a plate reader (Taglialatela et al., 1997).
Measurement of protein content
Protein content was determined by dye-binding method with BSA as the standard (Bradford, 1976).
Statistical analysis
Data were expressed as mean values or means±s.e.mean, and the significant difference was analysed with unpaired Student's t-test.
Results
Effect of maitotoxin on Ca2+ influx
Figure 1a shows the time courses of 45Ca2+ influx into C6-BU-1 cells after the addition of maitotoxin (10 ng ml−1) or high KCl (51.4 mM). The 45Ca2+ influx into C6-BU-1 cells was profoundly increased by matitotoxin after a brief period, but was not affected by high KCl. Maitotoxin induced a concentration-dependent increase in the 45Ca2+ influx into C6-BU-1 cells at concentrations above 1 ng ml−1 (Figure 1b).
Figure 1.
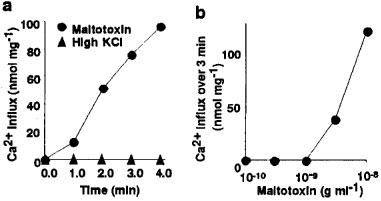
Stimulation of Ca2+ influx into C6-BU-1 cells by maitotoxin. (a) Time courses of 45Ca2+ influx by the treatment with maitotoxin (10 ng ml−1), and high KCl (51.4 mM). (b) Concentration-response curve for the effect of maitotoxin on the 45Ca2+ influx over 4 min. 45Ca2+ influx was initiated by adding 0.6 ml of (mM): sucrose 260, KCl 5.4, 45CaCl2 1.8, glucose 5.5 and HEPES 50, pH 7.3 supplemented with maitotoxin or KCl 46 mM. After desired incubation period at 37°C, the assay solution was removed to stop the influx, and then the cells were washed quickly four times. The cells were dissolved in 1% Triton X-100 and then the radioactivity was counted. The background 45Ca2+ influx (0.25 nmol mg−1 min−1) in the absence of maitotoxin and high KCl has been subtracted.
Substitution of sucrose with isotonic NaCl (130 mM) reduced the 45Ca2+ influx induced by maitotoxin to about one-twenty-fifth; however, the significant 45Ca2+ influx was still observed after treatment with maitotoxin (Figure 2a and b).
Figure 2.
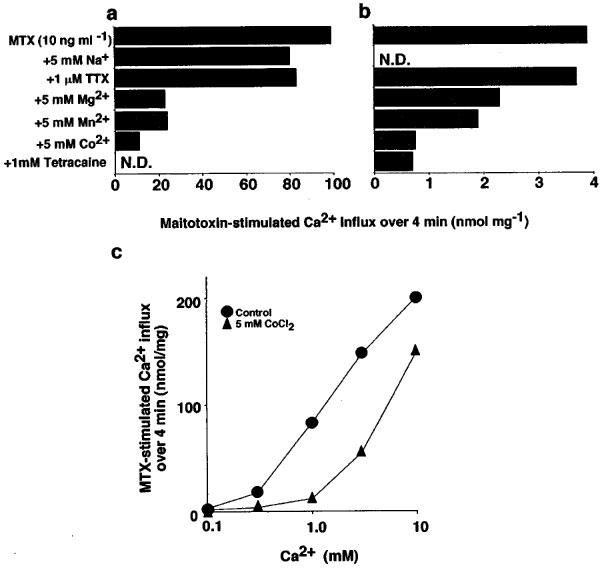
Characteristics of maitotoxin-induced 45Ca2+ influx. (a and b) Effects of several agents on the 45Ca2+ influx into C6-BU-1 cells induced by maitotoxin (MTX, 10 ng ml−1) in Na+-free (a) and Na+-containing solutions. (b) 45Ca2+ influx over 4 min was measured as described before, and the background influx (0.73 and 0.53 nmol mg−1 min−1 for a and b, respectively) has been subtracted from each set of values. The composition of Na+-containing solution was the same as that of assay solution described before except all the sucrose was substituted with 130 mM NaCl. (c) Effect of maitotoxin (MTX, 10 ng ml−1) on the log concentration response curve for Ca2+ in the presence or absence of Co2+. 45Ca2+ influx into C6-BU-1 cells over 4 min in the Na+-free assay solution was measured as described before and the background influx (0.22, 0.34, 0.58, 1.02 and 2.15 nmol mg−1 min−1 at 0.1, 0.3, 1, 3 and 10 mM of CaCl2, respectively) has been subtracted from each set of values. Results are mean of two determinations. N.D., not determined.
It is well known that the function of Ca2+ channels is blocked by inorganic Ca2+ channel blockers such as Mn2+, Mg2+ and Co2+ or by local anaesthetics, and that inhibitory effects of these Ca2+ channel blockers are competitively antagonized by external Ca2+ (Fleckenstein, 1971). Thus, the effects of various inorganic Ca2+ channel antagonists on maitotoxin-induced 45Ca2+ influx were studied both in Na+-containing and Na+-free solutions (Figure 2a and b). The 45Ca2+ influx induced by maitotoxin was markedly suppressed by divalent cations such as Mg2+, Mn2+ and Co2+ (5 mM) and tetracaine (1 mM), and was slightly suppressed by tetrodotoxin (1 μM) and Na+ (5 mM).
Figure 2c shows the inhibitory effect of Co2+ on the maitotoxin-stimulated 45Ca2+ influx into C6-BU-1 cells under various concentrations of external Ca2+. The average rate of the maitotoxin-stimulated 45Ca2+ influx over 4 min was increased upon increasing the concentration of external Ca2+ from 0.1 to 10 mM. In 1 mM Ca2+, Co2+ (5 mM) caused the inhibition of the 45Ca2+ influx by 89%, which was overcome by increasing the concentration of external Ca2+ to 10 mM.
Intracellular free Ca2+ concentration ([Ca2+]i) was measured by using fura 2. Maitotoxin (10 ng ml−1) caused [Ca2+]i elevation (Figure 3a). The [Ca2+]i elevation was weakly suppressed by pretreatment with Mg2+ (5 mM) (Figure 3b), and was completely inhibited by the removal of extracellular Ca2+ with EGTA (5 mM) (Figure 3c). High KCl (50 mM) caused neither change in basal [Ca2+]i nor in maitotoxin-induced [Ca2+]i elevation in C6-BU-1 cells (Figure 3e), indicating that the maitotoxin-induced [Ca2+]i elevation was attributed to Ca2+ influx through a voltage-insensitive Ca2+ channel. Since the addition of high KCl resulted in the hypertonic condition in this experiment, we also carried out the experiment using 50 mM NaCl instead of KCl, neglecting the hypertonic effect (Figure 3f).
Figure 3.
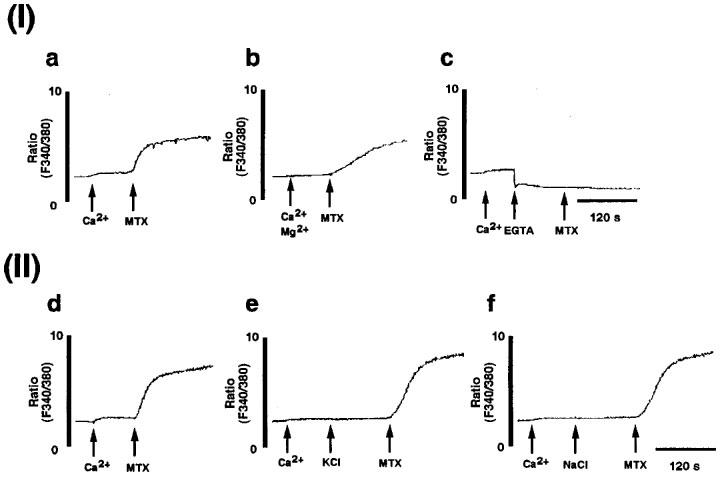
Maitotoxin-induced extracellular Ca2+-dependent voltage-insensitive Ca2+ influx. (I) Extracellular Ca2+ dependence of maitotoxin-induced Ca2+ influx in C6-BU-1 cells. [Ca2+]i was measured as described in Methods. The cells loaded with fura 2 acetoxy methylester were suspended in the Tyrode solution. (a) Maitotoxin (MTX, 10 ng ml−1) was added 90 s after addition of 1 mM Ca2+ (control for b and c). (b) Maitotoxin (MTX, 10 ng ml−1) was added 90 s after addition of 5 mM Mg2+ and 1 mM Ca2+. (c) Maitotoxin (MTX, 10 ng ml−1) was added after addition of 5 mM EGTA in the presence of 1 mM Ca2+. (II) Maitotoxin-induced Ca2+ influx through voltage-insensitive Ca2+ channel. (d) Maitotoxin (MTX, 10 ng ml−1) was added 90 s after addition of 1 mM Ca2+ (control for e and f). (e) Maitotoxin (MTX, 10 ng ml−1) was added 120 s after addition of high KCl (50 mM) in the presence of 1 mM Ca2+. (f) Maitotoxin (MTX, 10 ng ml−1) was added 120 s after addition of high NaCl (50 mM) in the presence of 1 mM Ca2+.
Effect of maitotoxin on NGF synthesis and secretion from C6-BU-1 cells
Since maitotoxin sometimes causes cell toxicity due to excess Ca2+ influx, cell viability was examined by the MTT-method (Taglialatela et al., 1997) in order to determine an appropriate incubation time and inappropriate maitotoxin concentration for NGF measurement. The result showed that almost all the cells remained alive under the following conditions: 3 ng ml−1 of maitotoxin for 24 h, and 10 ng ml−1 of maitotoxin for 3 h, while 50% of cells turned out to be dead with 10 ng ml−1 of maitotoxin for 24 h (data not shown). To examine the effect of maitotoxin on NGF synthesis and secretion, NGF content in the culture medium was measured by using ELISA. NGF contents in the conditioned medium were increased upon the treatment with maitotoxin (3 ng ml−1) and A-23187 (1 μM) as well as dbcAMP (0.5 mM) for 24 h (Figure 4). In addition, the NGF mRNA expression in C6-BU-1 cells was examined by using RT–PCR methods. Maitotoxin (10 ng ml−1) increased the expression of NGF mRNA, dependent on the extracellular Ca2+ concentration. EGTA abolished maitotoxin-induced the enhancement (Figure 5a). A-23187-induced NGF mRNA expression was also reversed by EGTA treatment as in the case of maitotoxin (Figure 5b). The results clearly demonstrated that NGF mRNA expression in C6-BU-1 cells was increased by maitotoxin in an extracellular Ca2+-dependent manner.
Figure 4.
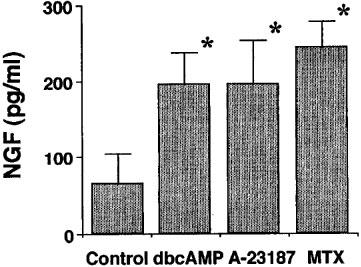
NGF secretion from C6-BU-1 cells in response to dbcAMP, A-23187 and maitotoxin. After incubation with dbcAMP (0.5 mM), A-23187 (1 μM) and maitotoxin (MTX, 3 ng ml−1) for 24 h, the medium from C6-BU-1 cell cultures were collected, and NGF content was measured using an ELISA. Values are the means±s.e.mean of three determinations. *P<0.05 vs control (without drug) in each cell.
Figure 5.
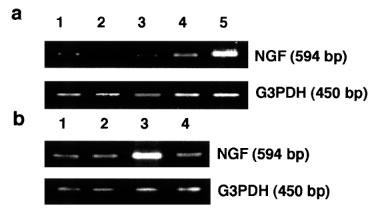
(a) NGF mRNA expression by maitotoxin. The cells were stimulated by maitotoxin for 3 h under various concentrations of extracellular Ca2+, then total RNA from C6-BU-1 cells was reverse transcribed followed by PCR as described before. Lane 1, control in 1.8 mM CaCl2; 2, control in 1.8 mM CaCl2+3.6 mM EGTA; 3, maitotoxin (10 ng ml−1) in 1.8 mM CaCl2+3.6 mM EGTA; 4, maitotoxin (10 ng ml−1) in 1.8 mM CaCl2+1.7 mM EGTA; 5, maitotoxin (10 ng ml−1) in 1.8 mM CaCl2. (b) NGF mRNA expression by A-23187. The cells were stimulated by A-23187 for 3 h in the presence or absence of 1.8 mM EGTA. Lane 1, control in 1.8 mM CaCl2; 2, control in 1.8 mM CaCl2+1.8 mM EGTA; 3, A-23187 (1 μM) in 1.8 mM CaCl2; 4, A-23187 (1 μM) in 1.8 mM CaCl2+1.8 mM EGTA.
Discussion
In the present experiments, maitotoxin induced a profound increase in Ca2+ influx into non-excitable glioma cells whereas high KCl treatment did not affect Ca2+ level at all. The effects of maitotoxin on C6-BU-1 cells were markedly suppressed by various Ca2+ channel blockers. The inhibitory effect of Co2+ was antagonized by external Ca2+ and became less obvious in the higher Ca2+ concentration range. These observations support the results that voltage-insensitive Ca2+ channels exist in the non-excitable plasma membranes of glioma cells, and that maitotoxin increases the Ca2+ permeability through these Ca2+ channels (Konoki et al., 1998; Murata et al., 1992). Recent lines of evidence suggest that maitotoxin activates non-selective cation channels (Bielfeldackermann et al., 1998; Dietl & Volkl, 1994; Estacion et al., 1996; Leech & Habener, 1997).
We demonstrated that maitotoxin and A-23187 as well as by dbcAMP caused NGF synthesis and secretion. In addition, the increase of NGF secretion by maitotoxin was initiated by its gene expression. Maitotoxin-induced NGF mRNA expression was completely reversed by the removal of extracellular Ca2+, indicating that Ca2+ influx is essential for NGF synthesis by maitotoxin. There have previously been a few reports regarding NGF mRNA expression enhanced by increasing [Ca2+]i. It was shown that N-methyl-D-aspartic acid (NMDA)-induced NGF production was reversed under the extracellular Ca2+-free condition in C6-BU-1 cells (Amano et al., 1992), suggesting that Ca2+ is necessary for NGF production. However, Jehan et al. (1995) showed that A-23187 inhibited the phorbol ester-induced production of the NGF mature protein in mouse primary astrocytes in spite of causing a weak NGF mRNA expression. It was shown that phorbol ester, cyclic AMP and Ca2+ ionophore expressed proto-oncogenes such as c-jun and c-fos (Jehan et al., 1995), the products of which might regulate the NGF mRNA expression. The Fos/Jun heterodimer complex known as activator protein-1 (AP-1), one of the major targets of protein kinase A (PKA) and PKC, could be a regulatory transcription factor for the expression of the NGF mRNA in vitro, which was supported by an identification of an AP-1 consensus sequence within downstream of the TATA box at the junction of the exon I/intron I region of rat and mouse NGF gene (D'Mello et al., 1991). In addition, it has been shown that the increase in AP-1 activity correlates with the induction of NGF mRNA (Colangelo et al., 1996).
It is reported that thapsigargin induces c-fos and c-jun expression mediated by increasing [Ca2+]i (Schonthal et al., 1991). On the other hand, it has been shown that PKA phosphorylates cyclic AMP responsive element (CRE)-binding protein (CREB) (Hagiwara et al., 1993). Since calmodulin kinase also activates CREB (Sheng et al., 1990), Ca2+ is assumed to cause the gene expression of proto-oncogenes such as c-fos via a CRE promoter. Therefore, it is supposed that the transcription activity of AP-1 is increased by the de novo synthesis of proto-oncogenesis via those pathways. In addition, since calmodulin kinase can activate adenylyl cyclase type I by phosphorylation (Impey et al., 1994), there is a possibility that increased [Ca2+]i by maitotoxin can activate PKA or CREB by cyclic AMP accumulation. Therefore, although further study is necessary, Ca2+ influx by maitotoxin causes NGF synthesis possibly through the expression of those proto-oncogenes or phosphorylation of AP-1 by PKA.
Exogenously administered NGF can serve as a neurotrophic factor in the brain, preventing neuronal death and activating neuronal function. However, it is difficult and inconvenient to administer NGF by intracerebral infusion, and peripherally administered NGF does not cross the blood–brain barrier. This is why the clarification of the mechanism of NGF synthesis in glial cells is indispensable for the development of new NGF inducers which pass through blood–brain barrier. These drugs will be useful for serious neuronal disorders such as Alzheimer's disease.
In conclusion, the present results suggest that maitotoxin activates the voltage-insensitive Ca2+ channel and accelerates NGF production in non-excitable C6-BU-1 glioma cells. Because maitotoxin is a powerful activator of voltage-insensitive Ca2+ channels, we believe that it will be a beneficial drug to clarify the role of Ca2+ in NGF synthesis and secretion.
Acknowledgments
We are grateful to Dr T. Amano for providing C6-BU-1 cells and his encouragement. This work was partly supported by a Grant-in-Aid from Scientific Research from the Ministry of Education, Science, Sport and Culture of Japan, Suzuken Memorial Foundation and the Research Foundation for Pharmaceutical Sciences.
References
- AMAND D., POTTAGE C., HENRY P., FAHNESTOCK M. Method for quantitation of low-abundance nerve growth factor mRNA expression in human nervous tissue using competitive reverse transcription polymerase chain reaction. DNA Cell Biol. 1996;15:415–422. doi: 10.1089/dna.1996.15.415. [DOI] [PubMed] [Google Scholar]
- AMANO T., YAMAKUNI T., OKABE N., KUWAHARA R., OZAWA F., HISHINUMA F. Regulation of nerve growth factor and nerve growth factor receptor production by NMDA in C6 glioma cells. Mol. Brain Res. 1992;14:35–42. doi: 10.1016/0169-328x(92)90007-x. [DOI] [PubMed] [Google Scholar]
- BENDA P., LIGHTBODY J., SATO G., LEVINE L., SWEET W. Differentiated rat glial cell strain in tissue culture. Science. 1968;161:370–371. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- BIELFELDACKERMANN A., RANGE C., KORMACHER C. Maitotoxin (MTX) activates a nonselective cation channel in xenopus laevis oocytes. Pflugers Arch. Eur. J. Physiol. 1998;436:329–337. doi: 10.1007/PL00008085. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;73:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CAMPBELL A.K. Intracellular Calcium, its Universal Role as Regulator. Chichester: John Wiley & Sons Ltd; 1983. [Google Scholar]
- CARMAN-KRZAN M., WISE B.C. Arachidonic acid lipoxygenation may mediate interleukin-1 stimulation of nerve growth factor secretion in astroglial cultures. J. Neurosci. Res. 1993;34:225–232. doi: 10.1002/jnr.490340210. [DOI] [PubMed] [Google Scholar]
- COLANGELO A.M., PANI L., MOCCHETTI I. Correlation between increased AP-1NGF binding activity and induction of nerve growth factor transcription by multiple signal transduction pathways in C6-2B glioma cells. Mol. Brain Res. 1996;35:1–10. doi: 10.1016/0169-328x(95)00171-n. [DOI] [PubMed] [Google Scholar]
- DIETL P., VOLKL H. Maitotoxin activates a non-selective cation channel and stimulates Ca2+ entry in MDCK renal epithelial cells. Mol. Pharmacol. 1994;45:300–305. [PubMed] [Google Scholar]
- D'MELLO S.R., HEINRICH G. Structural and functional identification of regulatory regions and cis elements surrounding the nerve growth factor gene promoter. Mol. Brain Res. 1991;11:225–264. doi: 10.1016/0169-328x(91)90034-u. [DOI] [PubMed] [Google Scholar]
- ESTACION M., NGUYEN H.B., GARGUS J.J. Calcium is permeable through a maitotoxin-activated nonselective cation channel in mouse L cells. Am. J. Physiol. 1996;270:C1145–C1152. doi: 10.1152/ajpcell.1996.270.4.C1145. [DOI] [PubMed] [Google Scholar]
- FLECKENSTEIN A. Calcium and Heart 1971London: Academic Press; 135–188.eds. Harris, P. & Opie, L.H. pp [Google Scholar]
- FUKUMOTO H., KAKIHANA M., SUNO M. Characterization of C6-10A glioma cells highly responsive to β-adrenergic receptor agonist-induced NGF synthesis/secretion. Glia. 1994;12:151–160. doi: 10.1002/glia.440120209. [DOI] [PubMed] [Google Scholar]
- GALVE-ROPERH I., HARO A., DIAZ-LAVIADA I. Induction of nerve growth factor synthesis by sphingomyelinase and ceramide in primary astrocyte cultures. Mol. Brain Res. 1997;52:90–97. doi: 10.1016/s0169-328x(97)00230-1. [DOI] [PubMed] [Google Scholar]
- GUSOVSKY F., DALY J.W. Maitotoxin: a unique pharmacological tool for research on calcium-dependent mechanisms. Biochem. Pharmacol. 1990;39:1633–1639. doi: 10.1016/0006-2952(90)90105-t. [DOI] [PubMed] [Google Scholar]
- HAGIWARA M., BRINDLE P., HAROOTUNIAN A., ARMSTRONG R., RIVER J., VALE W., TSIEN R., MONTMINY M.R. Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol. Cell Biol. 1993;13:4852–4859. doi: 10.1128/mcb.13.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANNUN Y.A. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- HEESE K., FIEBICH B.L., BAUER J., OTTEN U. NF-κB modulates lipopolysaccharide-induced microglial nerve growth factor expression. Glia. 1998;22:401–407. doi: 10.1002/(sici)1098-1136(199804)22:4<401::aid-glia9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- HONMA S., NAKAHATA N., OHIZUMI Y. Human astrocytoma cells express two thromboxane A2 receptor subtypes that communicate with Gq and G12. Prostaglandins and other lipid mediators. 1998;55:159–168. [Google Scholar]
- IMPEY S., WAYMAN G., WU Z., STORM D.R. Type I adenylyl cyclase functions as a coincidence detector for control of cyclic AMP response element-mediated transcription: synergistic regulation of transcription by Ca2+ and isoproterenol. Mol. Cell. Biol. 1994;14:8272–8281. doi: 10.1128/mcb.14.12.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEHAN F., NEVEU I., PHILIPPE N., NAVEIHAN P., WION D., BRACHET P. Interactions between second messenger pathways influence NGF synthesis in mouse primary astrocytes. Brain Res. 1995;672:128–136. doi: 10.1016/0006-8993(94)01337-h. [DOI] [PubMed] [Google Scholar]
- KALTSCHMIDT C., KALTSCHMIDT B., LANNES-VIEIRA J., KREUTZBERG G.W., WEKERLE H., BAEUERLE P.A., GEHRMANN J. Transcription factor NF-κB is activated in microglia during experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1994;55:99–106. doi: 10.1016/0165-5728(94)90151-1. [DOI] [PubMed] [Google Scholar]
- KONOKI K., HASHIMOTO M., NONOMURA T., SASAKI M., MURATA M., TACHIBANA K. Inhibition of maitotoxin-induced Ca2+ influx in rat glioma C6 cells by brevetoxins and synthetic fragments of maitotoxin. J. Neurochem. 1998;70:409–416. doi: 10.1046/j.1471-4159.1998.70010409.x. [DOI] [PubMed] [Google Scholar]
- LEECH C.A., HABENER J.F. Insulinotropic glucagon-like peptide-1-mediated activation of non-selective cation currents in insulinoma cells is mimicked by maitotoxin. J. Biol. Chem. 1997;272:17987–17993. doi: 10.1074/jbc.272.29.17987. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- LIN L.H., DOHERTY D.H., LILE J.D., BEKTESH S., COLLINS F. GDNF: A Glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- MOCCHETTI I., DE BERNARDI M.A., SZEKELY A.M., ALHO H., BROOKER G., COSTA E. Regulation of nerve growth factor biosynthesis by β-adrenergic receptor activation in astrocytoma cells: A potential role of c-Fos protein. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3891–3895. doi: 10.1073/pnas.86.10.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURATA M., GUSOVSKY F., YASUMOTO T., DALY J.W. Selective stimulation of Ca2+ flux in cells by maitotoxin. Eur. J. Pharmacol. 1992;227:43–49. doi: 10.1016/0922-4106(92)90140-q. [DOI] [PubMed] [Google Scholar]
- NEVEU I., JEHAN F., HOULGATTE R., WION D., BRACHET P. Activation of nerve growth factor synthesis in primary glial cells by phorbol 12-myristate 13-acetate: role of protein kinase C. Brain Res. 1992;570:316–322. doi: 10.1016/0006-8993(92)90596-2. [DOI] [PubMed] [Google Scholar]
- OHIZUMI Y. Application of physiologically active substances isolated from natural resources to pharmacological studies. Jpn. J. Pharmacol. 1997;73:263–289. doi: 10.1254/jjp.73.263. [DOI] [PubMed] [Google Scholar]
- OHKUBO S., NAKAHATA N., OHIZUMI Y. Thromboxane A2-mediated shape change: independent of Gq-phospholipase C-Ca2+ pathway in rabbit platelets. Br. J. Pharmacol. 1996;117:1095–1104. doi: 10.1111/j.1476-5381.1996.tb16702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAZAKI T., BIELAWSKA A., DOMAE N., BELL R.M., HANNUN Y.A. Characteristics and partial purification of a novel cytosolic, magnesium independent, neutral sphingomyelinase activated in the early signal transduction of 1α, 25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J. Biol. Chem. 1994;269:4070–4077. [PubMed] [Google Scholar]
- O'NEILL L.A.J., KALTSCHMIDT K. NF-κB: A crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- RUDGE J.S., PANSNIKOWSKI E.M., HOLST P., LINDSAY R.M. Changes in neurotrophic factor expression and receptor activation following exposure of hippocampal neuron/astrocyte cocultures to kainic acid. J. Neurosci. 1995;15:6856–6867. doi: 10.1523/JNEUROSCI.15-10-06856.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHONTHAL A., SUGARMAN J., HELLER-BROWN J., HANLEY M.R., FERAMISCO J.R. Regulation of c-fos and c-jun protooncogene expression by the Ca2+-ATPase inhibitor thapsigargin. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7096–7100. doi: 10.1073/pnas.88.16.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ J.P. Stimulation of nerve growth factor mRNA content in C6 glioma cells by a β-adrenergic receptor and by cyclic AMP. Glia. 1988;1:282–285. doi: 10.1002/glia.440010407. [DOI] [PubMed] [Google Scholar]
- SHENG M., THOMPSON M.A., GREENBERG M.E. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1990;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- TAGLIALATELA G., ROBINSON R., PEREZ-POLO J.R. Inhibition of nuclear factor kappa B (NFκB) activity induces nerve growth factor-resistant apoptosis in PC12. J. Neurosci. Res. 1997;47:155–162. [PubMed] [Google Scholar]
- WONG W.L., BROSTROM M.A., KUZNETSOV G., GMITTER-YELLEN D., BROSTROM C.O. Inhibition of protein synthesis and early protein processing by thapsigargin in cultured cells. Biochem. J. 1993;289:71–79. doi: 10.1042/bj2890071. [DOI] [PMC free article] [PubMed] [Google Scholar]


