Abstract
This study investigates the role of tachykinins in a repeated challenge with dinitrobenzene sulphonic acid (DNS) on the tracheal vascular permeability in dinitrofluorobenzene (DNFB)-sensitized mice.
DNFB-contact sensitization was followed by an intranasal (i.n.) challenge with DNS. A second challenge with DNS was administered 24 h after the first challenge. To assess changes in tracheal vascular permeability, Evans blue dye accumulation in tracheal tissue was measured.
A repeated challenge with DNS in DNFB-sensitized mice led to a 2.8 fold increase in tracheal vascular permeability when compared to DNFB-sensitized and vehicle-challenged mice or a 2.5 fold increase when compared to DNFB-sensitized single DNS-challenged mice (P<0.001, ANOVA).
RP67580 (10−9 mol mouse−1 i.v.) reduced the increased tracheal vascular permeability induced by a second exposure to DNS in DNFB-sensitized mice completely when injected 15 min before the second challenge (P<0.001, ANOVA).
The increased tracheal vascular permeability response induced by the second exposure to DNS could be mimicked with i.n. application of capsaicin (10−10 mol mouse−1) or substance P (SP) (10−12 mol mouse−1) to DNFB-sensitized and single DNS-challenged mice.
These results suggest that both tachykinin NK1 receptors and sensory nerves are involved in the development of vascular hyperpermeability changes found in the trachea of DNFB-sensitized mice after a repeated DNS-challenge.
Keywords: Tracheal vascular permeability, delayed-type hypersensitivity, sensory neuropeptides, tachykinins, capsaicin, tachykinin NK1-receptor
Introduction
Formation of airway wall oedema is a characteristic feature in lungs from patients with occupational as well as allergic asthma (Fabbri, 1994; Chung et al., 1990; Charan et al., 1997). Mucosal swelling is able to enhance airway reactivity to histamine (Brown et al., 1995). Oedema formation in the lungs not only reduces the airway compliance but it can also lead to shedding of the epithelium, generation of inflammatory mediators and activation of the sensory nerves (Goldie & Pedersen, 1995; Chung et al., 1990).
Tachykinins released from sensory nerves induce a wide range of inflammatory reactions. The inflammatory vascular effects are collectively referred to as neurogenic inflammation. These reactions are manifested by vasodilatation, increased vascular permeability and adherence of inflammatory cells to the endothelium thereby facilitating inflammatory cell infiltration (McDonald et al., 1991; McDonald, 1992). In addition, other actions of the tachykinins in the airways include bronchoconstriction, bronchial hyperresponsiveness and mucus secretion (Solway & Leff, 1991). It has been reported that the magnitude of the neurogenic inflammation can be altered by several factors. In rats, viral respiratory infections caused enhancement of the neurogenic inflammation induced by capsaicin and substance P (McDonald, 1988; 1992). This increased vascular responsiveness has been referred to as hyperpermeability.
Recently, a murine model for non-IgE mediated asthma has been developed and characterized in our laboratory (Buckley & Nijkamp, 1994a). In this model, a delayed-type hypersensitivity (DTH) reaction in the airways was induced by the low molecular weight compound dinitrofluorobenzene (DNFB), which was used as a skin-sensitizing agent. The sensitization was followed by an intra-airway challenge with dinitrobenzene sulphonic acid (DNS). Features of this DTH-induced pulmonary reaction included tracheal hyperreactivity to carbachol, mononuclear and neutrophilic cell infiltration and an increased mucosal exudation in the alveolar lumen at 24–48 h after the challenge (Buckley & Nijkamp, 1994a,1994b). Since the increase in mucosal exudation in the lungs was abolished by the depletion of the sensory nerves and pretreatment with the tachykinin NK1 receptor antagonist RP67580, the role of sensory neuropeptides and especially tachykinin NK1 receptors was evident for oedema formation found in this model (Buckley & Nijkamp, 1994b).
Although a single challenge with DNS in DNFB-sensitized mice did not reveal any changes in tracheal vascular permeability, we have found that a second challenge with DNS caused a profound increase in tracheal vascular permeability. The aim of the present study was to investigate the role of sensory nerves and the influence of capsaicin-induced release of neuropeptides on the tracheal vascular permeability. Furthermore, the role of tachykinin NK1-receptors are examined by the use of the selective tachykinin NK1 receptor antagonist RP67580, and its inactive enantiomer RP68651 and substance P (SP).
Methods
Animals
The experiments were performed on male Balb/c mice (5–7 weeks of age upon delivery) obtained from the Central Animal Laboratory, Utrecht, The Netherlands. The animals were allowed free access to tap-water and commercially available chow-food (Hope Farms, Woerden, The Netherlands). The mice were accommodated in macrolon cages and were rested at least 5 days before use. All experiments were approved by the Animal Care Committee of the Utrecht University.
Measurement of vascular permeability changes
Evans blue (25 mg kg−1) was used to assess changes in tracheal plasma extravasation and was administered intravenously (i.v.) to anaesthetized animals (sodium pentobarbitone 50 mg kg−1, intraperitoneally (i.p.)). During all experiments, Evans blue was administered 2 h before the animals were killed. Quantification of the extravasated Evans blue in the trachea provides a measurement of tracheal vascular permeability changes (Rogers et al., 1989; Udaka et al., 1970). Shortly before the animals were killed by an overdose of sodium pentobarbitone, they received heparin i.v. (10 U ml−1 blood) and blood samples were taken. Then the trachea was removed from the animal, dissected free of fat and connective tissue and was placed into formamide (250 μl). Evans blue was extracted overnight from the trachea at 40°C. Thereafter the tracheas were dried for 3 days at 40°C and dry weight was determined. The amount of Evans blue in formamide extracts and plasma samples were quantified by measuring the optical density at 595 nm with a Benchmark microplate reader (Biorad, CA, U.S.A.). The amount of Evans blue present in the trachea was calculated by dividing the Evans blue content in the formamide sample by the Evans blue content in 1 ml plasma. Changes in tracheal vascular permeability were expressed as μl leakage per mg tracheal dry weight or as percentage leakage of basal level in control animals.
Neuropeptide-induced changes in tracheal vascular permeability in naive mice; time-course and dose-response studies
The optimal time point for maximal plasma extravasation elicited by a dosage of capsaicin (10−7 mol mouse−1) or SP (10−10 mol mouse−1), both administered intranasally (i.n.), was established in anaesthetized naive mice. For capsaicin, a time-course study was carried out from zero to 120 min after the application. In case of SP, a range from zero to 60 min was examined. In another experiment changes in tracheal vascular permeability induced by increasing dosages of capsaicin (10−10–10−7 mol mouse−1, i.n.) or SP (10−13–10−10 mol mouse−1, i.n.) were investigated. In some animals RP67580 (10−9 mol mouse−1) was injected i.v. 15 min before i.n. application of capsaicin (10−8 mol mouse−1) or SP (10−10 mol mouse−1). In all experiments the i.n. applied volume was 50 μl.
Sensitization and challenge procedure
In a separate set of experiments mice were sensitized and challenged as described by Buckley & Nijkamp (1994a). Briefly, on days 0 and 1 of the experiment animals were skin-sensitized with either DNFB (5 mg ml−1) dissolved in a 4 : 1 mixture of acetone and olive oil (sensitized group) or vehicle (control group). During the procedure the animals were anaesthetized with i.p. injected sodium pentobarbitone (50 mg kg−1). On day 0, DNFB or vehicle was applied onto the shaved abdomen (50 μl) and the four paws (50 μl). On day 1, DNFB or vehicle was only applied onto the abdomen (50 μl).
On days 5 and 6, both sensitized and control groups were challenged i.n. under anaesthesia (sodium pentobarbitone 40 mg kg−1, i.p.) with either phosphate buffered saline (PBS) or DNS (6 mg ml−1 in PBS, 50 μl). The second challenge with DNS or PBS was applied 30 min before the animals were killed on day 6. The effect of a repeated challenge with vehicle or DNS was also examined in naive (non-sensitized) mice. The effect of the tachykinin NK1 receptor antagonist, RP67580, or its inactive enantiomer RP68651, on the changes in tracheal vascular permeability induced by a repeated challenge with DNS was investigated. RP67580 (10−9 mol mouse−1) or RP68651 (10−9 mol mouse−1) were injected i.v. 15 min before the second challenge with DNS. In further experiments the effect of capsaicin (10−10 mol mouse−1 i.n.) or substance P (10−13 and 10−12 mol mouse−1) was investigated on day 6 after the sensitization and challenge with DNS on day 5. Capsaicin and SP were administered at 30 and 15 min, respectively, before the mice were sacrificed 24 h after the first challenge with DNS on day 5.
Materials
DNFB was purchased from Sigma Chemical Co. (St. Louis, U.S.A.). DNS was obtained from Eastman Kodak Co. (Rochester, New York, U.S.A.). Evans blue and capsaicin were obtained from Fluka Chemika (Buchs, Switzerland). Substance P came from Calbiochem-Novabiochem (Lufelfingen, Switzerland). RP67580 ((3aR,7aR)-7,7-diphenyl-2-(1-imino-2-(2-methoxy-phenyl)ethyl)perhydroisoinol-4-one), and its inactive enantiomer RP68651 ((3aS,7aS)-7,7-diphenyl-2-(1-imino-2-(2-methoxy-phenyl)ethyl)perhydroisoinol-4-one) were gifts from Dr C. Garrett (Rhone-Poulenc Rorer, Vitry sur Seine, France). Sodium pentobarbitone (Nembutal) was purchased from Sanofi BV. (Maassluis, The Netherlands). Heparin was purchased from Leo Pharmaceutical Products (Ballerup, Denmark). Formamide was obtained from Merck (Darmstadt, Germany). Capsaicin was dissolved in a mixture of ethanol 96%, Tween 80 and saline (2 : 1 : 7) and was stored in a stock solution of 10−3 M and further dilutions were made in PBS. Substance P was dissolved in PBS. Both RP67580 and RP68651 were dissolved in ethanol 96% and stored in a stock solution of 10−2 M. Further dilutions were made in sterile saline.
Data analysis
The results are expressed as mean±s.e.mean. Differences between the groups were analysed by one-way analysis of variance (ANOVA) and followed by the Bonferroni's multiple comparison test or Dunnet's multiple comparison test (GraphPad Prism version 2.01, San Diego, U.S.A.). All P-values <0.05 were considered to reflect a statistically significant difference.
Results
Neuropeptide-induced tracheal vascular permeability changes in naive mice: time-course and dose-response studies
The time-course of the effect of capsaicin (10−7 mol mouse−1) or SP (10−10 mol mouse−1) on the changes in tracheal vascular permeability is summarized in Table 1. Capsaicin induced an increased tracheal vascular permeability over a period of 15–45 min after the challenge with a maximal response at 30 min (basal: 0.83±0.09 μl mg−1 tracheal dry weight, capsaicin: 2.27±0.30 μl mg−1 tracheal dry weight, P<0.001, ANOVA). The increase in vascular permeability returned to basal level after 60 min of exposure to capsaicin. SP reached a maximum at 15 min after exposure (basal: 0.20±0.14 μl mg−1 tracheal dry weight, SP: 0.61±0.22 μl mg−1 tracheal dry weight, P<0.01, ANOVA). The increase in tracheal vascular permeability returned to basal level 30 min after the application.
Table 1.
Results of a time-course study on the tracheal vascular permeability induced by capsaicin (10−7 mol mouse−1) or SP (10−10 mol mouse−1) administered i.n. to naive mice

For the dose-response studies, the animals were killed at the respective optimal time points for capsaicin and SP effects. Changes in tracheal vascular permeability elicited by several dosages of capsaicin only reached significance at the highest dose (Table 2). Although SP tended to increase tracheal vascular permeability in this experiment, no significant increase was found (Table 2).
Table 2.
Changes in the vascular permeability induced by different dosages of i.n. applied capsaicin or SP in the trachea of naive mice

The effect of RP67580 treatment on the neuropeptide-induced changes in tracheal vascular permeability
The effect of pretreatment with RP67580 (10−9 mol mouse−1) 15 min before application of capsaicin or SP is depicted in Figure 1. The increase in tracheal vascular permeability induced by capsaicin (10−8 mol mouse−1) could be blocked with RP67580 when injected i.v. (vehicle/PBS: 1.36±0.11 μl mg−1 tracheal dry weight, vehicle/capsaicin: 2.99±0.27 μl mg−1 tracheal dry weight, RP67580/capsaicin: 1.33±0.07 μl mg−1 tracheal dry weight, P<0.01, ANOVA). In addition, the increased tracheal vascular permeability induced by SP (10−10 mol mouse−1) could also be blocked by pretreatment with RP67580 (vehicle/PBS: 0.98±0.14 μl mg−1 tracheal dry weight, vehicle/substance P: 1.81±0.21 μl mg−1 tracheal dry weight, RP67580/SP: 0.91±0.15 μl mg−1 tracheal dry weight, P<0.01, ANOVA).
Figure 1.

The effect of RP67580 (10−9 mol mouse−1) injected i.v. on the tracheal vascular permeability changes induced by capsaicin (10−8 mol mouse−1, upper panel, black bars) or SP (10−10 mol mouse−1, lower panel, black bars) or PBS (white bars, both panels) in naive mice. Data are expressed μl leakage per mg tracheal dry weight 4–8 mice per group. *P<0.01 compared to the control groups, #P<0.01 compared to vehicle/SP or vehicle/capsaicin treated group, ANOVA, Bonferroni's multiple comparison test.
Effect of a repeated challenge with DNS on the tracheal vascular permeability in naive and DNFB-sensitized animals
In Figure 2 the effect of a repeated challenge with DNS on tracheal vascular permeability is depicted. This figure shows that a repeated challenge with DNS to naive mice had no influence on basal vascular permeability in the trachea (Figure 2, upper panel). However, a significant increase in tracheal vascular permeability was found after a repeated DNS challenge in the DNFB-sensitized group (Figure 2, lower panel). The change in tracheal vascular permeability induced by a repeated challenge with DNS was significantly increased compared to all sensitized control groups (DNFB/DNS/DNS: 2.09±0.23 μl mg−1 tracheal dry weight, DNFB/DNS/PBS: 0.81±0.07 μl mg−1 tracheal dry weight, DNFB/PBS/DNS: 0.85±0.04 μl mg−1 tracheal dry weight, DNFB/PBS/PBS: 0.74±0.13 μl mg−1 tracheal dry weight, P<0.001, ANOVA). In this experiment, a control group for a single challenge with DNS on either days 5 or 6 was included. The tracheal vascular permeability values of both single DNS-challenged DNFB-sensitized mice were comparable to the tracheal vascular permeability values observed in naive mice (Figure 2).
Figure 2.

Effect of a repeated challenge in naive (upper panel) and DNFB-sensitized (lower panel) mice 24 h after the first challenge. DNFB-sensitized animals were challenged from day 5 onwards. Mice were treated i.n. with two times PBS (open bars), an PBS and DNS-challenge (dotted bars), a challenge with DNS and PBS application (hatched bars) or two times DNS (closed bars). Changes in tracheal vascular permeability are expressed as μl leakage per mg tracheal dry weight for 5–7 mice per group and shown as mean±s.e.mean. *P<0.001 compared to all control groups, ANOVA, Bonferroni's multiple comparison test.
Effect of RP67580 on the tracheal vascular permeability due to a repeated DNS-challenge in DNFB-sensitized mice
The upper panel of Figure 3 shows that RP67580 (10−9 mol mouse−1) significantly blocked the DNFB/DNS/DNS-induced increase in tracheal vascular permeability injected 15 min before the second challenge with DNS (DNFB/DNS/vehicle/PBS: 0.72±0.08 μl mg−1 tracheal dry weight, DNFB/DNS/RP67580/PBS: 0.70±0.06 μl mg−1 tracheal dry weight, DNFB/DNS/vehicle/DNS: 1.97±0.13 μl mg−1 tracheal dry weight, DNFB/DNS/RP67580/DNS: 0.84±0.05 μl mg−1 tracheal dry weight, P<0.001, ANOVA). RP67580 itself had no effect on the tracheal vascular permeability in single DNS-challenged DNFB-sensitized mice. In contrast, treatment with the inactive enantiomer RP68651 did not affect the DNFB/DNS/DNS-induced increase in tracheal vascular permeability (Figure 3, lower panel).
Figure 3.
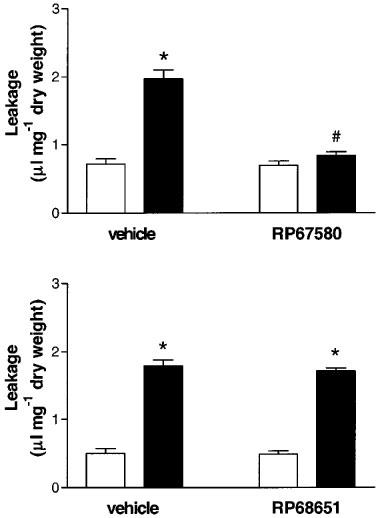
The effect of RP67580 (10−9 mol mouse−1, upper panel) or RP68651 (10−9 mol mouse−1, lower panel) injected i.v. on the increased vascular permeability after a repeated challenge with DNS in DNFB-sensitized mice. All animals were sensitized with DNFB and challenged with DNS on day 5. On day 6, mice were injected i.v. with either vehicle, RP67580 or RP68651 and exposed to a second challenge with DNS (black bars) or PBS (with bars). The changes in tracheal vascular permeability are expressed as μl leakage per mg tracheal dry weight for 7–10 mice per group and shown as mean±s.e.mean. *P<0.001 compared to the control groups, #P<0.001 compared to DNFB/DNS/DNS treated group, ANOVA, Bonferroni's multiple comparison test.
Effect of capsaicin and SP on the tracheal vascular permeability in vehicle-sensitized and DNFB-sensitized mice after single DNS challenge
Capsaicin (10−10 mol mouse−1) applied i.n. to DNFB-sensitized and single DNS-challenged mice resulted in a significant increase in tracheal vascular permeability (Figure 4, upper panel, DNFB/DNS/capsaicin: 2.11±0.17 μl mg−1 tracheal dry weight, DNFB/DNS/PBS: 1.05±0.04 μl mg−1 tracheal dry weight, vehicle/DNS/capsaicin: 0.71±0.10 μl mg−1 tracheal dry weight, vehicle/DNS/PBS: 0.79±0.06 μl mg−1 tracheal dry weight, P<0.001, ANOVA). The applied dose of capsaicin did not induce vascular permeability changes in naive mice or vehicle-sensitized and single DNS-challenged animals.
Figure 4.

Effect of capsaicin or SP in vehicle or DNFB-sensitized mice. At day 5, all animals were challenged with DNS. On day 6, mice were treated with 10−10 mol mouse−1 capsaicin (black bars, upper panel), 10−12 mol mouse−1 SP (black bars, lower panel) or PBS (white bars, in both panels). The changes in tracheal vascular permeability are expressed as μl per mg tracheal dry weight for 7–10 mice per group and shown as mean±s.e.mean. *P<0.001 compared to all control groups, ANOVA, Bonferroni's multiple comparison test.
SP was tested at two dosages of 10−13 and 10−12 mol mouse−1, respectively. Twenty-four h after the challenge with DNS, SP (10−13 mol mouse−1, i.n.) caused a small but significant increase in tracheal vascular permeability in DNFB-sensitized and single DNS-challenged mice (DNFB/DNS/SP: 0.85±0.06 μl mg−1 tracheal dry weight, DNFB/DNS/PBS: 0.57±0.02 μl mg−1 tracheal dry weight, vehicle/DNS/SP: 0.55±0.05 μl mg−1 tracheal dry weight, vehicle/DNS/PBS: 0.65±0.08 μl mg−1 tracheal dry weight, P<0.005, ANOVA). In addition, SP in a dose of 10−12 mol mouse−1 also increased the plasma extravasation in DNFB-sensitized and single DNS-challenged mice compared to vehicle-sensitized and single DNS-challenged animals (Figure 4, lower panel, DNFB/DNS/SP: 1.93±0.21 μl mg−1 tracheal dry weight, DNFB/DNS/PBS: 0.98±0.08 μl mg−1 tracheal dry weight, vehicle/DNS/SP: 0.86±0.03 μl mg−1 tracheal dry weight, vehicle/DNS/PBS: 0.99±0.09 μl mg−1 tracheal dry weight, P<0.001, ANOVA). No effect of SP was found in the vehicle-sensitized and single DNS-challenged group (Figure 4).
Discussion
This study shows that i.n. application of capsaicin or SP to naive mice increased the tracheal vascular permeability in a time-dependent and dose-dependent like manner. The increase in tracheal vascular permeability induced by capsaicin as well as by SP could be abolished by pretreatment with the tachykinin NK1 receptor antagonist, RP67580. This indicates that changes in tracheal vascular permeability due to capsaicin or SP are mediated through the activation of tachykinin NK1-receptors. These findings are consistent with data for tracheal permeability studies in rats. In rats, capsaicin or SP induced an increase in tracheal vascular permeability (Baluk et al., 1997; Eglezos et al., 1991; Germonpré et al., 1995). In case of capsaicin this increased response could be inhibited with a tachykinin NK1 receptor antagonist (Eglezos et al., 1991).
This study also demonstrates that the susceptibility of the tracheas to capsaicin, SP, and DNS is changed in a non-IgE mediated murine model for asthma. In DNFB-sensitized mice exposed to a repeated challenge with DNS, an increased tracheal vascular permeability was found that could be completely abolished by pretreatment with RP67580. This indicates that DNS may release SP that subsequently acts on the tachykinin NK1 receptor. Moreover, the increased permeability response to a repeated DNS challenge could be mimicked by i.n. application of capsaicin or SP to DNFB-sensitized and single DNS-challenged mice. The applied dosages of SP or capsaicin did not cause any increase in permeability in vehicle-sensitized single DNS-challenged mice or non-sensitized non-challenged (naive) mice. This change in susceptibility to neurogenic inflammation is not restricted to our model but has been reported before in rats infected with viral respiratory tract infections like Mycoplasma pulmonis (McDonald, 1988).
The increased tracheal vascular permeability induced by a repeated challenge with DNS or by i.n. application of capsaicin or SP to DNFB-sensitized and single DNS-challenged mice could be a result of several factors. Firstly, the increased tracheal vascular permeability could be a result from a decreased degradation or an increased release of tachykinin from the sensory nerves. It has been demonstrated that viral respiratory infections and inhalation of occupational irritants were capable in reducing the activity of enzymes like neutral endopeptidase which break down tachykinins or other inflammatory peptides (Bertrand et al., 1993; McDonald, 1992; Sheppard et al., 1988). This reduction in enzyme activity could increase the neuropeptide content in the airways leading to more pronounced effects elicited by tachykinins.
Secondly, the increased vascular permeability response to capsaicin or DNS could be the consequence of an increased sensitivity of the sensory nerves for various stimuli. It is known that cytokines as tumour necrosis factor (TNF)-α can alter the sensitivity of the sensory nerves for neurogenic stimulants. In ex vivo perfused rat tracheas, TNF-α enhanced the capsaicin-induced release of calcitonin gene-related peptide from sensory nerves (Hua et al., 1996). In addition, pretreatment of a sensory neurone culture with TNF-α not only increased the sensitivity of the neurones to the excitation produced by capsaicin but also enhanced the number of the cobalt-labelled capsaicin-sensitive neurones (Nicol et al., 1997).
In our study, not only the susceptibility of tracheas from mice to capsaicin after DNFB-sensitization and single DNS-challenge was increased but also the sensitivity to SP was enhanced. This indicates that not only an increase in sensitivity of the sensory nerves for capsaicin or DNS but also a pronounced effect via a receptor-coupled process could be another possible explanation for the increased tracheal permeability changes in this study. It is well documented that the tracheobronchiolar microvessels are part of the systemic circulation and form a dense network in the submucosa of the trachea (Goldie & Pedersen, 1995). Neovascularization of these tracheobronchiolar vessels with subsequent upregulation of tachykinin NK1 receptors could be a third explanation for the increased susceptibility of the tracheal vascular permeability in DNFB-sensitized mice. Rats infected with Mycoplasma pulmonis had more angiogenic blood vessels compared to pathogen-free rats (McDonald et al., 1991). Moreover, these newly formed blood vessels were abnormal sensitive to inflammatory mediators by upregulation of SP receptors (Baluk et al., 1997). In addition, patients with mild bronchial asthma have an increased number of blood vessels present in the trachea which indicates that a neovascularization process is evident in the trachea even in mild forms of asthma (Li & Wilson, 1997).
In summary, these present results indicate that the susceptibility of the tracheas to DNS, capsaicin, or SP is increased in DNFB-sensitized and single DNS-challenged mice. These increased tracheal vascular permeability responses are tachykinin NK1-receptor mediated.
Abbreviations
- DNFB
dinitrofluorobenzene
- DNS
dinitrobenzene sulphonic acid
- DTH
delayed-type hypersensitivity
- i.n.
intranasal
- SP
substance P
References
- BALUK P., BOWDEN J.J., LEFEVRE P.M., MCDONALD D.M. Upregulation of substance P receptors in angiogenesis associated with chronic airway inflammation in rats. Am. J. Physiol. 1997;273:L565–L571. doi: 10.1152/ajplung.1997.273.3.L565. [DOI] [PubMed] [Google Scholar]
- BERTRAND C., GEPPETTI P., BAKER J., YAMAWAKI I., NADEL J.A. Role of neurogenic inflammation in antigen-induced vascular extravasation in guinea-pig trachea. J. Immunol. 1993;150:1479–1485. [PubMed] [Google Scholar]
- BROWN R.H., ZERHOUNI E.A., MITZNER W. Airway edema potentiates airway reactivity. J. Appl. Physiol. 1995;79:12424–1248. doi: 10.1152/jappl.1995.79.4.1242. [DOI] [PubMed] [Google Scholar]
- BUCKLEY T.L., NIJKAMP F.P. Airways hyperreactivity and cellular accumulation in a delayed-type hypersensitivity reaction in the mouse: Modulation by capsaicin-sensitive nerves. Am. J. Respir. Crit. Med. 1994a;149:400–407. doi: 10.1164/ajrccm.149.2.8306037. [DOI] [PubMed] [Google Scholar]
- BUCKLEY T.L., NIJKAMP F.P. Mucosal exudation associated with a pulmonary delayed-type hypersensitivity reaction in the mouse: Role for the tachykinins. J. Immunol. 1994b;153:4169–4178. [PubMed] [Google Scholar]
- CHARAN N.B., BAILE E.M., PARÉ P.D. Bronchial vascular congestion and angiogenesis. Eur. Respir. J. 1997;10:1173–1180. doi: 10.1183/09031936.97.10051173. [DOI] [PubMed] [Google Scholar]
- CHUNG K.F., ROGERS D.F., BARNES P.J., EVANS T.W. The role of increased airway microvascular permeability and plasma exudation in asthma. Eur. Respir. J. 1990;3:329–337. [PubMed] [Google Scholar]
- EGLEZOS A., GIULIANI S., VITI G., MAGGI C.A. Direct evidence that capsaicin-induced plasma protein extravasation is mediated through tachykinin NK1 receptors. Eur. J. Pharmacol. 1991;209:277–279. doi: 10.1016/0014-2999(91)90183-q. [DOI] [PubMed] [Google Scholar]
- FABBRI L.M. Airway inflammation in occupational asthma. Am. J. Respir. Crit. Med. 1994;150:S80–S82. doi: 10.1164/ajrccm/150.5_Pt_2.S80. [DOI] [PubMed] [Google Scholar]
- GERMONPRÉ P.R., JOOS G.F., EVEREART E., KIPS J.C., PAUWELS R.A. Characterization of neurogenic inflammation in the airways of two highly inbred rat strains. Am. J. Respir. Crit. Care Med. 1995;152:1796–1804. doi: 10.1164/ajrccm.152.6.8520739. [DOI] [PubMed] [Google Scholar]
- GOLDIE R.G., PEDERSEN K.E. Mechanisms of increased airway microvascular permeability: role in airway inflammation and obstruction. Clin. Exp. Pharmacol. Physiol. 1995;22:387–396. doi: 10.1111/j.1440-1681.1995.tb02028.x. [DOI] [PubMed] [Google Scholar]
- HUA X.Y., CHEN P., FOX A., MYERS R.R. Involvement of cytokines in lipopolysaccharide-induced facilitation of CGRP release from capsaicin-sensitive nerves in the trachea; studies with interleukin-1 and tumor necrosis factor-α. J. Neurosci. 1996;16:4742–4748. doi: 10.1523/JNEUROSCI.16-15-04742.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI X., WILSON J.W. Increased vascularity of the bronchial mucosa in mild asthma. Am. J. Respir. Crit. Med. 1997;156:229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- MCDONALD D.M. Respiratory tract infections increase susceptibility to neurogenic inflammation in the rat trachea. Am. Rev. Respir. Dis. 1988;137:1432–1440. doi: 10.1164/ajrccm/137.6.1432. [DOI] [PubMed] [Google Scholar]
- MCDONALD D.M. Infections intensify neurogenic plasma extravasation in the airway mucosa. Am. Rev. Respir. Dis. 1992;146:S40–S44. doi: 10.1164/ajrccm/146.5_Pt_2.S40. [DOI] [PubMed] [Google Scholar]
- MCDONALD D.M., SCHOEB T.R., LINDSEY J.R. Mycoplasma pulmonis infections cause long-lasting potentiation of neurogenic inflammation in the respiratory tract of the rat. J. Clin. Invest. 1991;87:787–799. doi: 10.1172/JCI115082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICOL G.D., LOPSHIRE J.C., PAFFORD C.M. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J. Neurosci. 1997;17:975–982. doi: 10.1523/JNEUROSCI.17-03-00975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D.F., BOSCHETTO P., BARNES P.J. Plasma exudation: Correlation between Evans Blue dye and radiolabeled albumin in guinea pig airways in vivo. J. Pharmacol. Methods. 1989;21:309–315. doi: 10.1016/0160-5402(89)90068-5. [DOI] [PubMed] [Google Scholar]
- SHEPPARD D., THOMPSON J.E., SCYPINSKI L., DUSSER D., NADEL J.A., BORSON D.B. Toluene diisocyanate increases airway responsiveness to substance P and decreases airway neutral endopeptidase. J. Clin. Invest. 1988;81:1111–1115. doi: 10.1172/JCI113424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLWAY J., LEFF A.R. Sensory neuropeptides and airway function. J. Appl. Physiol. 1991;71:2077–2087. doi: 10.1152/jappl.1991.71.6.2077. [DOI] [PubMed] [Google Scholar]
- UDAKA K., TAKEUCHI Y., MOVAT H.Z. Simple method for quantification of enhanced vascular permeability. Proc. Soc. Exp. Biol Med. 1970;133:1384–1387. doi: 10.3181/00379727-133-34695. [DOI] [PubMed] [Google Scholar]


