Abstract
Isoproterenol relaxed KCl-precontracted rat bladder strips with a pD2 of 7.21 leaving a residual contractile response of 3.2% after 30 μM. The selective β1-agonist, T-0509 (pD2 : 6.24, 10.1% residual contraction after 100 μM), β2-agonist, terbutaline (pD2 : 5.43, 13.7% residual contraction after 100 μM), and β3-agonists, BRL 37344A (pD2 : 6.60, 17.3% residual contraction after 100 μM), and SR 58611A (pD2 : 5.15, 34.0% residual contraction after 100 μM), also relaxed bladder strips.
The relaxant response to isoproterenol was weakly but significantly antagonized by 1 μM propranolol which produced a 3 fold shift of the concentration-response curve to the right, and significantly antagonized by the β1-selective antagonist, metoprolol (10 μM, 3 fold shift), and the β2-selective antagonist, butoxamine (100 μM, 6 fold shift). A combination of 10 μM metoprolol and 100 μM butoxamine caused a 15 fold shift of the concentration-response curve for isoproterenol to the right. Incubation with the β3-antagonist, SR 59230A (1 μM), caused a 6 fold shift of the concentration response curve for isoproterenol to the right.
The non-conventional partial agonist, CGP 12177A, weakly relaxed KCl-precontracted bladder strips (pD2 : 3.31, 51.3% residual contraction after 300 μM); the relaxation was resistant to blockade by 1 or 10 μM propranolol.
In the presence of 200 μM propranolol, CGP 12177A (20 μM) or SR 59230A (10 μM) antagonized surmountably the relaxant effects of BRL 37344A.
The data suggest that rat urinary bladder body contains β1, β2, and β3-adrenoceptors, all of which mediate relaxation.
Keywords: Rat, bladder, muscle contraction, selective agonists, antagonists, β-adrenoceptors
Introduction
The function of the urinary bladder is the storage and periodic emptying of urine. This is regulated by the sacral parasympathetic (pelvic), thoracolumbar sympathetic (hypogastric), and sacral somatic (pudendal) nerves (de Groat et al., 1993). The primary role of the sympathetic pathways is to cause excitation of the urethra and bladder neck and maintain closure of the bladder outlet during the filling phase of the micturition cycle. In part, this occurs because of the regional distribution of adrenoceptors in the urinary bladder. α-Adrenoceptors mediating smooth muscle contraction, predominate in the bladder neck and urethra, but are sparsely distributed in the bladder body (Edvardsen & Setekleiv, 1968; Levin & Wein, 1979). The role of the sympathetic pathways in the bladder body is less clear. Sympathetic innervation of the bladder body is sparse (Lincoln & Burnstock, 1993), although β-adrenoceptors mediating relaxation are present and predominate over α-adrenoceptors. Some studies suggest that activation of β-adrenoceptors during bladder filling promotes relaxation of the bladder body and contributes to storage of urine at low intravesical pressure (Andersson, 1993; Vaughan & Satchell, 1995). However, the lack of effect of β-adrenoceptor antagonists on the human bladder in situ has been cited as evidence against β-adrenoceptors having any major functional role (Andersson, 1986).
Since the subdivision of β-adrenoceptors into the β1- and β2-subtypes by Lands and co-workers (Lands et al., 1967), evidence has accumulated that suggests that additional β-adrenoceptors exist. Thus, atypical β-adrenoceptors have been found in white and brown adipocytes, cardiac tissues, and the respiratory and gastrointestinal tracts (Arch & Kaumann, 1993). The evidence from these studies suggests that the classification of β-adrenoceptor subtypes must be expanded to include β3-adrenoceptors, as well as other atypical β-adrenoceptors (McLaughlin & MacDonald, 1990; Arch & Kaumann, 1993; Martin et al., 1994; Koike et al., 1995; Oriowo, 1995, 1997; Ten Berge et al., 1995; Kaumann & Molenaar, 1996). For an effect to be mediated through β3-adrenoceptors, the following criteria need to be met: (i) low sensitivity to antagonists with high affinity for β1- or β2-adrenoceptors, (ii) stimulation by β3-selective agonists, (iii) stimulation by non-conventional partial agonists (β-blocking agents that exhibit agonist effects at concentrations considerably greater than those causing blockade of β1- and β2-adrenoceptors), such as CGP 12177A, and (iv) sensitivity to β3-selective antagonists (Arch & Kaumann, 1993; Kaumann & Molenaar, 1996). Some early studies suggested that atypical β-adrenoceptors are present in the human urinary bladder, based on insensitivity of bladder strips to selective β1- and β2-antagonists (Nergårdh et al., 1977; Larsen, 1979). Recent pharmacological studies have concluded that the relaxant response of rat bladder to isoproterenol and other non-selective β-agonists is mediated by β2- and β3-adrenoceptors (Oshita et al., 1997; Yamazaki et al., 1998). Furthermore, mRNA for β1-, β2-, and β3-adrenoceptors has been detected in rat bladder using RT–PCR (Seguchi et al., 1998; Fujimura et al., 1999). However, β1-adrenoceptor-mediated relaxation has not been demonstrated. The objective of this study was to evaluate the relative effects of stimulation of β1-, β2-, and β3-adrenoceptors in the rat bladder.
Methods
Animals
Adult Sprague Dawley rats obtained from Charles River Laboratories were used throughout the study. All animals received food and water ad libitum. The protocols employed for this investigation were approved by the Albany College of Pharmacy Institutional Animal Care and Use Committee.
Tissue preparation
Rats were anaesthetized with Nembutal (50 mg kg−1, i.p.). The urinary bladder was removed and placed in ice-cold Krebs-Henseleit buffer of the following composition (mM): NaCl 113, KCl 4.8, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, dextrose 5.6, containing 10 μM indomethacin. Four equally-sized longitudinal strips of approximately 2×10 mm were cut from the bladder body, suspended on 000 sutures between a pair of platinum ring electrodes, 8 mm apart, and placed in 10 ml organ baths containing Krebs-Henseleit solution equilibrated with 95% O2, 5% CO2 at 37°C. The sutures were connected to Grass force displacement transducers (FT03) and the resting tension was adjusted to 2 g. Responses were recorded on a Grass Model 7E polygraph. All tissues were then given a 30 min equilibration period during which they were washed and the resting tension was adjusted every 10 min.
Contractile studies
After the equilibration period, strips were contracted by adding 5 μM carbachol or 40 mM KCl, 4 mM CaCl2 hypertonically to the organ baths. After a period of about 10 min, during which the contractile response stabilized, cumulative concentration-effect curves to isoproterenol or other agonists were generated. Drugs were added at 2–3 min intervals in 0.5 log unit increments as described by van Rossum (1963). After addition of the highest concentration of agonist, maximal relaxation was determined by addition of 10 μM pinacidil or cromakalim. Then tissues were washed at least twice with normal Krebs. Subsequent recontraction with KCl and relaxation with β-agonists was repeated after incubation with antagonists or Krebs buffer for 30 min. Responses to BRL 37344A were measured on unpaired strips because of the potential for tachyphylaxis.
Drugs
Butoxamine hydrochloride, carbamylcholine chloride (carbachol), cromakalim, indomethacin, (±)-isoproterenol hydrochloride, (±)-metoprolol tartrate, DL-propranolol hydrochloride, and terbutaline hemisulfate were obtained from Sigma Chemical Company (St. Louis, MO, U.S.A.). BRL 37344A ((±) - R*,R*) - [4 - [2 - [[2 - (3 - Chlorophenyl)-2-hydroxyetheyl]amino]propyl]phenoxy]-acetic acid sodium), (±)-CGP-12177A HCl (4-[3-[(1,1-Dimethylethyl)amino]-2-hydroxypropoxy]-1,3-dihydro-2H-benzimidazol-2-one hydrochloride), and pinacidil were obtained from RBI (Natick, MA, U.S.A.). SR 58611A (ethyl{(7S)-7-[(2R)-2-(3-chlorophenyl)-2-hydroxyethylamino] - 5,6,7,8 - tetrahydronaphthalen-2-yloxy}acetate hydrochloride) and SR 59230A ((3-(2-ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronaphth-1-ylamino]-2S-2-propanol oxalate) were kindly provided by Sanofi Recherche (Milan, Italy). T-0509 ([(−)-(R)-1-(3,4-dihydroxyphenyl)-2-[(3,4-dimethoxyphenethyl) amino]ethanol]-hydrochloride was kindly provided by Tanabe Seiyaku Co., Ltd. (Osaka, Japan).
Statistical analyses
Data are normalized relative to the contractile response to carbachol or KCl before addition of the β-agonist and the maximal relaxation in the presence of pinacidil or cromakalim, and are expressed as means±s.e.mean with N=number of rats and n=number of strips. Contractile responses were normalized for changes in tissue size by dividing the change in tension by the cross-sectional area. Cross sectional area was calculated from the equation; mm2=strip mass (g)/ density (1.05 g ml−1)×initial strip length (mm). Geometric mean EC50 values were obtained by probit analysis (Fleming et al., 1972; Kenakin, 1984) and are reported as pD2 values (−log EC50=pD2). Apparent pA2 values were estimated from the shift of an agonist concentration-effect curve by a single concentration of antagonist from the equation; pA2=log (CR-1) −log[antagonist]. Comparisons between responses before and after incubation with antagonists were done by the Student's t-test. Comparisons between groups were made using analysis of variance followed by Newman-Keul's multiple range test. In all cases, a P value <0.05 was considered significant.
Results
In preliminary experiments, we tested the relative ability of isoproterenol to relax KCl- or carbachol-precontracted rat bladder strips. There were no significant differences in the contractile responses to 40 mM KCl + 4 mM CaCl2 (20.33±1.83 mN/mm2; N=6, n=12) or to 5 μM carbachol (26.88±3.47 mN/mm2; N=6, n=12). However, while isoproterenol almost completely relaxed KCl-precontracted strips with a pD2 of 7.24, it was significantly less potent at relaxing carbachol-precontracted strips (pD2 : 5.32) and there was a 35% residual contraction at the highest concentration used (Figure 1). Over the same time period, there was approximately a 20% decline in the contractile response to KCl and to carbachol (Figure 1).
Figure 1.
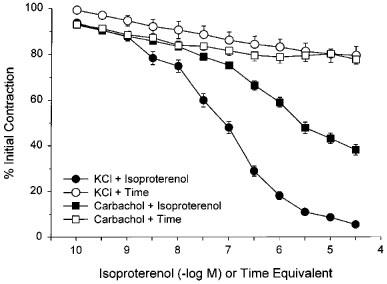
Responses of 40 mM KCl+4 mM CaCl2- or 5 μM carbachol-precontracted rat bladder body strips to time and to isoproterenol. Data are expressed as a percentage of the initial response to KCl or carbachol and maximal relaxation in the presence of pinacidil. Each point represents the mean±s.e.mean where N=6 and n=12.
Because of these differences in the ability of isoproterenol to stimulate relaxation in strips precontracted with carbachol or KCl, in all further studies relaxations were therefore measured after precontraction with 40 mM KCl+4 mM CaCl2. The contractile response to hypertonic addition of 40 mM KCl+4 mM CaCl2 to the organ baths was 24.98±1.19 mN/mm2 (N=26; n=99). After relaxation with maximal concentrations of β-adrenoceptor agonists, addition of 10 μM pinacidil or 10 μM cromakalim to the bath resulted in further relaxation to or below the basal tone level recorded before addition of KCl.
Isoproterenol relaxed KCl-precontracted bladder strips in a concentration-dependent manner (residual contraction 3.2±1.0% after 30 μM) with a pD2 of 7.21±0.07. The β1-agonist, T-0509, β2-agonist, terbutaline, and β3-agonists, BRL 37344A and SR 58611A, all relaxed KCl-precontracted bladder strips in vitro in a concentration-dependent manner (Figure 2). The rank order of agonist potency at relaxing bladder strips was isoproterenol=BRL 37344A=T-0509>terbutaline=SR 58611A (Table 1).
Figure 2.
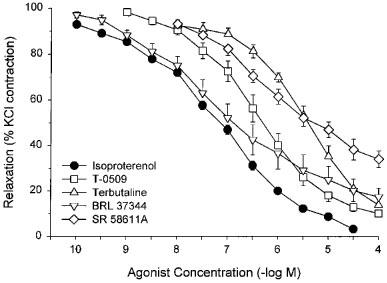
Responses of KCl-precontracted rat bladder body strips to isoproterenol, T-0509, terbutaline, BRL 37344A, and SR 58611A. Data are expressed as a percentage of the initial response to KCl and maximal relaxation in the presence of pinacidil or cromakalim. Each point represents the mean±s.e.mean where N=19, n=67 for isoproterenol, and N=n=5 or 6 for the other agonists.
Table 1.
Relative potencies of β-agonists at relaxing KCl-precontracted rat bladder body strips

Responses to isoproterenol were reproducible when repeated in the absence of antagonists (Figure 3). The responses to isoproterenol were weakly but significantly antagonized by 1 μM propranolol (Figure 3), which caused a 2.6 fold shift to the right of the pD2 values for paired strips giving an apparent pA2 of 6.19±0.06. Responses to isoproterenol were surmountably antagonized by the β3-antagonist, SR 59230A at a concentration of 1 μM (Figure 4), which caused a 5.7 fold shift to the right of the pD2 values for paired strips with an apparent pA2 of 6.54±0.13. The relaxant effects of isoproterenol were only partially blocked by high concentrations of the β1-antagonist, metoprolol (CR: 3.0±0.9; apparent pA2 : 5.02±0.25), and of the β2-antagonist, butoxamine (CR: 6.0±1.8; apparent pA2 : 4.66±0.15) (Figure 5). A combination of metoprolol and butoxamine caused a further rightward shift of the isoproterenol concentration curve (CR: 15.1±7.6) (Figure 5).
Figure 3.
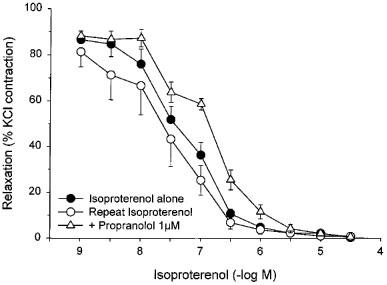
Responses of KCl-precontracted rat bladder body strips to cumulative addition of isoproterenol in the absence and presence of propranolol. The responses to isoproterenol before and after 30 min incubation in Krebs buffer containing vehicle (repeat) or 1 μM propranolol are shown. Data are expressed as a percentage of the initial response to KCl and maximal relaxation in the presence of cromakalim. Each point represents the mean±s.e.mean where N=5 and n=5–9.
Figure 4.
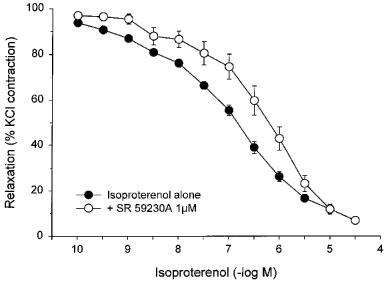
Responses of KCl-precontracted rat bladder body strips to cumulative addition of isoproterenol in the absence and presence of SR 59230A. The responses to isoproterenol before and after 30 min incubation with 1 μM SR 59230A are shown. Data are expressed as a percentage of the initial response to KCl and maximal relaxation in the presence of pinacidil. Each point represents the mean±s.e.mean where N=9 and n=7–29.
Figure 5.
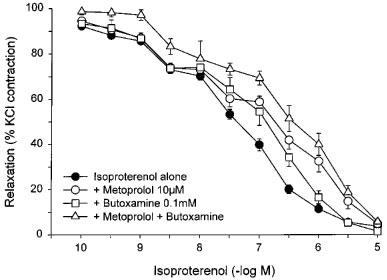
Responses of KCl-precontracted rat bladder body strips to cumulative addition of isoproterenol after β1- and β2-blockade. The responses to isoproterenol before and after 30 min incubation with 10 μM metoprolol, 0.1 mM butoxamine, or 10 μM metoprolol plus 0.1 mM butoxamine are shown. Data are expressed as a percentage of the initial response to KCl and maximal relaxation in the presence of pinacidil. Each point represents the mean±s.e.mean where N=7 and n=5–27.
The non-conventional partial agonist, CGP 12177A, weakly relaxed KCl-precontracted rat bladder strips with a pD2 of 3.31±0.20 (N=7, n=21), leaving a residual contraction of 51.3±1.9% (Figure 6). The response to CGP 12177A was resistant to inhibition by high concentrations of propranolol. After incubation with 1 and 10 μM propranolol, the pD2 values for CGP 12177A were 3.98±0.35 and 3.80±0.39, respectively.
Figure 6.
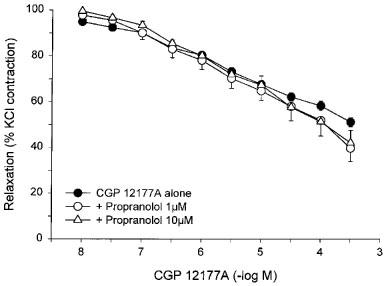
Responses of KCl-precontracted rat bladder body strips to cumulative addition of CGP 12177A. The responses to CGP 12177A before and after 30 min incubation with 1 μM or 10 μM propranolol are shown. Data are expressed as a percentage of the initial response to KCl and maximal relaxation in the presence of pinacidil. Each point represents the mean±s.e.mean where N=7 and n=6–21.
Relaxant responses to BRL 37344A were unaffected by 200 nM propranolol, a concentration which should block β1- and β2-adrenoceptors (Figure 7). However, a combination of propranolol and either 20 μM CGP 12177A or 10 μM SR 59230A caused significant surmountable antagonism, indicating a β3-adrenoceptor-mediated effect (Figure 7). The combination of propranolol and CGP 12177A caused a 9.9 fold shift and of propranolol and SR 59230A caused a 15.7 fold shift. Similar 12.8 and 10.7 fold shifts in the concentration-response curve to isoproterenol were produced by the combinations of propranolol and CGP 12177A and propranolol and SR 59230A (data not shown).
Figure 7.
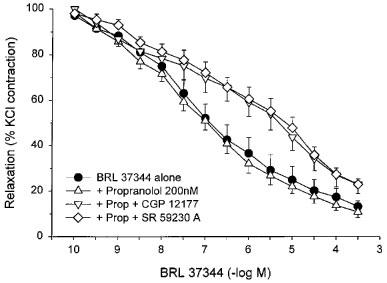
Responses of KCl-precontracted rat bladder body strips to cumulative addition of BRL 37344A after β1- and β2-blockade. The responses to BRL 37344A before and after 30 min incubation with 200 nM propranolol, 200 nM propranolol+20 μM CGP 12177A, and 200 nM propranolol+10 μM SR 59230A are shown. Data are expressed as a percentage of the initial response to KCl and maximal relaxation in the presence of pinacidil. Each point represents the mean±s.e.mean where N=6 and n=5 or 6.
Discussion
The results of several studies have suggested that atypical β-adrenoceptors are present in the urinary bladder. Larsen suggested that the human bladder contained neither β1- nor β2-adrenoceptors, based on the failure of 10 μM butoxamine or 10 μM practolol to alter the relaxant response to isoproterenol (Larsen, 1979. Similarly, Nergårdh and co-workers found the human bladder to be relatively insensitive to β1- or β2-selective agonists or antagonists (Nergårdh et al., 1977). In other species, isoproterenol-induced relaxation of the urinary bladder has been attributed to stimulation of predominantly the β1- [guinea-pig (Li et al., 1992) and cat (Nergårdh et al., 1977)], β2- [bovine (Garcia-Sacristán et al., 1985), horse (Labadia et al., 1988), human (Morita et al., 1993), pig (Larsen, 1979), rabbit (Morita et al., 1990, 1993), and rat (Elmér, 1974)], or both β1- and β2-adrenoceptors [dog (Morita et al., 1993) and sheep (Rivera et al., 1991; Morita et al., 1993)]. Recently, Oshita and co-workers and Yamazaki and co-workers suggested that β2- and β3-adrenoceptors were primarily responsible for β-adrenoceptor-induced relaxation in the rat bladder (Oshita et al., 1997; Yamazaki et al., 1998). Although β1-adrenoceptors have been identified in rat detrusor using reverse transcription polymerase chain reaction (RT–PCR) techniques (Seguchi et al., 1998; Fujimura et al., 1999), functional responses to β1-adrenoceptor stimulation have not been demonstrated. Our data confirm that β-adrenoceptor-mediated relaxation of rat bladder strips occurs in response to stimulation of β2- and β3-adrenoceptors, but also to stimulation of β1-adrenoceptors.
Our preliminary studies to find an appropriate agonist to precontract the bladder strips in order to monitor relaxant effects of the β-adrenoceptor agonists revealed some important differences between effectors. Isoproterenol was able to completely relax strips precontracted with KCl, but could only partly relax carbachol-precontracted strips with a 100 fold lower potency. The mechanisms responsible for these differences will be investigated in future studies, but one possible cause is a functional antagonism of β-adrenergic relaxation caused by stimulation of the muscarinic M2-receptor (Caulfield, 1993; Longhurst et al., 1995; Hegde et al., 1997). This has previously been proposed to occur in the rat bladder (Hegde et al., 1997). The dramatic difference in isoproterenol's efficacy under these two experimental conditions suggests that muscarinic agonists should not be used to precontract tissues containing heterogeneous populations of muscarinic receptors when responses to agonists which cause relaxation by increasing cyclic AMP levels are to be studied.
For an effect to be mediated through β3-adrenoceptors, we used the criteria described by Kaumann (Arch & Kaumann, 1993; Kaumann & Molenaar, 1996). Relaxant responses to isoproterenol and the β3-selective agonist, BRL 37344A, were resistant to blockade by concentrations of propranolol which normally block responses in tissues known to contain β1- and β2-adrenoceptors. In addition, isoproterenol-induced relaxation was resistant to the β1- and β2-selective antagonists, metoprolol and butoxamine, fulfilling criterion (i) of low sensitivity to antagonists with high affinities for β1- or β2-adrenoceptors. Although these compounds blocked responses at higher concentrations, the shifts in the concentration-response curves were minor in comparison with those normally found in tissues where β1- or β2-adrenoceptors predominate. The low apparent pA2 value for propranolol of 6.19 is similar to the values obtained in other smooth muscles where atypical β-adrenoceptors have been reported (Arch & Kaumann, 1993), as well as the value of 6.6 obtained in rat detrusor by Oshita et al. (1997).
BRL 37344A was a potent relaxant of rat bladder strips, as previously reported by Oshita et al. (1997), fulfilling criterion (ii) of stimulation by β3-selective agonists. We found that the potency of BRL 37344A was slightly less than that of isoproterenol, while Oshita et al. (1997) found BRL 37344A to be more potent. The differences may be due to the fact that the Krebs buffer used by Oshita et al. (1997) contained desmethylimipramine, deoxycorticosterone, and phentolamine, while ours contained only indomethacin. The response to BRL 37344A was resistant to pretreatment with low concentrations of propranolol but attenuated by both CGP 12177A and SR 59230A, a further indication of the involvement of β3-adrenoceptors. However, the β3-agonist, SR 58611A was considerably less potent at relaxing bladder strips than was either isoproterenol or BRL 37344A, with an EC50 value of 7.1 μM. There are relatively few reports on the efficacy of this compound in smooth muscle preparations. EC50 values of 6.85 μM and 5.48 μM have been reported for β3-adrenoceptor-induced relaxant effects of SR 58611A on guinea-pig common bile duct and colon (De Ponti et al., 1995b), and 7.6 μM on dog colon (De Ponti et al., 1995a); values which are similar to those obtained in this study. SR 58611A at concentrations up to 100 μM has been reported to have no effect on resting tone of guinea pig bladder (De Ponti et al., 1995b), yet in in our study SR 58611A caused 60% of maximal relaxation of KCl-precontracted rat bladder strips (Figure 2). However, EC50 values for SR 58611A in the nanomolar range have been reported in rat colon (Biancetti & Manara, 1990; Kaumann & Molenaar, 1996). The reasons for these differences in potency are not readily apparent, but have been suggested to result from β3-adrenoceptor heterogeneity and/or tissue or species-related differences due to variations in the β3-adrenoceptor amino acid sequence (Blin et al., 1994; De Ponti et al., 1995b). Therefore, the low potency of SR 58611A compared to BRL 37344A in our system may indicate that atypical β-adrenoceptors exist in rat bladder, in addition to the β3-subtype.
Oshita did not examine the responses to selective agonists for the other β-adrenoceptor subtypes. (Oshita et al., 1997). Instead, experiments demonstrating that adrenaline and noradrenaline relaxed rat bladder strips and that propranolol and ICI-118,551 caused only a slight inhibition of isoproterenol-induced relaxation were used as evidence for the presence of β2-adrenoceptors. A more detailed study by Yamazaki and co-workers looked at inhibition of rat bladder spontaneous activity by a number of β-agonists (Yamazaki et al., 1998). Our findings are similar to theirs, in that both β2- and β3-adrenoceptor agonists relaxed rat bladder strips. However, they found that the β1-agonist, dobutamine, was a poor relaxant of rat bladder smooth muscle. Although dobutamine is generally considered to be a β1-selective agonist, some studies have shown that it also has β2-agonist and α1-partial agonist actions (Ozaki et al., 1982; Ruffolo et al., 1984; Aikawa et al., 1996). In preliminary studies, we found that dobutamine caused a significant relaxation of KCl-precontracted rat bladder strips with an EC50 value of 6 μM (data not shown), similar to that obtained with terbutaline. However, because of the uncertain selectivity of dobutamine, we also examined the ability of the β1-selective antagonist, T-0509, to relax KCl-precontracted rat bladder strips. T-0509 was a potent full agonist, causing relaxation of rat bladder smooth muscle with an pD2 value of 6.24. Although the pD2 value is about one log unit less than that reported for T-0509-stimulated relaxation of guinea-pig trachea (Yabana et al., 1992), it was not significantly different from the pD2 values we obtained for isoproterenol on paired strips in the same experiment. Furthermore the pD2 value for isoproterenol-induced bladder strip relaxation under our experimental conditions was 2 log units less than that obtained in guinea-pig trachea by Yabana et al. (1992). Therefore, we believe that the data support the presence of functional β1-adrenoceptors in rat bladder.
The reason(s) for the wide discrepancies of both pD2 values as well as pA2 values between species and organ systems is unclear. In some instances it may result from the characteristics of the preparation used to measure the response (inhibition of spontaneous activity or relaxation of a precontracted strip). As shown in this study and others, agonists may selectively relax preparations precontracted with some agents (such as KCl) but not others (such as carbachol). This may be related to the transduction mechanisms activated by both the contracting and relaxing agonists and/or the relative magnitude of the physiological and biochemical response. In general, β-agonists appear to be more potent at effecting contractile responses, such as stimulation of cardiac muscles, than at relaxing smooth muscles (Arch & Kaumann, 1993). Similarly, β-agonists appear to be more potent at inhibiting spontaneous activity than relaxing precontracted smooth muscles. Therefore, although differences in potency ratio within a given species and tissue preparation can provide information on receptor characteristics, it is difficult to compare these values directly with those reported in the literature using different experimental conditions.
CGP 12177A was a partial agonist, causing approximately 50% relaxation of rat bladder strips and fulfilling criterion (iii) of stimulation by non-conventional partial agonists (β1- and β2-antagonists which are partial agonists at atypical β-adrenoceptors). The response to CGP 12177A was resistant to blockade by high concentrations of propranolol. In the presence of 200 nM propranolol, a concentration which would be expected to block β1- and β2-adrenoceptors, CGP 12177A caused significant rightward shifts of the concentration-response curves for both isoproterenol and BRL 37344A, indicating that the relaxation was mediated by β3-adrenoceptors.
The selective β3-antagonist, SR 59230A, inhibited both isoproterenol and BRL 37344A-induced relaxation of rat bladder strips fulfilling criterion (iv) of sensitivity to β3-antagonists. Like SR 58611A, there are relatively few reports of the effects of SR 59230A on β-adrenoceptor-induced smooth muscle relaxation. In human and rat colon preparations, SR 59230A inhibits β-agonist-stimulated inhibition of motility with pA2 values in the nanomolar range (De Ponti et al., 1995a, 1996). The concentrations of SR 59230A used in this study are similar to those used previously to characterize β3-adrenoceptors in rat colon and heart (Kaumann & Molenaar, 1996); μM concentrations inhibited colon motility but had little effect on the third cardiac β-adrenoceptor. Although μM concentrations of SR 59230A have been reported to affect β2-adrenoceptors, the cumulative evidence of responsiveness to BRL 37344A, SR 58611A, and CGP 12177A, as well as inhibition of responses by CGP 12177A and SR 59230A, suggests to us that the effects we are observing are not the result of stimulation of β2-adrenoceptors.
In conclusion, the resistance of isoproterenol-induced relaxation to propranolol, the relatively high potency of the β3-adrenoceptor agonist, BRL 37344A, the partial agonist actions of CGP 12177A, and the competitive antagonism of propranolol-resistant responses by SR 59230A and CGP 12177A suggest that the rat urinary bladder contains β3-adrenoceptors and/or atypical β-adrenoceptors. Furthermore, the responsiveness of the bladder strips to the β1-adrenoceptor agonist, T-0509, and the β2-adrenoceptor agonist, terbutaline, indicates that β1- and β2-adrenoceptors also mediate a functional response in the rat bladder. However, the functional significance of the β-adrenoceptor subtypes in the urinary bladder still remains to be determined.
Acknowledgments
This work was supported in part by USPHS grant DK51384.
Abbreviations
- BRL 37344A
((±)-R*,R*)-[4-[2-[[2-(3-Chlorophenyl)-2-hydroxyetheyl]amino]propyl]phenoxy]-acetic acid sodium)
- (±)-CGP-12177A
(4-[3-[(1,1-Dimethylethyl)amino]-2-hydroxypropoxy]-1,3-dihydro-2H-benzimidazol-2-one hydrochloride)
- CR
concentration-ratio
- RT–PCR
reverse transcription polymerase chain reaction
- SR 58611A
(ethyl{(7S)-7-[(2R)-2-(3-chlorophenyl)-2-hydroxyethylamino]-5,6,7,8-tetrahydronaphthalen-2-yloxy}acetate hydrochloride)
- SR 59230A
((3-(2-ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronaphth-1-ylamino]-2S-2-propanol oxalate)
- T-0509
([(−)-(R)-1-(3,4-dihydroxyphenyl)-2-[(3,4-dimethoxyphenethyl) amino]ethanol]-hydrochloride
References
- AIKAWA J., FUKAZAWA M., ISHIKAWA M., MOROI M., NAMIKI A., YAMAGUCHI T. Vascular smooth muscle relaxation by α-adrenoceptor blocking action of dobutamine in isolated rabbit aorta. J. Cardiovasc. Pharmacol. 1996;27:33–36. doi: 10.1097/00005344-199601000-00006. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.-E. Clinical relevance of some findings in neuro-anatomy and neurophysiology of the lower urinary tract. Clin. Sci. 1986;70 Supplement 14:21s–32s. doi: 10.1042/cs070s021. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.-E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol. Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- ARCH J.R.S., KAUMANN A.J. β3 and atypical β-adrenoceptors. Med. Res. Rev. 1993;13:663–729. doi: 10.1002/med.2610130604. [DOI] [PubMed] [Google Scholar]
- BIANCETTI L., MANARA L. In vitro inhibition of intestinal motility by phenylethanolaminotetralines: evidence of atypical β-adrenoceptors in rat colon. Br. J. Pharmacol. 1990;100:831–839. doi: 10.1111/j.1476-5381.1990.tb14100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIN N., NAHMIAS C., DRUMARE M.F., STROSBERG A.D. Mediation of most atypical effects by species homologues of the β3-adrenoceptor. Br. J. Pharmacol. 1994;112:911–919. doi: 10.1111/j.1476-5381.1994.tb13167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAULFIELD M.P. Muscarinic receptors–characterization, coupling and function. Pharmacol. Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- DE GROAT W.C., BOOTH A.M., YOSHIMURA N.Neurophysiology of micturition and its modification in animal models of human disease Nervous Control of the Urogenital System 1993Switzerland: Harwood Academic Publishers; 227–290.(ed. Maggi, C.A.) pp [Google Scholar]
- DE PONTI F., COSENTINO M., COSTA A., GIRANI M., GIBELLI G., D'ANGELO L., FRIGO G., CREMA F. Inhibitory effects of SR 58611A on canine colon motility: evidence for a role of β3-adrenoceptors. Br. J. Pharmacol. 1995a;114:1447–1453. doi: 10.1111/j.1476-5381.1995.tb13368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE PONTI F., GIBELLI G., CREMA F., LECCHINI S. Functional evidence for the presence of β3-adrenoceptors in the guinea-pig common bile duct and colon. Pharmacology. 1995b;51:288–297. doi: 10.1159/000139338. [DOI] [PubMed] [Google Scholar]
- DE PONTI F., GIBELLI G., CROCI T., ARCIDIACO M., CREMA F., MANARA L. Functional evidence of atypical β3-adrenoceptors in the human colon using the β3-selective adrenoceptor antagonist, SR 59230A. Br. J. Pharmacol. 1996;117:1374–1376. doi: 10.1111/j.1476-5381.1996.tb15294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDVARDSEN P., SETEKLEIV J. Distribution of adrenergic receptors in the urinary bladder of cats, rabbits and guinea pigs. Acta Pharmacol. et Toxicol. 1968;26:437–445. doi: 10.1111/j.1600-0773.1968.tb00462.x. [DOI] [PubMed] [Google Scholar]
- ELMÉR M. Inhibitory β-adrenoceptors in the urinary bladder of the rat. Life Sci. 1974;15:273–280. doi: 10.1016/0024-3205(74)90217-3. [DOI] [PubMed] [Google Scholar]
- FLEMING W.W., WESTFALL D.P., DE LA LANDE I.S., JELLETT L.B. Log-normal distribution of equieffective doses of norepinephrine and acetylcholine in several tissues. J. Pharmacol. exp. Ther. 1972;181:339–345. [PubMed] [Google Scholar]
- FUJIMURA T., TAMURA K., TSUTSUMI T., YAMAMOTO T., NAKAMURA K., KOIBUCHI Y., KOBAYASHI M., YAMAGUCHI O. Expression and possible functional role of the β3-adrenoceptor in human and rat detrusor muscle. J. Urol. 1999;161:680–685. [PubMed] [Google Scholar]
- GARCIA-SACRISTÁN A., LABADIÁ A., COSTA G. Influencia del sistema nervioso autónomo en la fisiología de la micción de los bóvidos. Med. Vet. 1985;2:475–480. [Google Scholar]
- HEGDE S.S., CHOPPIN A., BONHAUS D., BRIAUD S., LOEB M., MOY T.M., LOURY D., EGLEN R.M. Functional role of M2 and M2 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br. J. Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J., MOLENAAR P. Differences between the third cardiac β-adrenoceptor and the colonic β3-adrenoceptor in the rat. Br. J. Pharmacol. 1996;118:2085–2098. doi: 10.1111/j.1476-5381.1996.tb15648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENAKIN T.P. The classification of drugs and drug receptors in isolated tissues. Pharmacol. Rev. 1984;36:165–222. [PubMed] [Google Scholar]
- KOIKE K., HORINOUCHI T., TAKAYANAGI I. Effect of bupranolol on CGP 12177-induced relaxation and cAMP accumulation in the guinea-pig taenia caecum. Gen. Pharmacol. 1995;26:1791–1794. doi: 10.1016/0306-3623(95)00115-8. [DOI] [PubMed] [Google Scholar]
- LABADIA A., RIVERA L., COSTA G., GARCIA-SACRISTAN A. Influence of the autonomic nervous system in the horse urinary tract. Res. Vet. Sci. 1988;44:282–285. [PubMed] [Google Scholar]
- LANDS A.M., ARNOLD A., MCAULIFF J.P., LUDUENA F.P., BROWN T.G., JR Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967;214:597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- LARSEN J.-J. α- and β-Adrenoceptors in the detrusor muscle and bladder base of the pig and β-adrenoceptors in the detrusor muscle of man. Br. J. Pharmacol. 1979;65:215–222. doi: 10.1111/j.1476-5381.1979.tb07821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN R.M., WEIN A.J. Distribution and function of adrenergic receptors in the urinary bladder of the rabbit. Molec. Pharmacol. 1979;16:441–448. [PubMed] [Google Scholar]
- LI J.H., YASAY G.D., KAU S.T. β-Adrenoceptor subtypes in the detrusor of guinea-pig urinary bladder. Pharmacology. 1992;44:13–18. doi: 10.1159/000138868. [DOI] [PubMed] [Google Scholar]
- LINCOLN J., BURNSTOCK G.Autonomic innervation of the urinary bladder and urethra Nervous Control of the Urogenital System 1993Switzerland: Harwood Academic Publishers; 33–68.(ed. Maggi, C.A.) pp [Google Scholar]
- LONGHURST P.A., LEGGETT R.E., BRISCOE J.A.K. Characterization of the functional muscarinic receptors in the rat bladder. Br. J. Pharmacol. 1995;116:2279–2285. doi: 10.1111/j.1476-5381.1995.tb15065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN C.A.E., NALINE E., BAKDACH H., ADVENIER C. β3-adrenoceptor agonists, BRL 37344 and SR 58611A, do not induce relaxation of human, sheep and guinea-pig airway smooth muscle in vitro. Eur. Resp. J. 1994;7:1610–1615. doi: 10.1183/09031936.94.07091610. [DOI] [PubMed] [Google Scholar]
- MCLAUGHLIN D.P., MACDONALD A. Evidence for the existence of ‘atypical' β-adrenoceptors (β3-adrenoceptors) mediating relaxation in the rat distal colon in vitro. Br. J. Pharmacol. 1990;101:569–574. doi: 10.1111/j.1476-5381.1990.tb14122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORITA T., DOHKITA S., KONDO S., NISHIMOTO T., HIRANO S., TSUCHIDA S. Cyclic adenosine monophosphate production and contractile response induced by beta-adrenoceptor subtypes in rabbit urinary bladder smooth muscle. Urol. Int. 1990;45:10–15. doi: 10.1159/000281650. [DOI] [PubMed] [Google Scholar]
- MORITA T., ANDO M., KIHARA K., OSHIMA H. Species differences in cAMP production and contractile responses induced by β-adrenoceptor subtypes in urinary bladder smooth muscle. Neurourol. Urodynam. 1993;12:185–190. doi: 10.1002/nau.1930120213. [DOI] [PubMed] [Google Scholar]
- NERGÅRDH A., BORÉUS L.O., NAGLO A.-S. Characterization of the adrenergic beta-adrenoceptor in the urinary bladder of man and cat. Acta Pharmacol. et Toxicol. 1977;40:14–21. doi: 10.1111/j.1600-0773.1977.tb02049.x. [DOI] [PubMed] [Google Scholar]
- ORIOWO M.A. Different atypical β-adrenoceptors mediate isoprenaline-induced relaxation in vascular and non-vascular smooth muscles. Life Sci. 1995;56:PL269–PL275. doi: 10.1016/0024-3205(95)00076-3. [DOI] [PubMed] [Google Scholar]
- ORIOWO M.A. β3-Adrenoceptors mediate smooth muscle relaxation in the rat lower oesophageal sphincter. J. Auton. Pharmacol. 1997;17:175–182. doi: 10.1046/j.1365-2680.1997.00451.x. [DOI] [PubMed] [Google Scholar]
- OSHITA M., HIRAOKA Y., WATANABE Y. Characterization of β-adrenoceptors in urinary bladder: comparison between rat and rabbit. Br. J. Pharmacol. 1997;122:1720–1724. doi: 10.1038/sj.bjp.0701562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZAKI N., KAWAKITA S., TODA N. Effects of dobutamine on isolated canine cerebral, coronary, mesenteric, and renal arteries. J. Cardiovasc. Pharmacol. 1982;4:456–461. doi: 10.1097/00005344-198205000-00017. [DOI] [PubMed] [Google Scholar]
- RIVERA L., BENEDITO S., PRIETO D., HERNANDEZ M., LABADIA A., GARCIA-SACRISTAN A. α- and β-Adrenoceptors in the sheep urinary bladder. Res. Vet. Sci. 1991;50:259–263. doi: 10.1016/0034-5288(91)90120-d. [DOI] [PubMed] [Google Scholar]
- RUFFOLO R.R., JR, MESSICK K., HORNG J.S. Interactions of three inotropic agents, ASL-7022, dobutamine and dopamine, with α- and β-adrenoceptors in vitro. Naunyn-Schmied. Arch. Pharmacol. 1984;326:317–326. doi: 10.1007/BF00501436. [DOI] [PubMed] [Google Scholar]
- SEGUCHI H., NISHIMURA J., ZHOU Y., NIIRO N., KUMAZAWA J., KANAIDE H. Expression of β3-adrenoceptors in rat detrusor smooth muscle. J. Urol. 1998;159:2197–2201. doi: 10.1016/S0022-5347(01)63305-6. [DOI] [PubMed] [Google Scholar]
- TEN BERGE R.E.J., WEENING E.C., ROFFEL A.F., ZAAGSMA J. β2- but not β3-adrenoceptors mediate prejunctional inhibition of non-adrenergic non-cholinergic contraction of guinea pig main bronchi. Eur. J. Pharmacol. 1995;275:199–206. doi: 10.1016/0014-2999(94)00771-x. [DOI] [PubMed] [Google Scholar]
- VAN ROSSUM J.M. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch. int. Pharmacodyn. 1963;143:299–330. [PubMed] [Google Scholar]
- VAUGHAN C.W., SATCHELL P.M. Urine storage mechanisms. Prog. Neurobiol. 1995;46:215–237. [PubMed] [Google Scholar]
- YABANA H., WATANABE H., NARITA H., NAGAO T. Selective and full β1-adrenoceptor agonist action of a catechol derivative of denopamine (T-0509) in the guinea-pig cardiac muscle and trachea: comparison with denopamine, xamoterol and isoprenaline. Br. J. Pharmacol. 1992;106:335–341. doi: 10.1111/j.1476-5381.1992.tb14337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAZAKI Y., TAKEDA H., AKAHANE M., IGAWA Y., NISHIZAWA O., AJISAWA Y. Species differences in the distribution of β-adrenoceptor subtypes in bladder smooth muscle. Br. J. Pharmacol. 1998;124:593–599. doi: 10.1038/sj.bjp.0701870. [DOI] [PMC free article] [PubMed] [Google Scholar]


