Abstract
Effects of agents, which affect microtubule polymerization-depolymerization cycle, on Ba2+ current (IBa) flowing through voltage-gated Ca2+ channels and carbachol (CCh)-induced sustained suppression of IBa were examined in whole-cell voltage-clamped smooth muscle cells of guinea-pig ileum.
Colchicine (100 μM) and vinblastine (100 μM), microtubule depolymerizers, increased the ampitude of IBa. Lumicolchicine (100 μM), an inactive analogue of colchicine, had no effect on IBa.
Taxol (1–100 μM), a microtubule polymerizer, decreased IBa in a concentration-dependent manner and accelerated the rate of inactivation of IBa. Baccatin III (100 μM), an inactive analogue of taxol, had no effect on IBa.
Colchicine (100 μM) and vinblastine (100 μM), but not lumicolchicine (100 μM), decreased or abolished the sustained component of CCh (10 μM)-induced IBa suppression.
Pretreatment with taxol (10–100 μM) resulted in a concentration-dependent decrease in IBa and the action of CCh on IBa. The inhibitory effects of taxol and CCh on IBa were not additive.
Colchicine (100 μM) or taxol (100 μM) had no effect on voltage-gated K+ channel current or CCh-induced non-selective cationic channel current.
These results suggest that polymerization of microtubules leads to suppression of Ca2+ channel activity, and that muscarinic sustained suppression of Ca2+ channel current is mediated by a signal transduction element which involves microtubule cytoskeleton.
Keywords: Carbachol, Ca2+ channel current, microtubule cytoskeleton, colchicine, taxol, smooth muscle, guinea-pig ileum
Introduction
In guinea-pig ileal smooth muscle, muscarinic receptors couple with at least two types of GTP-binding protein (G protein) (Komori et al., 1992). One is pertussis toxin (PTX)-sensitive and mediates opening of non-selective cationic channels leading to the membrane depolarization (Benham et al., 1985; Inoue & Isenberg, 1990). The other is PTX-insensitive and activates phospholipase C (PLC), which leads to production of inositol 1,4,5,-trisphosphate (IP3) to release Ca2+ from intracellular stores (Komori & Bolton, 1991). We have shown in the same type of smooth muscle that muscarinic receptor stimulation induces suppression of Ca2+ current (ICa) through voltage-gated Ca2+ channels with an initial transient component followed by a sustained component (Unno et al., 1995; 1996). A PTX-insensitive G protein is involved in these two phases of ICa suppression. The initial transient phase of ICa suppression is brought about by operation of a Ca2+-induced inactivation mechanism due to release of stored Ca2+ by IP3. However, the sustained component of muscarinic action on ICa occurs independently of IP3-induced Ca2+ release (Unno et al., 1995; Beech, 1997). The signal transduction for the effect of muscarinic receptor stimulation remains unknown. No involvement of IP3, protein kinase C, cyclic AMP, cyclic GMP and arachidonic acid has been suggested, but an intermediate is likely because the sustained effect develops with a slow time course.
Recent evidence indicates that cytoskeletons, such as microfilament and microtubule, play a role not only for compartmentalization and anchoring of membrane proteins including receptors and ion channels, but also for regulation of their functions. Fukuda et al. (1981) suggested that in guinea-pig dorsal root ganglion neurons microtubule as well as microfilament regulates voltage-gated Na+ channel activity and voltage-gated Ca2+ channel activity. Microtubule cyto-skeleton has also been suggested to modulate voltage-gated Ca2+ channels in rat hippocampal pyramidal neurons (Johnson & Byerly, 1994) and chick embryonic ventricular myocytes (Galli & DeFelice, 1994).
A microtubule is a polymer composed of αβ heterodimers of tubulin, and polymerization and depolymerization of the dimers are dynamically regulated by cytosolic Ca2+, Mg2+, GTP and some cytoskeleton-associated proteins (for reviews see Dustin, 1984). It has been suggested that tubulin is capable of modulating signal transduction elements including G proteins (Leiber et al., 1993; Roychowdhury et al., 1993; Roychowdhury & Rasenick, 1994). Ravindra et al. (1996) and Popova et al. (1997) reported that PTX-insensitive Gq protein and tubulin can interact with each other and as a result of the interaction, subsequent activation of PLC and polymerization-depolymerization cycle of microtubule cytoskeleton are modified. In PC12 cells, stimulation of muscarinic receptors induces a modulation of microtubule polymerization which is suggested to be due to dephosphorylation of microtubule-associated protein, τ (Sadot et al., 1996).
In the present study, we investigated the effects of agents which influence microtubule cytoskeleton, on muscarinic receptor-mediated suppression of voltage-gated Ca2+ channels in guinea-pig ileal smooth muscle. The results suggest that polymerization of microtubules or recruitment of tubulin molecule for the signal transduction is likely to occur during the sustained phase of muscarinic suppression of ICa.
Methods
Preparation of cells
Male guinea-pigs, weighing 350–450 g were stunned and killed by exsanguination. Single smooth muscle cells were isolated from the longitudinal layer of small intestine by enzymatic procedures, as previously described (Komori et al., 1992), and suspended in physiological salt solution (PSS; for composition, see below) containing 0.5 mM Ca2+.
Pretreatment of cells with cytoskeletal agents
In general, agents, such as colchicine and taxol exert a time- and concentration-dependent action to form their complex with cytoskeletons. The action is exhibited slowly and it takes several hours to reach an equilibrium, especially, at a low concentration (1 μM or less) (Wilson et al., 1974; Dustin, 1984). In the present study, cells were pretreated with cytoskeletal agents at a high concentration of 100 μM for more than 1 h, unless otherwise stated.
The cell suspension in PSS containing 0.5 mM Ca2+ was divided into two or more parts. A cytoskeletal agent was added to one part of the cell suspension to give a required concentration and each cell suspension was placed on coverslips in a small aliquot and kept in a dark room at room temperature (21–25°C) until use on the same day. To evaluate the effect of a cytoskeletal agent, cells pretreated with the agent and cells incubated without the agent, but handled in an otherwise identical way, were used for recording membrane currents.
Recording of membrane currents
Membrane currents were recorded from single cells by use of the same patch-clamp techniques as described previously (Unno et al., 1995) and patch pipettes filled with a Cs+-based pipette solution (for composition, see below) with a resistance of 3–6 MΩ. For recording of membrane currents from cells pretreated with a cytoskeletal agent, the agent was added to both the bathing solution and the pipette solution and continued to be present throughout the experiments. Voltage-gated Ca2+ channel current (ICa) was elicited repeatedly by depolarizing pulses of a brief duration (30 ms) from the holding potential of −60 to 0 mV applied at a frequency of 0.25 Hz. After the amplitude of ICa was stabilized, the solution in the recording chamber was replaced with PSS containing Ba2+ to use as a charge carrier. Effects of cytoskeletal agents on ICa were evaluated using Ba2+ current (IBa) flowing through voltage-gated Ca2+ channels. Carbachol (CCh) was applied extracellularly 2 min after the replacement of Ca2+ with Ba2+ and the effect on IBa was investigated. Application of CCh was made by replacing the solution in the recording chamber with CCh-containing solution more than five times. The amplitude of IBa was estimated as the difference from the current level (IBa=0) obtained by application of the depolarizing pulse to the cell in the presence of 100 μM Cd2+ which was applied at the end of each experiment. For calculation of current density of IBa, membrane capacitance was measured from capacity current elicited by applying a 10 mV hyperpolarizing pulse.
When measuring K+ current (IK) flowing through voltage-gated K+ channels, PSS containing Mn2+ instead of Ca2+ was used as the solution in the recording chamber and K+-based pCa 6.5 solution (for composition, see below) as the pipette solution, and depolarizing pulses (2 s duration) to −40 mV or more positive potential (up to 80 mV) in 20 mV increments from the holding potential of −80 mV were applied. The amplitude of IK was estimated by subtracting a leakage component from the evoked outward current, as described previously (Unno et al., 1996).
In experiments where Ca2+-activated K+ channel current (IKCa) was recorded, PSS containing 2 mM Ca2+ was used as the solution in the recording chamber and K+-based solution (for composition, see below) as the pipette solution, and cells were held under voltage-clamp at 0 mV. A change in intracellular Ca2+ concentration brought about by Ca2+ release from intracellular stores induced by CCh was detected by monitoring IKCa (Komori et al., 1998).
The values in the text are presented as means±s.e.mean. Statistical significance was tested by Student's unpaired t-test and differences were considered significant when P<0.05.
Solutions and drugs
PSS used in the experiments had the following composition (mM): NaCl 126, KCl 6, CaCl2 2, MgCl2 1.2, glucose 14; HEPES 10.5. When Ba2+ and Mn2+ were used for extracellular solution, the CaCl2 was replaced with an equimolar solution of BaCl2 and MnCl2, respectively. Composition of patch-pipette solutions (mM) was as follows. Cs+-based solution: CsCl 134, MgCl2 1.2, MgATP 1, NaGTP 0.1, glucose 14, HEPES 10.5, EGTA 0.05 (titrated to pH 7.2 with CsOH). K+-based solution: KCl 134, MgCl2 1.2, MgATP 1, NaGTP 0.1, glucose 14, HEPES 10.5, EGTA 0.05 (titrated to pH 7.2 with KOH). K+-based pCa 6.5 solution: KCl 80, MgCl2 2.5, MgATP 1, NaGTP 0.1, glucose 14, HEPES 10.5, BAPTA 20, CaCl2 13.3 (titrated to pH 7.2 with KOH). In this solution, Ca-BAPTA buffer was used to maintain the ionized calcium concentration at a level of pCa 6.5.
Drugs and chemicals used were baccatin III, 1, 2-bis (2-aminophenoxy) ethane-N, N, N′, N′-tetraacetic acid (BAPTA), cytochalasin B (from Helminthosporium dematioideum), lumicolchicine, taxol (from Taxus brevifolia), vinblastine (all from Sigma, St Louis, MO, U.S.A.), carbachol chloride (CCh), colchicine, phalloidin (Wako, Osaka City, Osaka, Japan) and ethylene glycol-bis (β-aminoethyl ether) N, N, N′, N′-tetraacetic acid (EGTA; Dojin Kagaku, Kamimasushiro-gun, Kumamoto, Japan). Stock solutions of baccatin III, cytochalasin B, lumicolchicine, phalloidin and taxol were 100% DMSO and the final concentration of DMSO was reduced to less than 0.2%. Treatment of cells with 0.2% DMSO for up to 7 h had no effect on IBa itself and CCh-induced suppression of IBa. All other drugs were dissolved in distilled water as their stock solutions.
Results
Effects of colchicine, vinblastine and taxol on voltage-gated Ca2+ channel current
Cells were pretreated for 1–7 h with or without colchicine (100 μM), which binds to tubulin to inhibit polymerization of microtubules resulting in the disruption of the cytoskeleton. The mean treatment time was 3.9±0.4 h (n=20) for colchicine-treated cells and 4.1±0.4 h (n=18) for control cells. The pretreatment with colchicine increased somewhat the amplitude of IBa elicited by depolarizing pulses to 0 mV (Figures 1 and 2). Using the peak amplitude of IBa, recorded 10–15 min after achieving the whole-cell clamp configuration, and cell-capacitance measurement, the current density of IBa was estimated (see Methods). The current density of IBa varied between 4.5 and 14.6 pA·pF−1 among colchicine-treated cells with a mean of 8.2±0.6 pA·pF−1 (n=20), and between 3.5 and 9.0 pA·pF−1 among control cells with a mean of 5.3±0.4 pA·pF−1 (n=18). The difference between the two means was statistically significant, although the mean cell capacitance of 39.8±1.8 pF (n=20) in colchicine-treated cells was not different from that (40.7±1.9 pF, n=18) in control cells. When colchicine (100 μM) was applied extracellularly without pretreatment, the amplitude of IBa remained almost unaffected even 10 min after the application of colchicine (data not shown), indicating that colchicine takes a longer time to exhibit the action of a stimulant on IBa amplitude.
Figure 1.
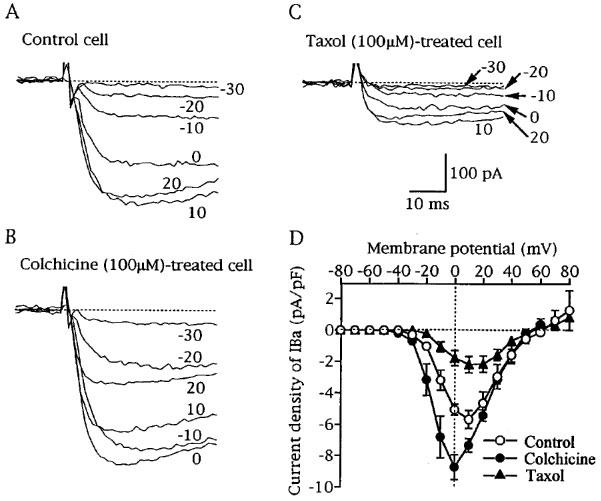
Effects of colchicine and taxol on current-voltage relationships of Ba2+ current (IBa). IBa was elicited by depolarizing pulses (2 s duration) to various potentials ranging from −70 to 80 mV in 10 mV increments from the holding potential of −80 mV. (A, B and C) Superimposed current traces recorded from a drug-untreated cell (control cell), a colchicine (100 μM)-treated cell and a taxol (100 μM)-treated cell, respectively. The attached figures near current traces represent the potentials attained by the depolarizing pulses. Leak currents were subtracted and interrupted lines indicate zero IBa levels. (D) current-voltage relationships of IBa obtained from control cells (n=7), colchicine-treated cells (n=8) and taxol-treated cells (n=7). The peak currents normalized to cell capacitance were averaged, and the mean current densities were plotted against membrane potentials attained by depolarizing pulses.
Figure 2.
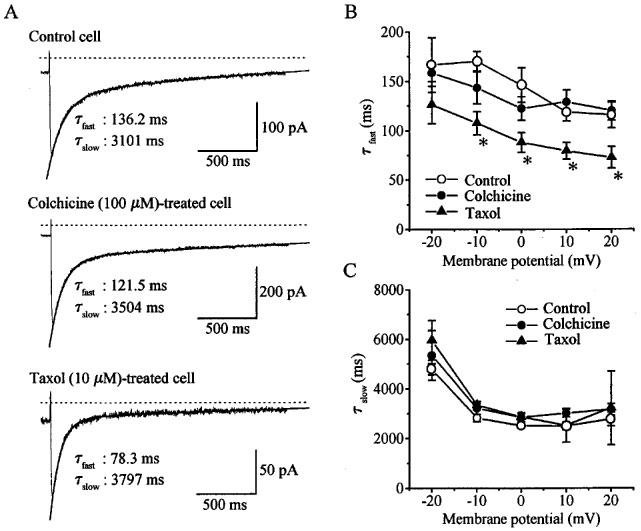
Effects of colchicine and taxol on the inactivation of IBa. IBa was elicited by depolarizing pulses (2 s duration) to various potentials ranging from −20 to 20 mV from the holding potential of −80 mV. (A) Current traces of IBa elicited by depolarizing pulses to 0 mV in a drug-untreated cell (control cell), a colchicine (100 μM)-treated cell and a taxol (10 μM)-treated cell. The smooth curves in the inactivation phase of IBa are the fitted two-exponential function and the time constants for the fast component (τ fast) and the slow component (τ slow) are shown. Note different calibration for each trace. (B and C) Voltage-dependence of τ fast and τ slow, respectively. Mean time constants averaged from seven control cells, eight colchicine-treated cells and seven taxol-treated cells were plotted against voltages attained by depolarizing pulses. *Statistically different (P<0.05) from the corresponding mean value for control. Taxol pretreatment decreased the time constants of inactivation for the fast component.
Pretreatment of cells with lumicolchicine (100 μM; mean treatment time: 4.2±0.5 h, n=8), an inactive analogue of colchicine, did not change the current density of IBa. The mean current density of IBa of 6.0±0.6 pA·pF−1 (mean cell capacitance: 42.6±2.7 pF) was not significantly different from that in control cells, but it was significantly smaller than that in colchicine-treated cells. In cells pretreated with another microtubule disrupter, vinblastine (100 μM; mean treatment time: 2.9± 0.5 h, n=7), the mean current density of IBa (8.0±0.9 pA·pF−1; mean cell capacitance: 37.1±3.6 pF) was also increased with a statistically significant difference from that in control cells.
The finding that disruption of microtubule cytoskeleton resulted in an increase of the activity of voltage-gated Ca2+ channels suggests that microtubule cytoskeleton may contribute to an inhibitory regulation of the Ca2+ channels.
Pretreatment of cells with taxol (100 μM), which polymerizes microtubule and stabilizes it, reduced markedly the amplitude of IBa (Figure 1C). The inhibitory effect of taxol on IBa was dependent on its treatment time. The pretreatment with 100 μM taxol for 30 min did not significantly change the mean current density of IBa (6.8±1.2 pA·pF−1 (n=4) in taxol-treated cells and 5.3±1.1 pA·pF−1 (n=5) in time-matched control cells). The reduction of IBa amplitude was evident when taxol was applied for 1 h and the inhibitory effect was maintained invariably up to 7 h. In cells pretreated with 1, 10 and 100 μM taxol for 1–7 h (mean treatment time: 3.7±0.6 h for 1 μM, 4.2±0.7 h for 10 μM, 4.4±0.4 h for 100 μM), the mean current density of IBa was 5.2±0.6 pA·pF−1 (n=5), 3.0±0.6 pA·pF−1 (n=8) and 1.5±0.3 pA·pF−1 (n=17), respectively. The latter two mean values were significantly smaller than the control (5.3±0.4 pA·pF−1, n=18), although the mean cell capacitances were unchanged by any concentrations of taxol. The concentration of taxol to produce 50% inhibition was estimated as 16.0 μM (Hill coefficient: 0.8) by fitting of the data with a modified Hill equation: Pcont/{1+([taxol]/IC50)n}, where Pcont, [taxol], IC50 and n are mean current density of IBa in control cells, taxol concentration, the concentration required 50% inhibition and the Hill coefficient, respectively.
When baccatin III (100 μM), a taxol analogue which has little effect on microtubules, was used instead of taxol (mean treatment time: 3.9±0.6 h, n=9), the mean current density of IBa (5.3±0.4 pA·pF−1; mean cell capacitance: 36.7±1.9 pF, n=9) was comparable to that in control cells.
These results suggest that polymerization of microtubule cytoskeleton may decrease the activity of voltage-gated Ca2+ channels.
Effects of colchicine and taxol on current (I)-voltage (V) relationships and the inactivation of voltage-gated Ca2+ channel current
IBa was elicited by depolarizing pulses from the holding potential of −80 mV to various membrane potentials ranging between −70 and 80 mV in 10 mV increments. In colchicine-treated cells, the amplitude of IBa increased over a range of membrane potentials from −20 to 10 mV (Figure 1A and B). As shown in Figure 1D, peak potential in the I-V curve in colchicine (100 μM)-treated cells was shifted by some 10 mV in the negative direction without changing the apparent reversal potential of IBa (around 60 mV). Such effects on IBa were not observed in lumicolchicine (100 μM)-treated cells (data not shown). In taxol (100 μM)-treated cells, the current density of IBa was reduced by the same extent over a wide range of membrane potentials (Figure 1D), indicating that the inhibitory effect of taxol on IBa was voltage-independent.
As can be seen from Figure 1B and C, colchicine and taxol had little or no effect on the activation time course of IBa. The time to peak of IBa at 0 mV of 16.7±1.6 ms (n=7) in colchicine-treated cells and that of 16.6±3.5 ms (n=7) in taxol-treated cells were not statistically different from 18.3±2.3 ms (n=7) in control cells.
Effects of colchicine and taxol on the inactivation kinetics of IBa elicited by depolarizing pulses of a long duration of 2 s were investigated. The decay time course of IBa could be approximated well by the sum of two exponentials (fast and slow components) (Figure 2A), as previously reported by Unno et al. (1996). The time constants for fast and slow components in control cells decreased as the stepping potential was increased over a range of potentials between −20 and 10 mV (Figure 2B and C). This was also true for cells pretreated with colchicine (100 μM). However, pretreatment with taxol (10 μM) resulted in a decrease in the time constant for the fast component but not the slow component (Figure 2B and C).
Figure 3 shows effects of colchicine and taxol on voltage-gated K+ channel current (IK). The IK was considered to represent the opening of both Ca2+-dependent and -independent K+ channels as described previously (Unno et al., 1996). Pretreatment of cells with colchicine (100 μM) or taxol (100 μM) did not change the current density of IK and the current-voltage relationship, indicating the agents have no effect on voltage-gated K+ channels.
Figure 3.
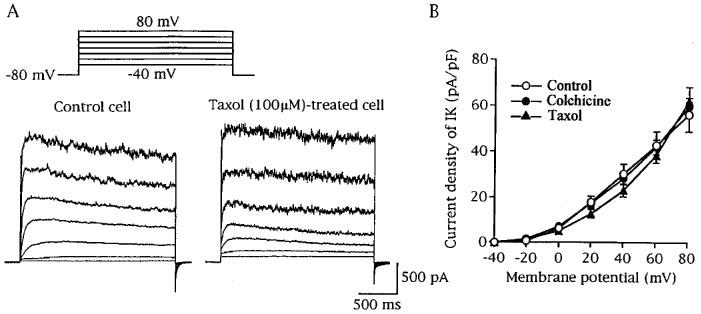
Effects of colchicine and taxol on K+ current (IK). IK was elicited by depolarizing pulses (2 s duration) to test potentials of −40 to 80 mV in 20 mV increments from the holding potential of −80 mV. (A) Superimposed current traces recorded from a drug-untreated cell (control cell) and a taxol (100 μM)-treated cell. (B) Current-voltage-relationships of IK obtained from control cells (n=8), colchicine (100 μM)-treated cells (n=7) and taxol (100 μM)-treated cells (n=7). The mean current densities were plotted against voltages attained by depolarizing pulses.
Effects of microtubule disrupters on carbachol-induced suppression of voltage-gated Ca2+ channel current
An attempt was made to see if microtubule cytoskeleton is involved in the muscarinic signal transduction responsible for carbachol (CCh)-induced suppression of IBa. CCh (10 μM), when applied extracellularly, induced a biphasic suppression of IBa, as previously reported by Unno et al. (1995); an initial transient component was followed by a sustained component persisting over the entire period of CCh presence in the bathing solution (Figures 4A and 5A).
Figure 4.
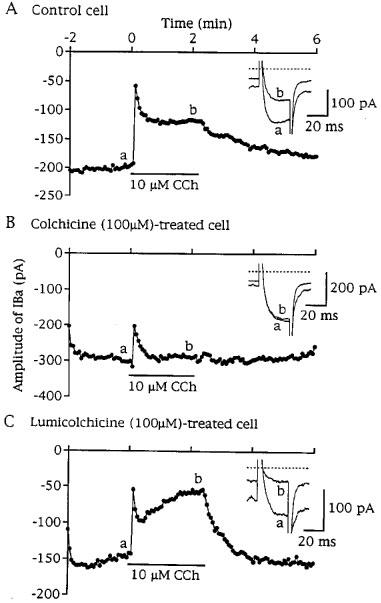
Effects of colchicine and lumicolchicine on transient and sustained components of carbachol (CCh, 10 μM)-induced suppression of IBa. IBa was repeatedly elicited by depolarizing pulses (30 ms duration) to 0 mV from a holding potential of −60 mV at 0.25 Hz. (A, B and C) Time-series plots for peak IBa amplitude (the beginning of CCh application was taken as zero) in a drug-untreated cell (control cell), a colchicine (100 μM)-treated cell and a lumicolchicine (100 μM)-treated cell, respectively. Examples of actual IBa traces for before (a) and during CCh application (b) are inserted in each plot. The downward deflection of holding current reflects the induction of non-selective cationic channel current induced by CCh. Pretreatment with colchicine, but not lumicolchicine, caused inhibition of the CCh-induced sustained suppression of IBa.
Figure 5.
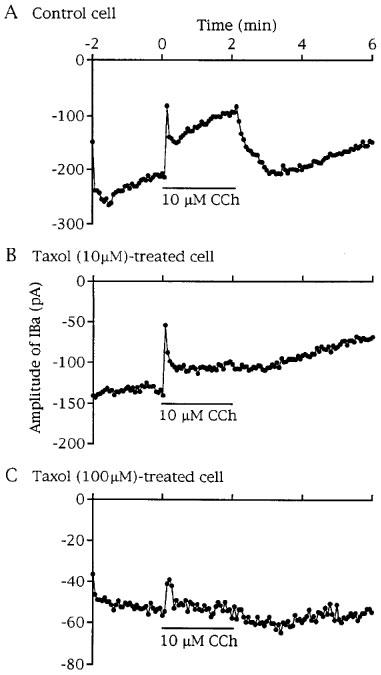
Effect of taxol on transient and sustained components of CCh (10 μM)-induced suppression of IBa. (A, B and C) Time-series plots for peak IBa amplitude in a drug-untreated cell (control cell), a 10 μM taxol- and a 100 μM taxol-treated cell, respectively. Taxol pretreatment resulted in concentration-dependent inhibition of CCh-induced sustained suppression of IBa.
In eight out of 13 colchicine (100 μM)-treated cells, CCh (10 μM) decreased IBa amplitude in the early period of 30 s, as shown in Figure 4B. After this period, IBa amplitude was only slightly reduced in five cells, remained almost unchanged in one cell, and rather increased in the remaining two cells (up to 19%). In the remaining five cells, CCh induced no IBa suppression irrespective of their transient and sustained components (n=3) or only a small sustained suppression which gradually developed (17.8%, n=2). In the seven colchicine-treated cells, which responded to CCh by a decrease in IBa during the corresponding period to the sustained phase of IBa suppression in control cells, the mean suppression of IBa was significantly smaller than the control (Table 1). Pretreatment of cells with vinblastine also decreased significantly the sustained component of CCh-induced IBa suppression (Table 1). Pretreatment of cells with lumicolchicine did not affect the action of CCh on IBa (Figure 4C and Table 1).
Table 1.
Effects of cytoskeletal agents on the transient and sustained components of carbachol (CCh)-induced Suppression of Ba2+ current (IBa)

These results indicate that the sustained component of IBa suppression induced by CCh is reduced after disruption of microtubules. Therefore, it is possible that CCh suppresses IBa through polymerization of microtubules or by a mechanism which simply requires the existence of functional microtubule cytoskeleton. In addition, the inhibitory effects of colchicine and vinblastine on the transient component of IBa suppression (Table 1) suggest a possible involvement of microtubule cytoskeleton in the signalling process for this action as well.
Effect of taxol on carbachol-induced suppression of voltage-gated Ca2+ channel current
Pretreatment of cells with 10 μM taxol had little or no effect on the transient component but significantly reduced the sustained component of IBa suppression induced by CCh (10 μM) (Figure 5B and Table 1). When taxol was used at 100 μM, the amplitude of IBa was reduced to one-third or less of the control and the sustained component of CCh action on IBa was abolished in seven out of ten cells. In the remaining three cells, a slight sustained IBa suppression was observed (Table 1). The initial transient phase of IBa suppression was still induced by CCh, although the extent was significantly smaller than the control (Figure 5C and Table 1). Baccatin III (100 μM) was without effect on the transient and sustained components of IBa suppressions induced by CCh (Table 1).
The inhibitory effects of taxol (100 μM) on the CCh action on IBa as well as IBa itself were not observed in cells pretreated for 30 min: These cells responded to CCh (10 μM) by IBa suppression with transient (49.2±17.4%, n=3) and sustained (58.4±10.9%, n=4) components, which were not significantly different from mean values in time-matched control cells (52.8±10.9%, n=4 and 41.7±9.1%, n=5). Thus, the inhibitory effects of taxol on the CCh action on IBa seemed to develop with a similar time course to that on IBa itself.
It is, therefore, likely that the CCh action on IBa is reduced in time when microtubule cytoskeleton has already been polymerized.
Effects of colchicine and taxol on carbachol-induced non-selective cationic channel current and Ca2+ release from intracellular stores
CCh not only suppressed IBa but also induced non-selective cationic channel current (ICCh) in a biphasic manner (Unno et al., 1995). The mean peak current densities of ICCh in the transient and sustained components were 3.3±0.6 pA·pF−1 (n=8) and 1.4±0.2 pA·pF−1 (n=13) in colchicine (100 μM)-treated cells and 5.5±1.9 pA·pF−1 (n=6) and 1.1±0.2 pA·pF−1 (n=10) in taxol (100 μM)-treated cells, respectively. These mean values were not significantly different from the corresponding mean values in control cells (4.7±0.6 pA·pF−1, n=11 and 1.3±0.2 pA·pF−1, n=12, respectively).
The transient component of ICCh is associated with IP3-induced Ca2+ release from intracellular stores (Unno et al., 1995). Therefore, both colchicine and taxol may not prevent this pathway for Ca2+ release. In fact, transient activation of Ca2+-activated K+ channel current (IKCa) induced by CCh, which can be used as an indicator of massive release of stored Ca2+ (Komori et al., 1998), remained unchanged after treatment with colchicine and taxol. The mean peak current density of CCh (10 μM)-induced IKCa was 100.1±15.0 pA·pF−1 (n=10) in colchicine (100 μM)-treated cells and 119.6±51.1 pA·pF−1 (n=5) in taxol (100 μM)-treated cells, which were not significantly different from 97.5±16.8 pA·pF−1 (n=9) in control cells.
Thus, the cytoskeletal agents do not prevent activation of non-selective cationic channels and release of Ca2+ from intracellular stores induced by CCh.
Effects of cytochalasin B and phalloidin on carbachol-induced suppression of voltage-gated Ca2+ channel current
Pretreatment of cells with cytochalasin B (100 μM; mean treatment time: 3.7±0.7 h, n=6) or phalloidin (100 μM; it was treated intracellularly for 10–15 min via the patch pipette), a disrupter and a stabilizer of actin microfilaments, respectively, did not reduce the current density of IBa and transient and sustained components of IBa suppression induced by CCh (Table 1). These results suggest that actin microfilaments are not involved in the muscarinic suppression of Ca2+ channel current.
Discussion
In the present study, we attempted to demonstrate a contribution of microtubule cytoskeleton to modulation of the activity of voltage-gated Ca2+ channels (VGCCs) in smooth muscle cells of the small intestine and to explore its possible involvement in suppression of the current through VGCCs induced by muscarinic receptor stimulation.
Pretreatment with colchicine, which inhibits tubulin assembly and dissociates microtubules into tubulin, increased VGCC current (IBa). Similar result was also obtained by another microtubule disrupter, vinblastine, which binds to tubulin at a different site from colchicine. Colchicine and vinblastine have been reported to increase intracellular cyclic AMP levels (Leiber et al., 1993) which possibly affect VGCC activity. However, adenylate cyclase activators and membrane permeable analogues of cyclic AMP are incapable of increasing VGCC activity in intestinal smooth muscle cells (Beech, 1997). This makes it difficult to explain the effects of colchicine and vinblastine on IBa by their action to increase cyclic AMP levels. In contrast to the action of microtubule disrupters, taxol, which promotes tubulin assembly and stabilizes microtubules, decreased IBa. The inhibitory effect of taxol on IBa is not attributable to its action to depolymerize actin microfilament (Antin et al., 1981), since cytochalasin B, an actin depolymerizer, had no effect on IBa. Colchicine and taxol appeared to be selective for VGCCs because the agents did not affect voltage-gated K channels and non-selective cationic channels induced by CCh (ICCh). Given these findings, it is likely that the VGCC activity is accelerated under disruption of microtubule cytoskeletons, but it is reduced under polymerization of them. The findings that inactive analogues of the cytoskeletal agents had no effect on IBa may support this view.
Microtubules, in addition to microfilaments, have been suggested to play a role for compartmentalization and anchoring of some membrane proteins, including receptors and ion channels (Froehner, 1993). Therefore, a conformational change of microtubule cytoskeleton is expected to have an influence on ion channel activity. In fact, it has been demonstrated in rat myotubes and neurons that the depolymerization of cytoskeleton decreased receptors and ion channels in number (Tilson et al., 1989; Froehner, 1993). However, this is not directly applicable to the present findings that the cytoskeletal depolymerization increased IBa. Furthermore, the current-voltage relationship and the inactivation curve of IBa were modified by the cytoskeletal agents, indicating that the observed modulation of the kinetics of IBa cannot be explained merely by changes in the number of VGCCs. Single channel recordings of VGCC activity in chick cardiac cells revealed that colchicine and taxol modulate inactivation kinetics of the VGCCs without changing the number of the channels (Galli & DeFelice, 1994).
A disruption of microtubule cytoskeleton might cause cell swelling as suggested in some cell types (Horie et al., 1983; Häussinger et al., 1993) and modulate ion channel activity. The activities of Ca2+-activated K+ channels in rabbit pulmonary artery (Kirber et al., 1992) and of VGCCs in guinea-pig stomach (Xu et al., 1996) are facilitated by cell inflation with a positive pressure applied via the patch pipette or a hypotonicity. Waniishi et al. (1997) demonstrated in the same type of smooth muscle cells as used in the present studies that a hypotonic cell swelling increased the amplitude of muscarinic non-selective cationic channel current as well as VGCC current. We have not observed increase in either Ca2+-activated K+ channel current or ICCh, which is more sensitive to the hypotonicity-induced cell swelling than VGCC current (Waniishi et al., 1997), but observed increase in VGCC current after treatment with colchicine and vinblastine. If the agents caused cell swelling, it would be expected that ICCh was also facilitated noticeably, so that the data are not positive to a role of cell swelling in the observed increase in VGCC current.
Taxol, but not colchicine, accelerated the inactivation of IBa (Figure 2A). The results were opposite to previous findings in cardiac muscle cells (Galli & DeFelice, 1994) where the inactivation of Ca2+ channel current was accelerated by colchicine and slowed by taxol. The explanation for the differences is not clear, but it seems likely that the molecular basis for the interaction between microtubule cytoskeleton and calcium channel protein in smooth muscle cells may be different in some way from that in cardiac muscle cells. In general, VGCC in cardiac and smooth muscle cells consists of α1-subunit forming channel pore and other auxiliary subunits including β- and α2/δ-subunits. The voltage-dependence of the channel is an intrinsic property of the α1-subunit which is affected by the auxiliary subunits (for reviews see McDonald et al., 1994). The β-subunit is a cytoskeleton-binding protein and directly interacts with a linker region of the α1-subunit protein (Pragnell et al., 1994) to modulate activation and inactivation kinetics. In smooth muscle cells, the type of β-subunit is thought to be different from that in cardiac muscle cells (β3 in smooth muscle which does not contain consensus protein kinase A phosphorylation sequences) (McDonald et al., 1994). Therefore, changes in physical state of microtubule cytoskeleton might affect conformation of the β-subunit differently between cardiac and smooth muscle cells and this might be responsible for the different regulation of the inactivation kinetics by the cytoskeleton.
It is interesting to think about a possible involvement of microtubule cytoskeleton in the sustained suppression of VGCC current induced by CCh. We have previously suggested that a cytosolic factor, which is well preserved during dialysis with the pipette solution, or a membrane-bound or associated factor is involved in exerting the effect of CCh, since the CCh effect can be observed some 30 min after achieving the conventional whole-cell clamp configuration (Unno et al., 1995). Microtubule cytoskeleton possesses such properties and is a potential candidate as a factor mentioned above. Pretreatment of cells with colchicine and vinblastine, but not lumicolchicine, resulted in a marked decrease or abolition of the sustained component of IBa suppression induced by CCh. In connection with the data, colchicine and vinblastine have been shown to increase the sensitivity to a muscarinic agonist in producing contraction in guinea-pig ileal tissue (Famaey et al., 1977). These findings lend support to an involvement of microtubule cytoskeleton in the CCh action on IBa.
The inhibitory effects of colchicine and taxol on the CCh action are not considered to result from a decrease in the number of muscarinic receptors, since the binding studies revealed that these agents did not produce changes in the number of muscarinic receptors and in the binding of a muscarinic agonist to the receptor (McKay et al., 1985; 1991). In fact, ICCh and CCh-induced Ca2+ release from intracellular stores, mediated by Gi/o type G protein coupled with M2 subtype of muscarinic receptor and Gq type G protein coupled with M3 subtype of muscarinic receptor, respectively (Zholos & Bolton, 1997; Komori et al., 1998), remained unchanged after treatment with colchicine and taxol. The results also indicate that the agents do not disturb muscarinic receptor-G protein coupling in a non-specific manner.
Considering that tubulin is a common target for the cytoskeletal agents used in the present study (colchicine, vinblastine and taxol), it is conceivable that tubulin-related mechanisms may be involved in the muscarinic signalling linked to VGCCs. Tubulin molecule exists in membrane-bound and cytosolic forms, and recent evidence provides the molecule with variety of roles in different signal transduction pathways (Roychowdhury et al., 1993; Roychowdhury & Rasenick, 1994; Popova et al., 1997). Tubulin has GTPase activity which enables its interaction with several signal transduction elements, such as receptor-coupled G proteins and protein kinases (Rasenick et al., 1989; Huby et al., 1995; Ravindra et al., 1996; Garcia-Rocha et al., 1997). If muscarinic signal transduction utilizes tubulin as an intermediate, the cytoskeletal agents could interact directly with tubulin resulting in perturbation of the signal transduction. It has been demonstrated that colchicine and taxol are capable of inhibiting GTPase activity of tubulin molecule independently of their ability to depolymerize or polymerize microtubules (Ravindra & Aronstam, 1993; and the references therein). Therefore, the finding that both colchicine and taxol, which have opposite effects on microtubule cytoskeleton, inhibit the CCh action on IBa may be explained by such a dual action of the agents on tubulin molecule itself and microtubules.
Another possible interpretation of the present findings is that CCh polymerizes microtubule to suppress IBa. The inhibitory effect of taxol on IBa was accompanied by acceleration of the channel inactivation. This is similar to that obtained with CCh (Unno et al., 1996). Furthermore, microtubule requires low but certain levels of [Ca2+]i (<1 μM) for its polymerization (Dustin, 1984), as does the inhibitory effect of CCh on IBa (Unno et al., 1996). The effects of CCh and taxol on IBa were not additive, and after treatment with taxol at 100 μM high enough to produce the maximal effect on IBa, CCh could no longer suppress IBa. The results are explained if CCh and taxol are working through the same mechanism. Colchicine and vinblastine may act to prevent the polymerization induced by CCh. There are some reports which lead us to suppose signalling pathways between muscarinic receptors and microtubule polymerization. It has been suggested that Gq type G protein can interact directly with tubulin and stimulate its assembly to microtubules (Ravindra et al., 1996). Popova et al. (1997) have reported that phosphatidyl inositol bisphos-phate (PIP2) can bind to tubulin molecule and modulate tubulin assembly. If so, activation of Gq type of G protein by muscarinic receptor stimulation produces a reduction of PIP2 levels resulting from activation of phospholipase C and thereby free tubulin is released from PIP2-bound tubulin, which in turn may facilitate formation of microtubules leading to a decrease in VGCC activity. As suggested in other cell types (Sadot et al., 1996; Fromm et al., 1997; Schmidt et al., 1997) muscarinic receptor stimulation might activate a small molecular weight G protein or a phosphatase which are both known to promote polymerization of cytoskeletons (Murthy & Flavin, 1983; Yamamoto et al., 1988; Takai et al., 1995). Thus, it seems likely that microtubule polymerization is involved in the signal transduction of the muscarinic inhibitory effect on the VGCC activity.
The transient component of IBa suppression induced by CCh, which is brought about by Ca2+ release from intracellular stores, was also inhibited by the cytoskeletal agents to a smaller extent, compared with the sustained component. This may result from a role of cytoskeleton in the Ca2+-induced inactivation process of VGCCs, as suggested in hippocampus pyramidal neurons of adult rats (Johnson & Byerly, 1994) and cardiac cells of chick embryos (Galli & DeFelice, 1994).
In summary, the present studies suggest that microtubule cytoskeleton may modulate the activity of VGCCs in ileal smooth muscle cells of the guinea-pig and that muscarinic receptor stimulation induces sustained suppression of the VGCC current through a process, which is probably polymerization of microtubules or recruitment of tubulin molecule for the signal transduction. To determine the full significance of our results, more direct evidence, such as visualization of the state of microtubule, or a biochemical measurement of tubulin assembly, may be needed.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (08760274; 09760266) from the Ministry of Education, Science and Culture, Japan. We thank Dr I. Greenwood for giving helpful comment and criticism.
Abbreviations
- BAPTA
1, 2-bis (2-aminophenoxy) ethane-N, N, N′, N′-tetraacetic acid
- CCh
carbachol
- EGTA
ethylene glycol-bis (β-aminoethyl ether) N, N, N′, N′-tetraacetic acid
- G protein
GTP binding protein
- IBa
Ba2+ current
- ICa
Ca2+ current
- ICCh
non-selective cationic channel current
- IK
K+ current
- IKCa
Ca2+-activated K+ current
- IP3
inositol 1,4,5,-trisphosphate
- PIP2
inositol bisphosphate
- PLC
phospholipase C
- PSS
physiological salt solution
- PTX
pertussis toxin
- VGCCs
voltage-gated Ca2+ channels
References
- ANTIN P.B., FORRY-SCHAUDIES S., FRIEDMAN T.M., TAPSCOTT S.J., HOLTZER H. Taxol induces postmitotic myoblasts to assemble integrating microtubule-myosin arrays that exclude actin filaments. J. Cell Biol. 1981;90:300–308. doi: 10.1083/jcb.90.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEECH D.J. Actions of neurotransmitters and other messengers on Ca2+ channels and K+ channels in smooth muscle cells. Pharmacol. Ther. 1997;73:91–119. doi: 10.1016/s0163-7258(97)87271-3. [DOI] [PubMed] [Google Scholar]
- BENHAM C.D., BOLTON T.B., LANG R.J. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985;316:345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- DUSTIN P.General physiology of tubulins and microtubules Microtubules 1984New York: Springer-Verlag; 94–126.ed. Dustin, P. pp [Google Scholar]
- FAMAEY J.P., FONTAINE J., REUSE J. Smooth muscle sensitization induced by colchicine: is it an in vivo property of antitubulin agents. Agents Actions. 1977;7:305–309. doi: 10.1007/BF01969989. [DOI] [PubMed] [Google Scholar]
- FROEHNER S. Regulation of ion channel distribution at synapses. Annu. Rev. Neurosci. 1993;16:347–368. doi: 10.1146/annurev.ne.16.030193.002023. [DOI] [PubMed] [Google Scholar]
- FROMM C., COSO O.A., MONTANER S., XU X., GUTKIND J.S. The small GTP-binding protein Rho links G protein-coupled receptors and Gα12 to the serum response element and to cellular transformation. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10098–10103. doi: 10.1073/pnas.94.19.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUDA J., KAMEYAMA M., YAMAGUCHI K. Breakdown of cytoskeletal filaments selectively reduces Na and Ca spikes in cultured mammal neurons. Nature. 1981;294:82–85. doi: 10.1038/294082a0. [DOI] [PubMed] [Google Scholar]
- GALLI A., DEFELICE L. Inactivation of L-type Ca channels in embryonic chick ventricle cells: dependence of the cytoskeletal agents colchicine and taxol. Biophys, J. 1994;67:2296–2304. doi: 10.1016/S0006-3495(94)80715-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-ROCHA M., AVILA J., LOZANO J. The zeta isozyme of protein kinase C binds to tubulin through the pseudosubstrate domain. Exp. Cell Res. 1997;230:1–8. doi: 10.1006/excr.1996.3364. [DOI] [PubMed] [Google Scholar]
- HÄUSSINGER D., SAHA N., HALLBRUCKER C., LANG F., GEROK W. Involvement of microtubules in the swelling-induced stimulation of transcellular taurocholate transport in perfused rat liver. Biochem. J. 1993;291:355–360. doi: 10.1042/bj2910355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIE H., TAKENAKA T., KAIHO M. Effects of disruption of microtubules on translocation of particles and morphology in tissue cultured neurones. Brain Res. 1983;288:85–93. doi: 10.1016/0006-8993(83)90083-5. [DOI] [PubMed] [Google Scholar]
- HUBY R.D., CARLILE G.W., LEY S.C. Interactions between the protein-tyrosine kinase ZAP-70, the proto-oncoprotein Vav, and tubulin in Jurkat T cell. J. Biol. Chem. 1995;270:30241–30244. doi: 10.1074/jbc.270.51.30241. [DOI] [PubMed] [Google Scholar]
- INOUE R., ISENBERG G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J. Physiol. 1990;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON B.D., BYERLY L. Ca2+ channel Ca2+-dependent inactivation in a mammalian central neuron involves the cytoskeleton. Pflügers Arch. 1994;429:14–21. doi: 10.1007/BF02584025. [DOI] [PubMed] [Google Scholar]
- KIRBER M.T., ORDWAY R.W., CLAPP L.H., WALSH J.V., JR, SINGER J.J. Both membrane stretch and fatty acids directly activate large conductance Ca2+-activated K+ channels in vascular smooth muscle cells. FEBS Lett. 1992;297:24–28. doi: 10.1016/0014-5793(92)80319-c. [DOI] [PubMed] [Google Scholar]
- KOMORI S., BOLTON T.B. Calcium release induced by inositol 1,4,5-trisphosphate in single rabbit intestinal smooth muscle cells. J. Physiol. 1991;433:495–517. doi: 10.1113/jphysiol.1991.sp018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., KAWAI M., TAKEWAKI T., OHASHI H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J. Physiol. 1992;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., UNNO T., NAKAYAMA T., OHASHI H. M2 and M3 muscarinic receptors couple, respectively, with activation of nonselective cationic channels and potassium channels in intestinal smooth muscle cells. Jpn. J. Pharmacol. 1998;76:213–218. doi: 10.1254/jjp.76.213. [DOI] [PubMed] [Google Scholar]
- LEIBER D., JASPER J.R., ALOUSI A.A., MARTIN J., BERNSTEIN D., INSEL A.A. Alteration in Gs-mediated signal transduction in S49 lymphoma cells treated with inhibitors of microtubules. J. Biol. Chem. 1993;268:3833–3837. [PubMed] [Google Scholar]
- MCDONALD T.F., PELZER S., TRAUTWEIN W., PELZER D.J. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol. Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- MCKAY D.B., ARONSTAM R.S., SCHNEIDER A.S. Interaction of microtubule-active agents with nicotinic acetylcholine receptors: relationship to their inhibition of catecholamine secretion by adrenal chromaffin cells. Mol. Pharmacol. 1985;28:10–16. [PubMed] [Google Scholar]
- MCKAY D.B., LOPEZ I., SANCHEZ P.A., ENGLISH J.L., WALLACE L.J. Characterization of muscarinic receptors of bovine adrenal chromaffin cells: binding, secretion and anti-microtubule drug effects. Gen. Pharmacol. 1991;22:1185–1189. doi: 10.1016/0306-3623(91)90599-2. [DOI] [PubMed] [Google Scholar]
- MURTHY A.S.N., FLAVIN M. Microtubule assembly using the microtubule-associated protein MAP-2 prepared in defined states of phosphorylation with protein kinase and phosphatase. Eur. J. Biochem. 1983;137:37–46. doi: 10.1111/j.1432-1033.1983.tb07792.x. [DOI] [PubMed] [Google Scholar]
- POPOVA J.S., GARRISON J.C., RHEE S.G., RASENICK M.M. Tubulin, Gq, and phosphatidylinositol 4,5-bisphosphate interact to regulate phospholipase Cβ1 signaling. J. Biol. Chem. 1997;272:6760–6765. doi: 10.1074/jbc.272.10.6760. [DOI] [PubMed] [Google Scholar]
- PRAGNELL M., WAARD M.D., MORI Y., TANABE T., SNUTCH T.P., CAMPBELL K.P. Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- RASENICK M.M., YAN K., WANG N.Tubulin as a G protein The Guanine Nucleotide-Binding Protein 1989New York: Plenum Publishing Corp; 391–402.eds. Boch, L, Krall, B. & Parmeggiani, A. pp [Google Scholar]
- RAVINDRA R., ARONSTAM R.S. Effect of colchicine and taxol on stimulation of G protein GTPase activity in anterior pituitary lobe of rats by gonadotrophin- and thyrotrophin-releasing hormones. J. Reproduct. Fertil. 1993;97:27–33. doi: 10.1530/jrf.0.0970027. [DOI] [PubMed] [Google Scholar]
- RAVINDRA R., KUNAPULI S.P., FORMAN L.J., NAGELE R.G., FOSTER K.A., PATEL S.A. Effect of transient overexpression of Gqα on soluble and polymerized tubulin pools in GH3 and AtT-20 cells. J. Cell. Biochem. 1996;61:392–401. doi: 10.1002/(SICI)1097-4644(19960601)61:3%3C392::AID-JCB6%3E3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- ROYCHOWDHURY S., RASENICK M.M. Tubulin-G protein association stabilizes GTP binding and activates GTPase: cytoskeletal participation in neuronal signal transduction. Biochemistry. 1994;33:9800–9805. doi: 10.1021/bi00198a052. [DOI] [PubMed] [Google Scholar]
- ROYCHOWDHURY S., WANG N., RASENICK M.M. G protein binding and G protein activation by nucleotide transfer involve distinct domains on tubulin: regulation of signal transduction by cytoskeletal elements. Biochemistry. 1993;32:4955–4961. doi: 10.1021/bi00069a034. [DOI] [PubMed] [Google Scholar]
- SADOT E., GURWITZ D., BARG J., BEHAR L., GINZBURG I., FISHER A. Activation of m1 muscarinic acetylcholine receptor regulates τ phosphorylation in transfected PC12 cells. J. Neurochem. 1996;66:877–880. doi: 10.1046/j.1471-4159.1996.66020877.x. [DOI] [PubMed] [Google Scholar]
- SCHMIDT M., RUMENAPP U., KELLER J., LOHMANN B., JAKOBS K.H. Regulation of phospholipase C and D activities by small molecular weight G protein and muscarinic receptors. Life Sci. 1997;60:1093–1100. doi: 10.1016/s0024-3205(97)00052-0. [DOI] [PubMed] [Google Scholar]
- TAKAI Y., SASAKI T., TANAKA K., NAKANISHI H. Rho as a regulator of the cytoskeleton. Trends Biochem. Sci. 1995;20:227–231. doi: 10.1016/s0968-0004(00)89022-2. [DOI] [PubMed] [Google Scholar]
- TILSON H.A., SCHWARTZ R.D., ALI S.F., MCLAMB R.L. Colchicine administered into the area of the nucleus basalis decreases cortical nicotinic cholinergic receptors labelled by [3H]-acetylcholine. Neuropharmacology. 1989;28:855–861. doi: 10.1016/0028-3908(89)90178-0. [DOI] [PubMed] [Google Scholar]
- UNNO T., KOMORI S., OHASHI H. Inhibitory effect of muscarinic receptor activation on Ca2+ channel current in smooth muscle cells of guinea-pig ileum. J. Physiol. 1995;484:567–581. doi: 10.1113/jphysiol.1995.sp020687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNNO T., KOMORI S., OHASHI H. Some evidence against the involvement of arachidonic acid in muscarinic suppression of voltage-gated calcium channel current in guinea-pig ileal smooth muscle cells. Br. J. Pharmacol. 1996;119:213–222. doi: 10.1111/j.1476-5381.1996.tb15973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANIISHI Y., INOUE R., ITO Y. Preferential potentiation by hypotonic sell swelling of muscarinic cation current in guinea pig ileum. Am. J. Physiol. 1997;272:C240–C253. doi: 10.1152/ajpcell.1997.272.1.C240. [DOI] [PubMed] [Google Scholar]
- WILSON L., BAMBURG J.R., MIZEL S.B., GRISAHM L.M., CRESWELL K.M. Interaction of drugs with microtubule proteins. Fed. Proc. 1974;33:158–166. [PubMed] [Google Scholar]
- XU W.X., KIM S.J., KIM S.J., SO I., KANG T.M., RHEE J.C., KIM K.W. Effect of stretch on calcium channel currents recorded from the antral circular myocytes of guinea-pig stomach. Pflügers Arch. 1996;432:159–164. doi: 10.1007/s004240050119. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO H., SAITOH Y., FUKUNAGA K., NISHIMIRA H., MIYAMOTO E. Dephosphorylation of microtubule proteins by brain protein phosphatatases 1 and 2A, and its effect on microtubule assembly. J. Neurochem. 1988;50:1614–1623. doi: 10.1111/j.1471-4159.1988.tb03051.x. [DOI] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. Br. J. Pharmacol. 1997;122:885–893. doi: 10.1038/sj.bjp.0701438. [DOI] [PMC free article] [PubMed] [Google Scholar]


