Abstract
We tested whether nociceptin (NCE), the endogenous ligand of the opioid receptor-like 1 (ORL1) receptor, and nocistatin (NST), which reverses central NCE effects when applied intrathecally (i.t.), affect small-diameter afferent fibre-mediated vasodilatation in rat hairless skin.
Female Wistar rats were vagotomized. Ongoing sympathetic vasoconstrictor activity was abolished by bilateral section of the lumbar sympathetic trunk between ganglia L2 and L3. Sensory axons were selectively stimulated in the dorsal root L5 by 20 electrical impulses supramaximal for activating C-fibres at 1 Hz. Blood flow was measured on the plantar skin of the left hind paw in the L5 dermatome using laser Doppler flowmetry.
NCE injected intravenously (i.v.) as single boluses (1, 10 and 100 nmol kg−1) 7–8 min before dorsal root stimulation (n=6) dose-dependently decreased blood pressure and local vascular resistance and suppressed antidromic vasodilatation maximally by 47% (P<0.01). When NCE was injected 2 min before stimulation (n=3), antidromic vasodilatation was reduced by 64% after NCE (1 nmol kg−1) and totally, or almost totally, abolished after the two higher doses.
NST (1–100 nmol kg−1 i.v., n=6) was without significant effect on blood pressure and cutaneous vascular resistance. Applied 5 (n=6) or 2 min (n=3) before stimulation it also did not affect antidromic vasodilatation. NST (100 nmol kg−1 i.v.) applied shortly before an equimolar dose of NCE did not antagonize NCE effects on vascular resistance, blood pressure and antidromic vasodilatation (n=4).
In conclusion, NCE inhibits antidromic vasodilatation, a component of neurogenic inflammation, in rat skin while NST is without effect. NST, at the small-diameter sensory ending, is not an effective antagonist of NCE.
Keywords: Nociceptin, orphanin FQ, nocistatin, opioid receptor-like 1, sensory neurones, neurogenic inflammation, sensory neuropeptides, antidromic vasodilatation, skin blood flow, laser Doppler
Introduction
Nociceptin (NCE), also called orphanin FQ, has structural similarities to dynorphin A and is an endogenous ligand of the opioid receptor-like 1 (ORL1) receptor which is a member of the opioid receptor family (Meunier et al., 1995; Reinscheid et al., 1995). The ORL1 receptor does not bind the known opioids with high affinity and the effects of NCE are not antagonized by naloxone (Darland et al., 1998). NCE seems to have, as yet only in part well-defined, effects on a number of biological systems, e.g. on the nociceptive system and on the cardiovascular system (Darland et al., 1998). Thus, when injected intracerebroventricularly (i.c.v.) or intrathecally (i.t.), it elicits hyperalgesia and allodynia in mice (Meunier et al., 1995; Reinscheid et al., 1995; Hara et al., 1997) and reverses opioid-mediated antinociception (Mogil et al., 1996). In contrast, in the spinal dorsal horn NCE rather seems to have an inhibitory effect on nociceptive transmission (Lai et al., 1997; Liebel et al., 1997; Yamamoto et al., 1997a,1997b). NCE also evokes decreases in systemic blood pressure, apparently by influencing transmitter release from autonomic neurones supplying heart and resistance vessels (Giuliani et al., 1997). Nocistatin (NST) is a neuropeptide which is derived from the same precursor molecule as NCE (Okuda-Ashitaka et al., 1998). Administered i.t. in mice it antagonizes the hyperalgesia and allodynia generated by NCE (Minami et al., 1998; Okuda-Ashitaka et al., 1998), although it does not bind to the ORL1 receptor and is not coupled to the same second messenger cascade (Okuda-Ashitaka et al., 1998).
Small-diameter primary afferent neurones express opioid receptors (Smith & Buchan, 1984; Lundberg, 1996). Although opioids may not necessarily decrease the excitability of the nociceptive ending to physiological stimuli (Shakhanbeh & Lynn, 1993) they reduce the release of neuropeptides from afferent endings resulting in a suppression of neurogenic inflammation, i.e. antidromic vasodilatation (Lembeck & Donnerer, 1985; Holzer et al., 1991; Shakhanbeh & Lynn, 1993; Holzer, 1998) and plasma extravasation (Lembeck & Donnerer, 1985; Holzer et al., 1991; Towler & Brain, 1998). NCE is also capable of inhibiting neurogenic plasma extravasation in skin (Helyes et al., 1997). In the isolated guinea-pig renal pelvis and the rat trachea NCE inhibits the release of neuropeptides (Giuliani & Maggi, 1996; Nemeth et al., 1998). Neurogenic plasma extravasation is mainly mediated by neurokinins whereas small-diameter afferent fibre-induced vasodilatation is predominantly mediated by calcitonin gene-related peptide (CGRP) (Lundberg, 1996; Holzer, 1998; Häbler et al., 1999a). Therefore, the inhibition of plasma extravasation by NCE does not necessarily imply that antidromic vasodilatation is also reduced by this neuropeptide. Whether NST affects neurogenic inflammation is unknown.
The purpose of this study was to test (i) whether NCE inhibits antidromic vasodilatation in hairless skin, (ii) whether NST has any effect on antidromic vasodilatation and (iii) whether NST can reverse the effects elicited by NCE. Preliminary results have been published recently (Häbler et al., 1999b).
Methods
Experimental procedures have been described in detail recently (Häbler et al., 1999a). Briefly, female Wistar rats (Charles River GmbH, Sulzfeld, Germany) (230–290 g) were anaesthetized with pentobarbital sodium i.p. (Nembutal®, 60 mg kg−1, additional doses 10 mg kg−1 every hour i.v.). The adequacy of anaesthesia was judged from the absence of withdrawal reflexes, gross fluctuations of blood pressure and heart rate and the absence of longlasting blood pressure effects upon brief noxious stimuli applied to the skin. Before starting experimental protocols animals were paralyzed (Pancuronium, Organon, 1 mg kg−1 initially, maintenance with 0.4 mg kg−1 when necessary) and artificially ventilated with O2-enriched room air (ventilation-pump RUS-1300, FMI, Egelsbach, Germany). At intervals we let muscular paralysis wear off and assured that withdrawal reflexes were absent. Blood gases and blood acid-base status were measured at intervals (ABL 5, Radiometer, Copenhagen, Denmark). Arterial blood pressure was continuously recorded in the ventral caudal artery. Rectal temperature was kept constant close to 37°C by means of a servo-controlled heating blanket.
All efforts were made to minimize animal suffering and to reduce the number of animals used. At the end of the experiments rats were killed under deep anaesthesia by i.v. injection of a saturated solution of potassium chloride. All experiments had been approved by the local animal care committee of the state administration and were conducted in accordance with German Federal Law.
All rats were bilaterally vagotomized. Using a retroperitoneal approach, both lumbar sympathetic trunks (LST) together with the white rami L3 were then cut caudally to ganglion L2 before starting experimental protocols to abolish on-going vasoconstrictor activity to the hindlimb which was verified by a rise in skin temperature and blood flow after the intervention (Häbler et al., 1997). After a lumbosacral laminectomy the left dorsal root (DR) L5 was identified, cut proximally and stimulated electrically with a train of 20 pulses supramaximal for activating C-fibres (20 V, pulse width 0.5 ms) at a frequency of 1 Hz to induce antidromic vasodilatation. A pool was formed from skin flaps and the exposed tissue was covered with warm mineral oil.
Superficial blood flow was measured within the L5 innervation territory on central plantar glabrous skin (proximal to the pads) of the left hindpaw using a laser Doppler flowmeter device (MBF3D, Moor Instruments, Axminster, Devon, U.K.). Flux signals were low pass filtered with the time constant set to 3 s.
NCE and NST (Bachem, Bubendorf, Switzerland) were dissolved in physiological saline containing 1 mg ml−1 bovine serum albumin to prevent adsorption of the peptides and given as single i.v. boluses (0.2 ml) of 1, 10 and 100 nmol kg−1 in ascending order. In six rats, after 7–8 min following NCE injection, in order to allow blood pressure and flow to return to baseline, and 5 min after NST injection, antidromic vasodilatation was evoked electrically. In another three rats, DR stimulation was performed 2 min after NCE (1–100 nmol kg−1) and NST (100 nmol kg−1). Control and test vasodilatations were performed strictly in a paired manner for each dose of neuropeptide. The test vasodilatation was elicited 10–15 min after the control stimulation. Between two successive doses of neuropeptide at least 30 min were allowed and at least 1 h between the last dose of NCE and the first of NST, respectively. In four experiments, stimulation-induced vasodilatation was tested after a 100 nmol kg−1 dose of NST injected 30 s before an equimolar dose of NCE.
Antidromic vasodilatation was quantified by determining the peak increase of blood flow following stimulation. Integrated flow responses (area under the curve) were determined over 1, 2 and 3 min after stimulation. Vascular resistance in hairless skin was calculated in arbitrary units from mean arterial pressure (MAP) and blood flow. All responses were expressed as percentage changes of control flow and resistance, respectively, averaged within a time period of 60 s before stimulation. Data are expressed as means±s.e.mean or means±s.d. as indicated. Statistical significance was assessed using two-tailed paired t-test and t-test as appropriate.
Results
NCE induced a dose-dependent transient decrease of systemic blood pressure (Figure 1A) which returned to baseline within 6–8 min after injection of the neuropeptide. In parallel, there was a significant decrease of cutaneous vascular resistance which was also dose-dependent (Figure 1B). Because both LST were cut this was not due to changes in sympathetic vasoconstrictor activity.
Figure 1.
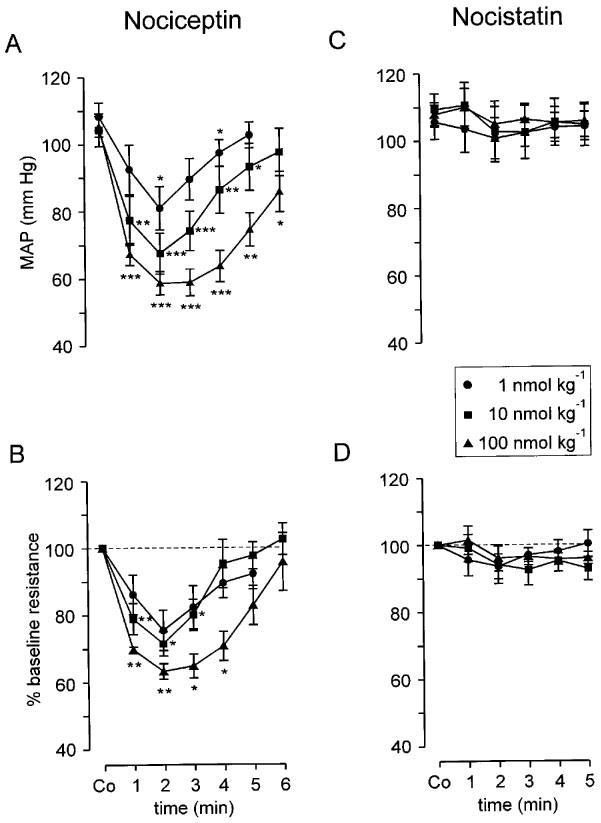
Cardiovascular effects elicited by single i.v. bolus injections of NCE (A, B) and NST (C, D). NCE (A) but not NST (C) dose-dependently and transiently decreased mean arterial blood pressure (MAP). Vascular resistance in hairless skin (calculated from MAP and laser Doppler flow; baseline denoted by broken line) was dose-dependently reduced by NCE (B) but not affected by NST (D). A vagal contribution to MAP responses was excluded by bilateral vagotomy. The decrease of vascular resistance in (B) was non-neurogenic because the LST was cut bilaterally. Co on abscissa indicates control. Data (means±s.e.mean) from n=6 animals in both groups, *P<0.05, **P<0.01, ***P<0.001 (paired t-test).
Stimulation-induced antidromic vasodilatation was inhibited by NCE given i.v. as a single bolus. The inhibition increased with each logarithmic increment in the dose of the neuropeptide (Figures 2,3,4) without changing the overall shape of antidromic vasodilatation. When the DR was stimulated 2 min after NCE application (n=3), i.e. while NCE-induced reductions of MAP and local vascular resistance were near to maximal, antidromic vasodilatation was totally abolished at the highest dose and almost totally suppressed after 10 nmol kg−1 (Figure 2B and C). At the lowest dose tested, stimulation-induced vasodilatation was substantially and significantly reduced (Figures 2A and 4, Table 1).
Figure 2.
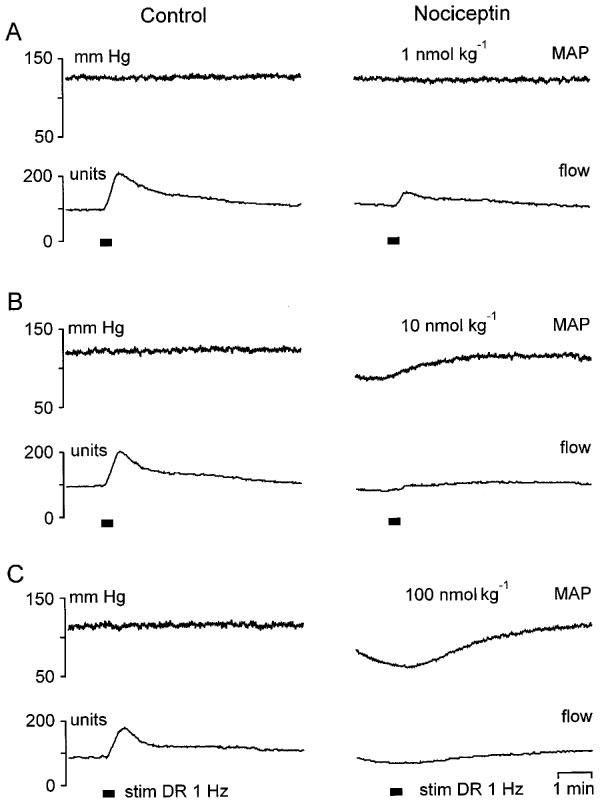
Strong suppression of antidromic vasodilatation by NCE injected 2 min prior to dorsal root L5 stimulation (20 s, 1 Hz, supramaximal for C-fibres; stim DR 1 Hz). Compared with control (left panel) NCE substantially inhibited stimulation-induced vasodilatation at the lowest dose tested (A). At the two higher doses, antidromic vasodilatation was almost totally suppressed (B) and completely abolished (C). Note that test stimulations were performed during NCE-induced decreases of MAP and cutaneous vascular resistance. The flow increases after DR stimulation in (B) and (C) (right panels) largely parallel the increase of MAP towards baseline and therefore are not neurogenic.
Figure 3.
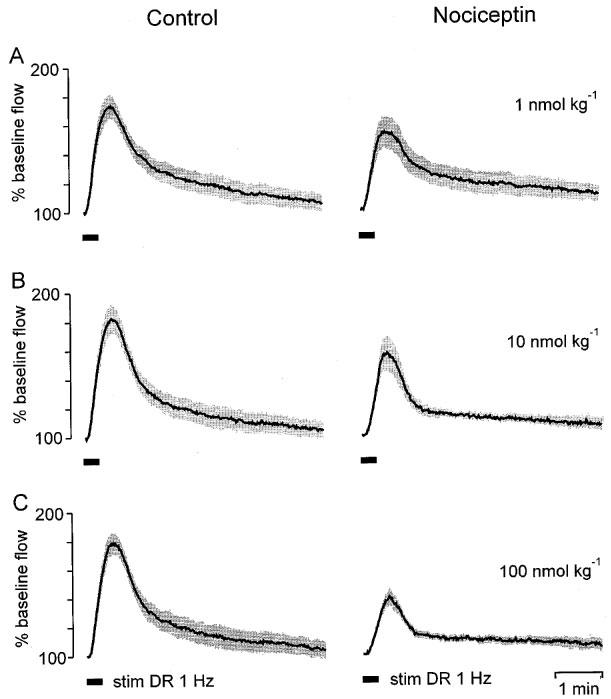
Dose-dependent suppression of antidromic vasodilatation by NCE injected i.v. The dorsal root L5 was stimulated for 20 s at a frequency of 1 Hz (stim DR 1 Hz) 7–8 min after applying NCE when blood pressure and skin blood flow had returned to baseline. Stimulation-induced (bar) vasodilator responses from six animals were normalized and averaged (shaded areas are means±s.e.mean). Compared with the control dilatations (left panels) NCE decreased the magnitude but did not change the shape of antidromic vasodilatation (right panels).
Figure 4.
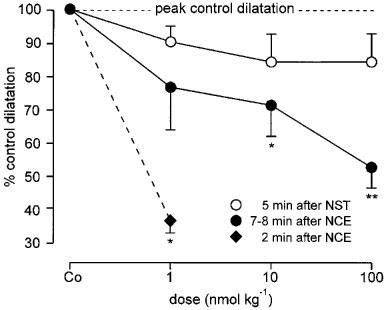
Reduction of the peak amplitude of antidromic vasodilatation by NCE and NST (applied 7–8 min and 5 min, respectively, before DR L5 stimulation). NCE dose-dependently reduced peak vasodilatation, at the highest dose almost by 50%. The inhibition by NST was small and non-significant. Co indicates control. Data (means±s.e.mean) from n=6 animals in both groups. For comparison, the much stronger effect of the lowest NCE dose applied 2 min before DR stimulation is also shown (data from three rats). *P<0.05, **P<0.01 (paired t-test).
Table 1.
Effects of nociceptin and nocistatin on antidromic vasodilatation (AVD)
When antidromic vasodilatation was evoked 7–8 min after NCE injection, i.e. after MAP and vascular resistance had returned to baseline, peak vasodilatation and the flow responses integrated over 1–3 min after stimulation (area under the curve) were still significantly reduced compared with the controls after the two higher doses of NCE (Figures 3B, C and 4, Table 1). The reduction of stimulation-induced vasodilatation was no longer significant after the lowest dose of NCE (Figures 3A and 4, Table 1). About 20 min after the 100 nmol kg−1 dose of NCE, antidromic vasodilatation had fully recovered to control magnitude.
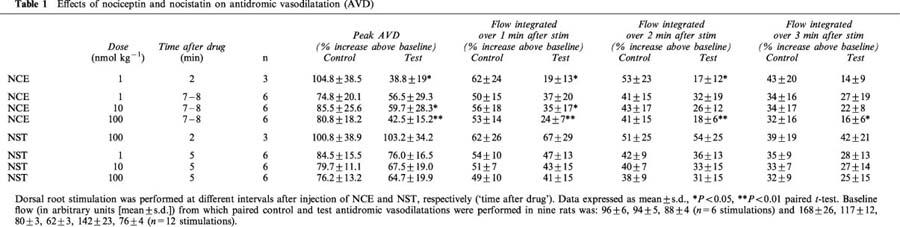
NST in any dose tested did not significantly change MAP nor vascular resistance in hairless skin (Figure 1C and D). When elicited 5 min after NST application, antidromic vasodilatation was somewhat reduced but not significantly so and not in a dose-dependent manner (Figures 4 and Figures 5, Table 1). Peak vasodilatation and integrated flow responses over 1–3 min after stimulation were not significantly smaller than controls (Table 1). NST (100 nmol kg−1), injected 2 min before DR stimulation also did not reduce the resulting vasodilatation (Table 1).
Figure 5.
Lack of a significant effect on antidromic vasodilatation of NST. Stimulation-induced vasodilator responses from six animals were normalized and averaged (shaded areas are means±s.e.mean). The dorsal root L5 was stimulated for 20 s at a frequency of 1 Hz (stim DR 1 Hz) 5 min after applying NST. Compared with the control dilatations (left panels) NST reduced the magnitude of antidromic vasodilatation only little (right panels).
A 100 nmol kg−1 bolus of NST, applied shortly before an equimolar dose of NCE, failed to reverse the inhibition of stimulation-induced antidromic vasodilatation elicited by NCE. Thus, peak vasodilatation (Figure 6A), maximum decrease of cutaneous vascular resistance (Figure 6B) and maximum decrease of MAP (Figure 6C) after combined administration of both peptides were similar to those evoked by NCE (100 nmol kg−1) alone.
Figure 6.
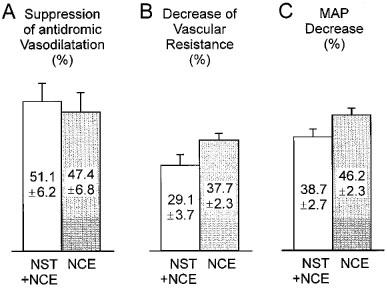
Effect of NST on NCE-induced changes of antidromic vasodilatation, skin vascular resistance and blood pressure. DR L5 was stimulated 7–8 min after injection of NCE. NST (100 nmol kg−1) applied 30 s before NCE (100 nmol kg−1) did not significantly affect NCE-mediated suppression of stimulation-induced vasodilatation (expressed relative to the controls, P>0.05, t-test) (A) nor the maximum decrease of vascular resistance (relative to baseline) after NCE (P>0.05) (B) nor the decrease of systemic blood pressure (relative to baseline pressure) due to NCE (P>0.05) (C). Data (means±s.e.mean) from n=4 animals receiving NST and NCE (open columns) and n=6 animals (shaded columns) receiving only NCE.
Discussion
The results of the present study show that NCE (orphanin FQ), the endogenous ligand of the ORL1-receptor, applied i.v. inhibits small-diameter afferent fibre-mediated antidromic vasodilatation in rat hairless skin and relaxes cutaneous blood vessels by a non-neurogenic mechanism. However, the profound inhibition of antidromic vasodilatation 2 min after NCE injection was greatly attenuated 5 min later indicating that the NCE effects are rather short-lived. In contrast, NST, which when administered i.t. has been shown to be a functional antagonist of NCE because it was capable of reversing a number of central NCE effects (Minami et al., 1998; Nicol et al., 1998; Okuda-Ashitaka et al., 1998), produced only a small, non-significant, inhibition of antidromic vasodilatation and had no significant effects on blood pressure and vascular resistance in the cutaneous bed. Furthermore, NST was not capable of antagonizing the NCE effects observed in the present study. These observations suggest that (i) NCE acting via ORL1 receptors on the peripheral endings of thin afferents has considerable anti-inflammatory potency and might be useful for the treatment of inflammation, (ii) NCE effects on antidromic vasodilatation, systemic blood pressure and blood vessels are similar to those exerted by opioids (Lembeck & Donnerer, 1985; Holzer et al., 1991; Shakhanbeh & Lynn, 1993; Champion et al., 1997b; Czapla et al., 1998; Holzer, 1998) and (iii) NST, at the level of the peripheral afferent ending, is not an effective antagonist of NCE. Although NCE had a considerable potency at low doses, it is doubtful whether the effects of NCE observed in the present study play any role under physiological conditions. In humans, NCE appears in the plasma but only at a concentration of about 10 pg ml−1 (Brooks et al., 1998). However, during peripheral inflammation NCE is upregulated in related dorsal root ganglia (Andoh et al., 1997). Under this condition NCE, after axonal transport into the periphery, may be released in the vicinity of nociceptor endings and inhibit neurogenic vasodilatation.
These results complement those reported by Helyes et al. (1997) who found that NCE inhibited neurogenic plasma extravasation, the other component of neurogenic inflammation, in rat skin in vivo. However, although substance P (SP) and/or neurokinin A and calcitonin gene-related peptide (CGRP) are involved in both plasma extravasation and antidromic vasodilatation (Häbler et al., 1999a) it is predominantly SP which mediates plasma extravasation whereas CGRP plays the major role in antidromic vasodilatation (Lundberg, 1996; Holzer, 1998). The present results together with those of Helyes et al. (1997) therefore suggest that NCE suppresses neurokinin and CGRP release from small-diameter afferent endings in skin. This is consistent with previous evidence from in vitro experiments showing that NCE inhibits the release of both SP and CGRP from capsaicin sensitive nerve fibres supplying guinea-pig and rat airways (Fischer et al., 1998; Nemeth et al., 1998; Shah et al., 1998) and guinea-pig atrium and renal pelvis (Giuliani & Maggi, 1996; 1997). Thus NCE in general appears to attenuate the efferent function of small-diameter afferent nerve fibres, at least when they are activated electrically. There is evidence that NCE does not attenuate neuropeptide release due to the activation of capsaicin receptors (Shah et al., 1998) indicating that voltage dependent Ca2+ channels, but not the capsaicin-controlled cation channel may be the target of NCE (Connor et al., 1996; Knoflach et al., 1996). Our and previous results, which indicate inhibition of neuropeptide release by NCE, are in contrast to a recent report presenting evidence that NCE injected into the plantar skin of mice induced the release of SP. This in turn elicited a flexor reflex, which was interpreted as indicative of pain (Inoue et al., 1998).
NST which was capable of reversing the hyperalgesia due to NCE applied i.c.v. or i.t. in mice (Okuda-Ashitaka et al., 1998) was not an effective antagonist of NCE in preventing the inhibition of antidromic vasodilatation. This is in line with the observation that NST did not bind to the ORL1 receptor nor did it affect the NCE-induced decrease of intracellular cyclic AMP (Okuda-Ashitaka et al., 1998). Thus it appears that NST does not interfere with small-diameter afferents in the periphery.
Our results confirm earlier findings showing that NCE has profound cardiovascular effects (Champion & Kadowitz, 1997; Champion et al., 1997a; Czapla et al., 1997b; Giuliani et al., 1997). It has been shown that the decrease in systemic arterial pressure is the consequence of two component responses, (i) a bradycardia mediated by both the vagus and the cardiac sympathetic nerves and (ii) a decrease of peripheral vascular resistance probably mediated by inhibition of transmitter release from sympathetic vasoconstrictor fibres (Giuliani et al., 1997; Bucher, 1998). Elimination of the neurogenic influence by vagotomy and intravenous guanethidine abolished the NCE-induced MAP decrease (Giuliani et al., 1997). Since NCE has been localized to brainstem regions which are involved in the regulation of central autonomic functions (Darland et al., 1998), NCE may also play a role during on-going regulation of blood pressure. Thus, micro-injection of NCE into the rostral ventolateral medulla (Chu et al., 1998), where ‘pre-sympathetic' neurones are located, decreased blood pressure and heart rate in rats. However, since NCE applied i.v. is unlikely to cross the blood brain barrier this mechanism does not account for the MAP decrease in the present study.
We studied whether in addition to the effects brought about by the autonomic nervous system there is also a non-neurogenic vasodilator effect by NCE in the cutaneous vascular bed. To this end we eliminated on-going sympathetic vasoconstrictor activity by sectioning the LST bilaterally. Despite sympathetic denervation we saw a considerable dose-dependent decrease of vascular resistance suggesting that NCE has also a direct vasorelaxant effect in blood vessels supplying hairless skin. This observation is consistent with previous reports showing that NCE induced vasodilatation in the denervated rat hindlimb (Czapla et al., 1997a) and relaxation of phenylephrine-precontracted rings of various cat arteries in vitro (Gumusel et al., 1997). This direct relaxant effect of NCE may be mediated by the blockade of voltage-dependent Ca2+ channels in vascular smooth muscle, in analogy to the blockade of similar channels in neurones. Another possibility may be that the vascular endothelium expresses ORL1 receptors whose activation may increase the production of nitric oxide (NO) resulting in vasodilatation. There is evidence that this mechanism is used by opioids (Stefano et al., 1995; Champion & Kadowitz, 1998; Wilderman & Armstead, 1998). However, recent evidence suggests that NCE is a vasodilator independent of endothelial NO (Champion et al., 1998).
In conclusion, the present study shows that NCE is an effective inhibitor of small-diameter afferent fibre-mediated vasodilatation and may thus be useful as an anti-inflammatory agent. NCE has also profound depressant cardiovascular effects part of which seem to be mediated by a non-neurogenic action on smooth muscle. NST, which was shown recently to block some anti-opioid effects of NCE was almost totally ineffective in influencing antidromic vasodilatation and blood pressure. It also did not reverse NCE effects on antidromic vasodilatation, blood pressure and local vascular resistance and thus does not seem to be a functional antagonist of NCE neither at the peripheral endings of small-diameter primary afferents nor at cutaneous blood vessels.
Acknowledgments
We thank Eike Tallone for making the illustrations and Sigrid Augustin for technical help in the experiments. This work was supported by the Deutsche Forschungsgemeinschaft.
Abbreviations
- DR
dorsal root
- LST
lumbar sympathetic trunk
- MAP
mean arterial pressure
- NCE
nociceptin
- NST
nocistatin
- ORL1
opioid receptor-like 1
References
- ANDOH T., ITOH M., KURAISHI Y. Nociceptin gene expression in rat dorsal root ganglia induced by peripheral inflammation. Neuroreport. 1997;8:2793–2796. doi: 10.1097/00001756-199708180-00028. [DOI] [PubMed] [Google Scholar]
- BROOKS H., ELTON C.D., SMART D., ROWBOTHAM D.J., MCKNIGHT A.T., LAMBERT D.G. Identification of nociceptin in human cerebrospinal fluid: comparison of levels in pain and non-pain states. Pain. 1998;78:71–73. doi: 10.1016/S0304-3959(98)00130-4. [DOI] [PubMed] [Google Scholar]
- BUCHER B. ORL1 receptor-mediated inhibition by nociceptin of noradrenaline release from perivascular sympathetic nerve endings of the rat tail artery. Naunyn Schmied. Arch. Pharmacol. 1998;358:682–685. doi: 10.1007/pl00005312. [DOI] [PubMed] [Google Scholar]
- CHAMPION H.C., BIVALACQUA T.J., FRIEDMAN D.E., ZADINA J.E., KASTIN A.J., KADOWITZ P.J. Nitric oxide release mediates vasodilator responses to endomorphin 1 but not nociceptin/OFQ in the hindquarters vascular bed of the rat. Peptides. 1998;19:1595–1602. doi: 10.1016/s0196-9781(98)00110-7. [DOI] [PubMed] [Google Scholar]
- CHAMPION H.C., CZAPLA M.A., KADOWITZ P.J. Nociceptin, an endogenous ligand for the ORL1 receptor, decreases cardiac output and total peripheral resistance in the rat. Peptides. 1997a;18:729–732. doi: 10.1016/s0196-9781(97)00003-x. [DOI] [PubMed] [Google Scholar]
- CHAMPION H.C., KADOWITZ P.J. Nociceptin, an endogenous ligand for the ORL1 receptor, has novel hypotensive activity in the rat. Life Sci. 1997;60:PL241–PL245. doi: 10.1016/s0024-3205(97)00087-8. [DOI] [PubMed] [Google Scholar]
- CHAMPION H.C., KADOWITZ P.J. D-[Ala2]endomorphin 2 and endomorphin 2 have nitric oxide-dependent vasodilator activity in rats. Am. J. Physiol. 1998;274:H1690–H1697. doi: 10.1152/ajpheart.1998.274.5.H1690. [DOI] [PubMed] [Google Scholar]
- CHAMPION H.C., ZADINA J.E., KASTIN A.J., HACKLER L., GE L.J., KADOWITZ P.J. Endomorphin 1 and 2, endogenous ligands for the mu-opioid receptor, decrease cardiac output, and total peripheral resistance in the rat. Peptides. 1997b;18:1393–1397. doi: 10.1016/s0196-9781(97)00210-6. [DOI] [PubMed] [Google Scholar]
- CHU X., XU N., LI P., WANG J.Q. Profound inhibition of cardiomotor neurons in the rat rostral ventrolateral medulla by nociceptin (orphanin FQ) Neuroreport. 1998;9:1081–1084. doi: 10.1097/00001756-199804200-00022. [DOI] [PubMed] [Google Scholar]
- CONNOR M., YEO A., HENDERSON G. The effect of nociceptin on Ca++ channel current and intracellular Ca++ in the SH-SY5Y human neuroblastoma cell line. Br. J. Pharmacol. 1996;118:205–207. doi: 10.1111/j.1476-5381.1996.tb15387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CZAPLA M.A., CHAMPION H.C., KADOWITZ P.J. Nociceptin, an endogenous ligand for the ORL1 receptor, has vasodilator activity in the hindquarters vascular bed of the rat. Peptides. 1997a;18:793–795. doi: 10.1016/s0196-9781(97)00014-4. [DOI] [PubMed] [Google Scholar]
- CZAPLA M.A., CHAMPION H.C., KADOWITZ P.J. Decreases in systemic arterial and hindquarters perfusion pressure in response to nociceptin are not inhibited by naloxone in the rat. Peptides. 1997b;18:1197–1200. doi: 10.1016/s0196-9781(97)00178-2. [DOI] [PubMed] [Google Scholar]
- CZAPLA M.A., CHAMPION H.C., ZADINA J.E., KASTIN A.J., HACKLER L., GE L.J., KADOWITZ P.J. Endomorphin 1 and 2, endogenous mu-opioid agonists, decrease systemic arterial pressure in the rat. Life Sci. 1998;62:PL175–PL179. doi: 10.1016/s0024-3205(98)00048-4. [DOI] [PubMed] [Google Scholar]
- DARLAND T., HEINRICHER M.M., GRANDY D.K. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- FISCHER A., FORSSMANN W.G., UNDEM B.J. Nociceptin-induced inhibition of tachykinergic neurotransmission in guinea pig bronchus. J. Pharmacol. Exp. Ther. 1998;285:902–907. [PubMed] [Google Scholar]
- GIULIANI S., MAGGI C.A. Inhibition of tachykinin release from peripheral endings of sensory nerves by nociceptin, a novel opioid peptide. Br. J. Pharmacol. 1996;118:1567–1569. doi: 10.1111/j.1476-5381.1996.tb15576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIULIANI S., MAGGI C.A. Prejunctional modulation by nociceptin of nerve-mediated inotropic responses in guinea-pig left atrium. Eur. J. Pharmacol. 1997;332:231–236. doi: 10.1016/s0014-2999(97)01076-5. [DOI] [PubMed] [Google Scholar]
- GIULIANI S., TRAMONTANA M., LECCI A., MAGGI C.A. Effect of nociceptin on heart rate and blood pressure in anaesthetized rats. Eur. J. Pharmacol. 1997;333:177–179. doi: 10.1016/s0014-2999(97)01128-x. [DOI] [PubMed] [Google Scholar]
- GUMUSEL B., HAO Q., HYMAN A., CHANG J.K., KAPUSTA D.R., LIPPTON H. Nociceptin: an endogenous agonist for central opioid like1 (ORL1) receptors possesses systemic vasorelaxant properties. Life Sci. 1997;60:PL141–PL145. doi: 10.1016/s0024-3205(96)00696-0. [DOI] [PubMed] [Google Scholar]
- HÄBLER H.-J., TIMMERMANN L., STEGMANN J.-U., JÄNIG W. Involvement of neurokinins in antidromic vasodilatation in hairy and hairless skin of the rat hindlimb. Neuroscience. 1999a;89:1259–1268. doi: 10.1016/s0306-4522(98)00322-4. [DOI] [PubMed] [Google Scholar]
- HÄBLER H.-J., TIMMERMANN L., STEGMANN J.-U., JÄNIG W.Effects of nociceptin on antidromic vasodilatation in rat skin Pflügers Arch. 1999b437supplR133(abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- HÄBLER H.-J., WASNER G., JÄNIG W. Interaction of sympathetic vasoconstriction and antidromic vasodilatation in the control of skin blood flow. Exp. Brain Res. 1997;113:402–410. doi: 10.1007/pl00005594. [DOI] [PubMed] [Google Scholar]
- HARA N., MINAMI T., OKUDA-ASHITAKA E., SUGIMOTO T., SAKAI M., ONAKA M., MORI H., IMANISHI T., SHINGU K., ITO S. Characterization of nociceptin hyperalgesia and allodynia in conscious mice. Br. J. Pharmacol. 1997;121:401–408. doi: 10.1038/sj.bjp.0701146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELYES Z., NEMETH J., PINTER E., SZOLCSANYI J. Inhibition by nociceptin of neurogenic inflammation and the release of SP and CGRP from sensory nerve terminals. Br. J. Pharmacol. 1997;121:613–615. doi: 10.1038/sj.bjp.0701209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- HOLZER P., LIVINGSTON E.H., GUTH P.H. Sensory neurons signal for an increase in rat gastric mucosal blood flow in the face of pending acid injury. Gastroenterology. 1991;101:416–423. doi: 10.1016/0016-5085(91)90020-l. [DOI] [PubMed] [Google Scholar]
- INOUE M., KOBAYASHI M., KOZAKI S., ZIMMER A., UEDA H. Nociceptin/orphanin FQ-induced nociceptive responses through substance P release from peripheral nerve endings in mice. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10949–10953. doi: 10.1073/pnas.95.18.10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOFLACH F., REINSCHEID R.K., CIVELLI O., KEMP J.A. Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. J. Neurosci. 1996;16:6657–6664. doi: 10.1523/JNEUROSCI.16-21-06657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAI C.C., WU S.Y., DUN S.L., DUN N.J. Nociceptin-like immunoreactivity in the rat dorsal horn and inhibition of substantia gelatinosa neurons. Neuroscience. 1997;81:887–891. doi: 10.1016/s0306-4522(97)00251-0. [DOI] [PubMed] [Google Scholar]
- LEMBECK F., DONNERER J. Opioid control of the function of primary afferent substance P fibres. Eur. J. Pharmacol. 1985;114:241–246. doi: 10.1016/0014-2999(85)90365-6. [DOI] [PubMed] [Google Scholar]
- LIEBEL J.T., SWANDULLA D., ZEILHOFER H.U. Modulation of excitatory synaptic transmission by nociceptin in superficial dorsal horn neurones of the neonatal rat spinal cord. Br. J. Pharmacol. 1997;121:425–432. doi: 10.1038/sj.bjp.0701149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG J.M. Pharmacology of cotransmission in the autonomic nervous system: Integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol. Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- MEUNIER J.-C., MOLLEREAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.-C., FERRARA P., MONSARRAT B., MAZARGUIL H., VASSART G., PARMENTIER M., COSTENTIN J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MINAMI T., OKUDA-ASHITAKA E., NISHIUCHI Y., KIMURA T., TACHIBANA S., MORI H., ITO S. Anti-nociceptive responses produced by human putative counterpart of nocistatin. Br. J. Pharmacol. 1998;124:1016–1018. doi: 10.1038/sj.bjp.0701995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOGIL J.S., GRISEL J.E., REINSCHEID R.K., CIVELLI O., BELKNAP J.K., GRANDY D.K. Orphanin FQ is a functional anti-opioid peptide. Neuroscience. 1996;75:333–337. doi: 10.1016/0306-4522(96)00338-7. [DOI] [PubMed] [Google Scholar]
- NEMETH J., HELYES Z., OROSZI G., THAN M., PINTER E., SZOLCSANYI J. Inhibition of nociceptin on sensory neuropeptide release and mast cell-mediated plasma extravasation in rats. Eur. J. Pharmacol. 1998;347:101–104. doi: 10.1016/s0014-2999(98)00216-7. [DOI] [PubMed] [Google Scholar]
- NICOL B., LAMBERT D.G., ROWBOTHAM D.J., OKUDA-ASHITAKA E., ITO S., SMART D., MCKNIGHT A.T. Nocistatin reverses nociceptin inhibition of glutamate release from rat brain slices. Eur. J. Pharmacol. 1998;356:R1–R3. doi: 10.1016/s0014-2999(98)00545-7. [DOI] [PubMed] [Google Scholar]
- OKUDA-ASHITAKA E., MINAMI T., TACHIBANA S., YOSHIHARA Y., NISHIUCHI Y., KIMURA T., ITO S. Nocistatin, a peptide that blocks nociceptin action in pain transmission. Nature. 1998;392:286–289. doi: 10.1038/32660. [DOI] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J., JR, CIVELLI O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- SHAH S., PAGE C.P., SPINA D. Nociceptin inhibits non-adrenergic non-cholinergic contraction of guinea-pig airway. Br. J. Pharmacol. 1998;125:510–516. doi: 10.1038/sj.bjp.0702068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAKHANBEH J., LYNN B. Morphine inhibits antidromic vasodilatation without affecting the excitability of C-polymodal nociceptors in the skin of the rat. Brain Res. 1993;607:314–318. doi: 10.1016/0006-8993(93)91522-t. [DOI] [PubMed] [Google Scholar]
- SMITH T.W., BUCHAN P. Peripheral opioid receptors located on the rat saphenous nerve. Neuropeptides. 1984;5:217–220. doi: 10.1016/0143-4179(84)90066-0. [DOI] [PubMed] [Google Scholar]
- STEFANO G.B., HARTMAN A., BILFINGER T.V., MAGAZINE H.I., LIU Y., CASARES F., GOLIGORSKY M.S. Presence of mu3 opiate receptor in endothelial cells. Coupling to nitric oxide production and vasodilation. J. Biol. Chem. 1995;270:30290–30293. doi: 10.1074/jbc.270.51.30290. [DOI] [PubMed] [Google Scholar]
- TOWLER P.K., BRAIN S.D. Activity of tachykinin NK1 and bradykinin B2 receptor antagonists, and an opioid ligand at different stimulation parameters in neurogenic inflammation in the rat. Neurosci. Lett. 1998;257:5–8. doi: 10.1016/s0304-3940(98)00770-8. [DOI] [PubMed] [Google Scholar]
- WILDERMAN M.J., ARMSTEAD W.M. Role of endothelial nitric oxide synthase in hypoxia-induced pial artery dilatation. J. Cereb. Blood Flow Metab. 1998;18:531–538. doi: 10.1097/00004647-199805000-00008. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., NOZAKI-TAGUCHI N., KIMURA S. Analgesic effect of intrathecally administered nociceptin, an opioid receptor-like1 receptor agonist, in the rat formalin test. Neuroscience. 1997a;81:249–254. doi: 10.1016/s0306-4522(97)00166-8. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO T., NOZAKI-TAGUCHI N., KIMURA S. Effects of intrathecally administered nociceptin, an opioid receptor-like1 (ORL1) receptor agonist, on the thermal hyperalgesia induced by carageenan injection into the rat paw. Brain Res. 1997b;754:329–332. doi: 10.1016/s0006-8993(97)00186-8. [DOI] [PubMed] [Google Scholar]


