Abstract
Based on our finding that melatonin decreased the lower limit of cerebral blood flow autoregulation in rat, we previously suggested that melatonin constricts cerebral arterioles. The goal of this study was to demonstrate this vasoconstrictor action and investigate the mechanisms involved.
The effects of cumulative doses of melatonin (10−10 to 10−6 M) were examined in cerebral arterioles (30–50 μM) of male Wistar rats using an open skull preparation. Cerebral arterioles were exposed to two doses of melatonin (3×10−9 and 3×10−8 M) in the absence and presence of the mt1 and/or MT2 receptor antagonist, luzindole (2×10−6 M) and the Ca2+-activated K+ (BKCa) channel blocker, tetraethylammonium (TEA+, 10−4 M). The effect of L-nitro arginine methyl ester (L-NAME, 10−8 M) was examined on arterioles after TEA+ superfusion. Cerebral arterioles were also exposed to the BKCa activator, NS1619 (10−5 M), and to sodium nitroprusside (SNP, 10−8 M) in the absence and presence of melatonin (3×10−8 M).
Melatonin induced a dose-dependent constriction with an EC50 of 3.0±0.1 nM and a maximal constriction of −15±1%. Luzindole abolished melatonin-induced vasoconstriction. TEA+ induced significant vasoconstriction (−10±2%). No additional vasoconstriction was observed when melatonin was added to the aCSF in presence of TEA+, whereas L-NAME still induced vasoconstriction (−10±1%). NS1619 induced vasodilatation (+11±1%) which was 50% less in presence of melatonin. Vasodilatation induced by SNP (+12±2%) was not diminished by melatonin.
Melatonin directly constricts small diameter cerebral arterioles in rats. This vasoconstrictor effect is mediated by inhibition of BKCa channels following activation of mt1 and/or MT2 receptors.
Keywords: Melatonin, cerebral arterioles, potassium channels, cerebral blood flow autoregulation
Introduction
We recently reported that melatonin increased the autoregulatory cerebrovascular dilatory capacity and improved the cerebrovascular security margin in rats (Régrigny et al., 1998), suggesting a potential protective effect of melatonin against hypotension-induced cerebral ischemia (Shuaib, 1992; Daffertshofer & Hennerici, 1995; Skoog, 1997). We suggested that melatonin increases the cerebrovascular dilatory capacity by constricting not only large cerebral influx arteries but also cerebral microvessels (Régrigny et al., 1998). Melatonin is known to constrict large-diameter cerebral arteries in vitro, following G protein-dependent inhibition of Ca2+ activated large-conductance K+ (BKCa) channels (Geary et al., 1997). In contrast, little is known of the effects of melatonin on small diameter cerebral arterioles which are involved in cerebral blood flow autoregulation.
The first goal of this study was to investigate the contractile effects of melatonin on small diameter (less than 50 μm) cerebral arterioles. The second goal was to determine whether the contractile effect of melatonin on small diameter cerebral arterioles was mediated by inhibition of BKCa channels following activation of melatonin (mt1 and/or MT2) receptors, as previously described for large-diameter cerebral arteries (Geary et al., 1997). Experiments were performed using an open skull preparation in anaesthetized rats.
Methods
Animals and open skull preparation
Experiments were performed on adult, male Wistar rats (Ico : WI, IOPS AF/Han; Iffa-Credo, l'Arbresle, France, body weight=538±22 g; 8–9 months). Animals were allowed free access to food and water, and housed at 24°C under controlled conditions of a 12 h light–dark cycle, lights on at 0600 and off at 1800). Experiments were performed in accordance with the guidelines of the European Union and the French Ministry of Agriculture.
Animals were anaesthetized with sodium pentobarbitone (60 mg kg−1, i.p.) at 0900 h. A polyethylene cannula (Merck Biotrol, Chennevieres, France) was introduced into the left femoral artery, and connected to a low volume, strain-gauge transducer (Baxter, Bentley Laboratories, Europe) for measurement of blood pressure and heart rate. A polyethylene cannula was introduced into the right femoral artery to withdraw blood for measurement of arterial blood gases (pH, PaO2, and PaCO2, mmHg; 170 pH/Blood Gas Analyzer, Corning Medical, Medfield, U.S.A). A silicone catheter (Sigma Medical, Nanterre, France) was introduced into the left femoral vein, and connected to a pump (Bioblock Scientific, Paris, France) for infusion of sodium pentobarbitone (at a rate of 0.25 ml h−1; 20 mg kg−1 h−1) throughout the experiment to maintain anaesthesia. Animals were intubated and mechanically ventilated with room air (60 strokes min−1; 10 ml kg−1). Paralysis of skeletal muscle was obtained with gallamine triethiodide (20 mg kg−1 i.v.) repeated every hour. Because the animals were paralyzed, the depth of anaesthesia was evaluated by applying pressure to the tail and observing changes in heart rate and blood pressure. Rectal temperature was maintained at 37–38°C with a heating pad. To comply with Home Office regulations, at the end of the experiment, animals were killed while under general anaesthesia.
Measurement of arteriolar diameter
We measured the diameter of first order arterioles of the cerebrum (Harper & Bohlen, 1984) through an open skull preparation (Chillon et al., 1996; 1997). The head was placed in an adjustable head holder, and a 1 cm incision was made in the skin to expose the skull. The skin edges were retracted with sutures, and ports placed for inflow and outflow of artificial cerebrospinal fluid (aCSF). Craniotomy was performed over the left parietal cortex, and the dura was incised to expose cerebral vessels. The exposed brain was continuously suffused (3 ml min−1) with aCSF, warmed to 37–38°C and equilibrated with a gas mixture of 5% CO2–95% N2. The composition of the aCSF was (mM) KCl, 3.0; MgCl2, 0.6; CaCl2, 1.5; NaCl, 131.9; NaHCO3, 24.6; urea, 6.7; and glucose, 3.7 (Chillon et al., 1996; 1997).
Arterioles were monitored through a microscope (Stemi 2000-C, Carl Zeiss Jena GmbH, Jena, Germany) connected to a closed-circuit video system with a final magnification of ×400. Images of arterioles were digitized using a video frame. Arteriolar diameter was measured from the digitized images with image analysis software (Saisam®, Microvision Instruments, Evry, France). The precision of this system is 0.5 μm (1 pixel=0.5 μm).
Experimental protocols
In a first experiment, we determined the effects of melatonin on cerebral arterioles. We examined latency and duration of the response induced by melatonin. For this we measured cerebral arteriolar diameter (n=5) every 5 min during perfusion with aCSF plus melatonin (10−6 M). After 25 min, melatonin perfusion was stopped and we measured return in diameter to baseline. A cumulative concentration–response curve for melatonin (n=8) was constructed by adding increasing concentrations of melatonin in the range of 10−10 to 10−6 M. For each rat, a sigmoid curve fit was then calculated with the least squares method:
where: Y=diameter variation (μm), X=dose of melatonin (M), min=minimum, max=maximum, EC50=the half-maximum effect, and slope=the slope of the curve.
The averages of min, max, EC50 and slopes were then used to determine the average response curve. For the remainder of the experiments, two doses of melatonin, 3×10−9 M (EC50, see results) and 3×10−8 M (inframaximal, see Results) were used.
In a second set of experiments, we explored the mechanisms by which melatonin induced contraction of cerebral arterioles. The effects of the melatonin receptor (mt1 and MT2) antagonist, luzindole (Dubocovich, 1995) (n=7), and the BKCa channel blocker, tetraethylammonium (Brayden & Nelson, 1992) (TEA+, n=6) on arteriolar vasoconstriction induced by melatonin were determined. Cerebral arterioles were exposed to two cumulative doses of melatonin (3×10−9 and 3×10−8 M) prior to and after 20 min of superfusion of the cranial window with aCSF plus luzindole (2×10−6 M) or TEA+ (10−4 M). In order to verify that cerebral arterioles were still able to constrict after TEA+, we repeated this experiment (n=4) with L-nitro arginine methyl ester (L-NAME, 10−8 M, a concentration which induced constriction of amplitude similar to that induced by melatonin, 3×10−8 M) instead of melatonin. The effect of melatonin on the vasodilatation of cerebral arterioles induced by the BKCa channel activator NS1619 (Holland et al., 1996) (n=6) was also assessed in the same rats. For this, cerebral arterioles were exposed to NS1619 (10−5 M) prior to and 15 min after addition of melatonin at 3×10−8 M in the aCSF. To verify that the effect of melatonin on NS1619-induced vasodilatation was specific, we repeated this experiment (n=4) with another vasodilatator, sodium nitroprusside (SNP, 10−8 M, a concentration which induced a similar degree of dilatation to that induced by NS1619). Finally, to verify that NS1619 acts via BKCa channel in this preparation, we studied the effect of TEA+ (10−4 M) on NS1619-induced vasodilatation (10−5 M, n=6). For this, cerebral arterioles were exposed to NS1619 prior to and 15 min after addition of TEA+ in the aCSF.
All experiments started 30 min after completion of surgery which lasted about 2 h. All drugs, individually or in combination, were added to the aCSF at their final concentration and superfused in the cranial window until a stable response was obtained. For each drug concentration, diameters of cerebral arterioles, arterial blood pressure, heart rate, and blood gases were measured prior to the infusion of the drug and after stabilization of the diameter under drug infusion (about 20 min). Responses of cerebral arterioles to the various drugs were defined as the differences in diameters measured at the same point on the cerebral arteriole prior to and during drug infusion. Responses of cerebral arterioles were expressed either as absolute change in diameter (μm) or as percentage change, using the diameter measured prior to drug infusion as baseline. The cranial window was continuously superfused with aCSF between drug exposure for 30 min to washout drugs and allow the diameter of the cerebral arterioles to return to baseline.
Substances used
Melatonin, luzindole, TEA+, NS1619, SNP, L-NAME and gallamine triethiodide were purchased from Sigma Chemical Company (St. Louis, MO, U.S.A). Melatonin, luzindole and NS1619 were dissolved in aCSF plus absolute ethanol (0.1% v v−1) and protected from light. Control studies showed that the solvent had no effect on cerebral arteriolar diameter (43±3 vs baseline 45±2 μm). Doses are expressed as base. Gases were purchased from Air Liquide (Nancy, France). Sodium pentobarbitone was purchased from Sanofi Santé Animale (Libourne, France). KCl, MgCl2, CaCl2, NaCl, NaHCO3, urea and glucose were purchased from Merck KGaA (Darmstadt, Germany).
Statistical analysis
Results are expressed as means±s.e.mean. The experimental protocol was designed to use one-way analysis of variance (ANOVA). Significant differences between means were determined using the Bonferroni test. The probability level chosen was P⩽0.05.
Results
Cumulative concentration-response curve for melatonin
Melatonin induced dose-dependent constriction of cerebral arterioles (Figures 1, 2 and 3). The constriction of cerebral arterioles was maximal after 10 min perfusion with aCSF plus melatonin (10−6 M). Thirty minutes after the end of the melatonin superfusion, cerebral arteriolar diameter returned to baseline values (Figure 3). The responses of cerebral arterioles to cumulative doses of melatonin were fit to a sigmoid curve (Y=(−5.6≈thinsp;+ 0.2) + ((5.7≈thinsp;+ 0.5) / (1+(108.5±0.2/10logX) )1.0±0.1); R2=0.98; P<0.01) with an EC50 of 3.0±0.1 nM, a maximum constriction of −5.6±0.2 μm (−15±1% vs baseline 38± 2 μm) and a slope of 1.0±0.1 μm.mmol−1.L.
Figure 1.
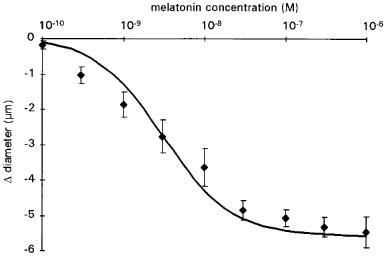
Cumulative concentration-response curve for melatonin. Results are expressed as change in diameter from baseline. Values are means±s.e.mean; n=8. The sigmoid curve was calculated as the average of the individual curves obtained by least squares analysis of each data set (see Methods).
Figure 2.
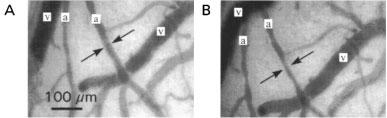
Cerebral arterioles (a) and cerebral veins (v) superfused with aCSF (A) or with aCSF plus melatonin (10−6 M; B). Arrows show the reduction in arteriolar diameter induced by melatonin.
Figure 3.
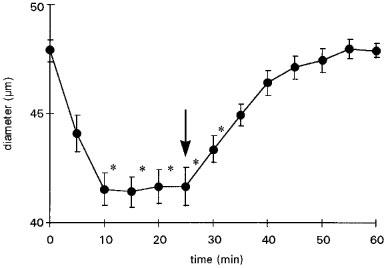
Latency and duration effect of melatonin (10−6 M) on cerebral arteriolar diameter. Arrow shows the end of the melatonin perfusion. Values are means±s.e.mean; n=5. *P⩽0.05 vs baseline.
Effects of luzindole on melatonin-induced constriction
Luzindole (2×10−6 M), had no significant effect on cerebral arteriolar diameter (46±2 μm vs baseline diameter: 46±2 μm) but abolished the constriction induced by melatonin (Figure 4).
Figure 4.
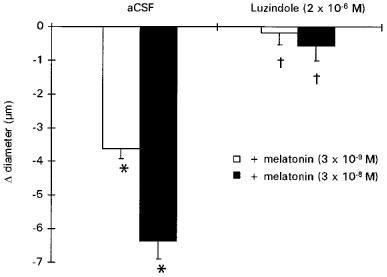
Effect of melatonin (3×10−9 and 3×10−8 M) prior to (aCSF) and after luzindole (2×10−6 M). Values are means±s.e.mean; n=7. *P⩽0.05 vs baseline. †P⩽0.05 vs responses induced by melatonin alone.
Effects of TEA+ on melatonin-induced constriction
The BKCa channel blocker, TEA+ (10−4 M), induced significant constriction of cerebral arterioles (−10±2% vs baseline 48±2 μm) of a similar amplitude to that induced by melatonin (3×10−8 M : −14±1% vs baseline 47±3 μm). No additional vasoconstriction was observed when melatonin (3 ×10−9 and 3 ×10−8 M) was added to the aCSF in presence of TEA+ (Figure 5).
Figure 5.
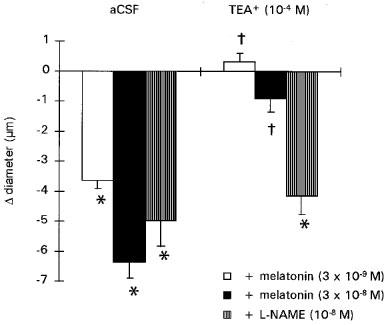
Effect of melatonin (3×10−9 and 3×10−8 M; n=6) or L-NAME (10−8 M; n=4) prior to (aCSF) and after TEA+ (10−4 M). Values are means±s.e.mean. *P⩽0.05 vs baseline. †P⩽0.05 vs responses induced by melatonin alone.
Effects of L-NAME on TEA+-induced constriction
The absence of vasoconstriction in cerebral arterioles preconstricted with TEA+ was specific for melatonin as L-NAME (10−8 M) in the presence of TEA+ induced an additional, significant vasoconstrictor response (Figure 5).
Effects of melatonin on NS1619- or SNP-induced dilatation of cerebral arterioles
The BKCa channel activator NS1619 (10−5 M) induced a significant increase in cerebral arteriolar diameter (+11±1%; vs baseline 45±3 μm; Figure 6). Melatonin (3×10−8 M) induced significant constriction of cerebral arterioles (−13±1% vs baseline 45±3 μm). NS1619-induced vasodilatation in the presence of melatonin was reduced by 50% (+5±1% vs baseline 39±3 μm; Figure 6). TEA+ significantly reduced vasodilatation induced by NS1619 (+16±2% vs +8±1%).
Figure 6.
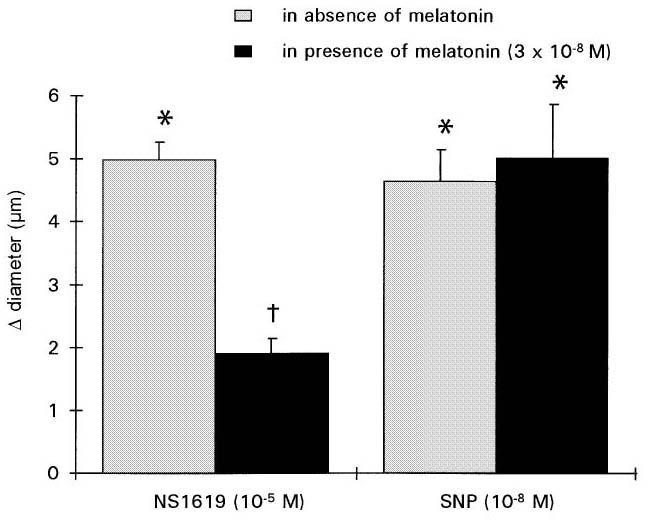
Effect of melatonin (3×10−8 M) on the vasodilatory response to NS1619 (10−5 M; n=6) or SNP (10−8 M; n=4). Values are means±s.e.mean. *P⩽0.05 vs baseline. †P⩽0.05 vs responses induced by NS1619.
SNP (10−8 M) induced significant vasodilatation (+12±2%; vs baseline 39±6 μm; Figure 6). Melatonin (3×10−8 M) induced significant constriction of cerebral arterioles (−12±1% vs baseline 37±6 μm). The vasodilator response to SNP in the presence of melatonin (+17±4% vs baseline 33±6 μm) was equal to that obtained prior to melatonin superfusion (Figure 6).
Luzindole, melatonin, TEA+, NS1619, L-NAME or SNP in the cranial window had no significant effect on systemic haemodynamics or blood gas parameters as heart rate (357±14 bpm), mean arterial blood pressure (120±2 mmHg), pH (7.39±0.01), PaO2 (96±3 mmHg) and PaCO2 (36±1 mmHg) did not change significantly throughout the experiment.
Discussion
The present study shows that melatonin constricts small diameter cerebral arterioles in situ in anaesthetized rats. This effect appears to be mediated by the binding of melatonin on mt1 and/or MT2 receptors as the melatoninergic receptor (mt1 and MT2) antagonist, luzindole, abolished the response of cerebral arterioles to melatonin. Furthermore, our results suggest that the vasoconstriction induced by melatonin is due to inhibition of BKCa channels as melatonin failed to elicit vasoconstriction when BKCa channels were already inhibited by TEA+ and reduced the vasodilatation induced by the BKCa activator, NS1619.
We previously suggested that melatonin constricts not only large cerebral influx arteries but also cerebral arterioles (Régrigny et al., 1998). A direct vasoconstrictor effect of melatonin on large-diameter cerebral arteries has already been established (Geary et al., 1997). Our hypothesis that melatonin would vasoconstrict small diameter cerebral arterioles was based on the following observations. First, the cerebrovascular response to carbon dioxide, which is a measure of the dilatory capacity of cerebral microvessels (Heistad et al., 1980; Wei et al., 1980; Jones et al., 1989), was increased in a concentration-dependent manner following melatonin infusion (Régrigny et al., 1998). Second, melatonin infusion shifted the lower limit of cerebral blood flow autoregulation towards a lower level of blood pressure (Régrigny et al., 1998), an effect that, according to the hypothesis of Paulson & Waldemar (1990), would only be observed if melatonin constricted not only large diameter cerebral arteries but also cerebral microvessels.
The present results confirm our hypothesis that melatonin constricts small diameter cerebral arterioles. In our study, melatonin constricted cerebral arterioles in situ with a potency (EC50=3.0±0.1 nM) similar to that described for large diameter cerebral arteries in vitro pressurized to physiological levels (EC50=2.7 nM) (Geary et al., 1997) or for pressurized tail arteries (0.7–3.2 nM) (Ting et al., 1997). Furthermore, the maximal decrease in diameter observed in our study (−15%) was also similar to that measured in large-diameter cerebral (10–17%) (Geary et al., 1997) or in tail arteries (19–20%) (Ting et al., 1997). The response to melatonin was similar to that produced by maximum dose of another vasoconstrictor, endothelin (22±5%) (Faraci, 1989).
The contractile effect of melatonin on cerebral arterioles was abolished by pretreatment with luzindole, a melatoninergic receptor antagonist (Krause & Dubocovich, 1991) at a concentration that has been reported to antagonize melatonin-induced vasoconstriction in isolated middle cerebral arteries (Geary et al., 1997). This result supports the hypothesis that, as in large-diameter cerebral arteries (Geary et al., 1997), melatonin acts on a vascular mt1 and/or MT2 receptor. However, as melatonin is known to be an anti-oxidant (Reiter, 1997) and as free radicals are known to dilate blood vessels via effects on potassium channels (Archer et al., 1994) we cannot rule out the possibility that such a mechanism is at least partly responsible for melatonin-induced constriction in our experiments.
In large diameter cerebral arteries, such as the middle cerebral artery, melatonin induces vasoconstriction by blockade of receptor-coupled BKCa (Geary et al., 1997). The present results suggest that the same mechanism operates in arterioles. Melatonin failed to elicit further vasoconstriction in cerebral arterioles preconstricted with the BKCa channel blocker TEA+, suggesting that the two drugs act via the same pathway. This was confirmed when we examined the effect of a vasoconstrictor with a different mechanism of action, L-NAME, on cerebral arterioles preconstricted with TEA+. L-NAME significantly constricted cerebral arterioles preconstricted with TEA+. An additional indication that melatonin-induced vasoconstriction in cerebral arterioles is mediated by the BKCa channels is given by the results of the experiment using NS1619. NS1619, a BKCa channel opener, induced vasodilatation in cerebral arterioles and this was antagonized by melatonin and TEA+. This effect of melatonin appears to be specific for the BKCa channel opener, NS1619, as melatonin failed to antagonize vasodilatation of cerebral arterioles induced by a vasodilatory agent with a different mechanism of action, SNP. NS1619 has been shown to inhibit L-type Ca2+ channels and voltage-activated K+ channels (Holland et al., 1996), however the fact that TEA+ inhibits NS1619 vasodilatation is consistent with an effect of NS1619 on BKCa channels in our preparation.
Implications of our findings
In conclusion, the present results show that melatonin vasoconstricts small diameter cerebral arterioles. This direct constrictor effect of melatonin in small diameter cerebral arterioles appears to be mediated by melatoninergic (mt1 and/or MT2) receptors. The results also suggest that, as observed in large-diameter cerebral arteries (Geary et al., 1997), vasoconstriction induced by activation of the receptor is mediated by inhibition of BKCa channels. This direct vasoconstrictor effect of melatonin observed in vivo on cerebral arterioles, together with the vasoconstrictor effect of melatonin observed in vitro on large-diameter cerebral arteries (Geary et al., 1997), may explain the increase in the cerebrovascular dilatory capacity and the decrease of the lower limit of cerebral blood flow autoregulation that we have previously described (Régrigny et al., 1998).
Acknowledgments
This study was funded by grants from the French Ministry of Education and Research (EA/JE2166, Paris, France), the Regional Development Committee (Metz, France), the Greater Nancy Urban Council (Nancy, France), the Henri Poincaré University (Nancy, France) and Institut de Recherches Internationales Servier (Courbevoie, France). Part of these results was presented at the Winter Meeting of the British Pharmacological Society, Brighton, U.K. (Chillon et al., 1999).
Abbreviations
- aCSF
artificial cerebrospinal fluid
- BKCa
Ca2+ activated large-conductance K+ channel
- L-NAME
L-nitro arginine methyl ester
- SNP
sodium nitroprusside
- TEA+
tetraethylammonium
References
- ARCHER S.L., HUANG J.M., HAMPL V., NELSON D.P., SHULTZ P.J., WEIR E.K. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7583–7587. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAYDEN J.E., NELSON M.T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- CHILLON J.M., GHONEIM S., BAUMBACH G.L. Effects of chronic nitric oxide synthase inhibition on cerebral arterioles in rats. Hypertension. 1997;30:1097–1104. doi: 10.1161/01.hyp.30.5.1097. [DOI] [PubMed] [Google Scholar]
- CHILLON J.M., HEISTAD D.D., BAUMBACH G.L. Effects of endothelin receptor inhibition on cerebral arterioles in hypertensive rats. Hypertension. 1996;27:794–798. doi: 10.1161/01.hyp.27.3.794. [DOI] [PubMed] [Google Scholar]
- CHILLON J.M., RÉGRIGNY O., LARTAUD-IDJOUADIENE I., DELAGRNAGE P., SCALBERT E., ATKINSON J. Melatonin constricts rat cerebral arterioles in vivo by blocking Ca2+-activated K+ (BKCa) channels. Br. J. Pharmacol. 1999;126:34P. doi: 10.1038/sj.bjp.0702714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAFFERTSHOFER M., HENNERICI M. Cerebrovascular regulation and vasoneuronal coupling. J. Clin. Ultrasound. 1995;23:125–138. doi: 10.1002/jcu.1870230207. [DOI] [PubMed] [Google Scholar]
- DUBOCOVICH M.L. Melatonin receptors: are there multiple subtypes. Trends Pharmacol. Sci. 1995;16:50–56. doi: 10.1016/s0165-6147(00)88978-6. [DOI] [PubMed] [Google Scholar]
- FARACI F.M. Effects of endothelin and vasopressin on cerebral blood vessels. Am. J. Physiol. 1989;257:H799–H803. doi: 10.1152/ajpheart.1989.257.3.H799. [DOI] [PubMed] [Google Scholar]
- GEARY G.G., KRAUSE D.N., DUCKLES S.P. Melatonin directly constricts rat cerebral arteries through modulation of potassium channels. Am. J. Physiol. 1997;273:H1530–H1536. doi: 10.1152/ajpheart.1997.273.3.H1530. [DOI] [PubMed] [Google Scholar]
- HARPER S.L., BOHLEN H.G. Microvascular adaptation in the cerebral cortex of adult spontaneously hypertensive rats. Hypertension. 1984;6:408–419. doi: 10.1161/01.hyp.6.3.408. [DOI] [PubMed] [Google Scholar]
- HEISTAD D.D., MARCUS M.L., PIEGORS D.J., ARMSTRONG M.L. Regulation of cerebral blood flow in atherosclerotic monkeys. Am. J. Physiol. 1980;239:H539–H544. doi: 10.1152/ajpheart.1980.239.4.H539. [DOI] [PubMed] [Google Scholar]
- HOLLAND M., LANGTON P.D., STANDEN N.B., BOYLE J.P. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br. J. Pharmacol. 1996;117:119–129. doi: 10.1111/j.1476-5381.1996.tb15163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES S.C., BOSE B., FURLAN A.J., FRIEL H.T., EASLEY K.A., MEREDITH M.P., LITTLE J.R. CO2 reactivity and heterogeneity of cerebral blood flow in ischemic, border zone, and normal cortex. Am. J. Physiol. 1989;257:H473–H482. doi: 10.1152/ajpheart.1989.257.2.H473. [DOI] [PubMed] [Google Scholar]
- KRAUSE D.N., DUBOCOVICH M.L. Melatonin receptors. Ann. Rev. Pharmacol. Toxicol. 1991;31:549–568. doi: 10.1146/annurev.pa.31.040191.003001. [DOI] [PubMed] [Google Scholar]
- PAULSON O.B., WALDEMAR G.Inhibition of the renin angiotensin system and the autoregulation of cerebral circulation Coronary and Cerebrovascular Effects of Antihypertensive Drugs 1990London: Transmedica; 129–144.ed. Atkinson, J., Capdeville, C. & Zannad, F. pp [Google Scholar]
- RÉGRIGNY O., DELAGRANGE P., SCALBERT E., ATKINSON J., LARTAUD-IDJOUADIENE I. Melatonin improves cerebral circulation security margin in rats. Am. J. Physiol. 1998;275:H139–H144. doi: 10.1152/ajpheart.1998.275.1.H139. [DOI] [PubMed] [Google Scholar]
- REITER R.J. Antioxidant actions of melatonin. Advances in Pharmacology. 1997;38:103–117. doi: 10.1016/s1054-3589(08)60981-3. [DOI] [PubMed] [Google Scholar]
- SHUAIB A. Alteration of blood pressure regulation and cerebrovascular disorders in the elderly. Cerebrovasc. Brain Met. Rev. 1992;4:329–345. [PubMed] [Google Scholar]
- SKOOG I. The relationship between blood pressure and dementia: a review. Biomed. Pharmacother. 1997;51:367–375. doi: 10.1016/s0753-3322(97)89428-0. [DOI] [PubMed] [Google Scholar]
- TING K.N., DUNN W.R., DAVIES D.J., SUGDEN D., DELAGRANGE P., GUARDIOLA-LEMAÎTRE B., SCALBERT E., WILSON V.G. Studies on the vasoconstrictor action of melatonin and putative melatonin receptor ligands in the tail artery of juvenile Wistar rats. Br. J. Pharmacol. 1997;122:1299–1306. doi: 10.1038/sj.bjp.0701511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEI E.P., KONTOS H.A., PATTERSON J.L.J. Dependence of pial arteriolar response to hypercapnia on vessel size. Am. J. Physiol. 1980;238:H697–H703. doi: 10.1152/ajpheart.1980.238.5.H697. [DOI] [PubMed] [Google Scholar]


