Abstract
Experimental hypertension is associated with several functional alterations of vascular endothelium and smooth muscle, but relatively few studies have examined the control of arterial tone in isolated vascular preparations from patients with essential hypertension. Therefore, we compared functional characteristics in vitro of distal ring segments of the mesenteric artery from 17 hypertensive and 22 normotensive humans.
Arterial constrictor responses induced by cumulative addition of Ca2+ in the presence of noradrenaline (NA) were more effectively inhibited by the Ca2+ entry blocker nifedipine (0.5 nM) in hypertensive than normotensive subjects (by 55.4±4.9, n=17 and 35.0±5.2%, n=22, respectively). Also the contractions elicited by high concentrations of KCl were more effectively inhibited by nifedipine in arterial rings from hypertensive than normotensive patients (by 38.9±3.7, n=17 and 20.2±4.6%, n=22, respectively). However, the concentration-response curves of contractions to NA, serotonin and KCl in the absence of nifedipine were similar between the study groups.
The concentration-response curves of endothelium-dependent relaxations to acetylcholine and Ca2+ ionophore A23187, as well as of endothelium-independent relaxations to the nitric oxide donor nitroprusside, β-adrenoceptor agonist isoprenaline and K+ channel opener cromakalim did not show any differences between the groups. Moreover, the nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester (0.1 mM) almost abolished the relaxations to acetylcholine and Ca2+ ionophore in both groups, indicating that these responses were largely mediated by nitric oxide. The function of arterial sodium pump was evaluated by relaxations elicited by the return of K+ upon contractions induced by K+-free solution. The rate of K+-relaxation was similar in hypertensive and normotensive arteries (for all these responses n=20–22 in the normotensive and 15–17 in the hypertensive group).
These results suggest abnormal function of voltage-dependent Ca2+ channels in arterial smooth muscle of hypertensive patients, whereas vascular responses to endothelium-dependent and -independent vasodilators and classical contractile agents were similar between hypertensive and normotensive subjects. The present findings support the view that blockade of voltage-dependent Ca2+ channels is an effective means of reducing arterial tone in essential hypertension.
Keywords: Arterial smooth muscle, endothelium, essential hypertension, nifedipine, nitric oxide
Introduction
Vascular endothelium plays an important role in the regulation of arterial tone via the production of powerful vasoactive mediators which include nitric oxide (NO), prostacyclin (PGI2) and the endothelium-derived hyperpolarizing and contractile factors (EDHF and EDCF, respectively) (Cohen & Vanhoutte, 1995; Moncada et al., 1991; Lüscher & Vanhoutte, 1986). Both essential and experimental hypertension have been shown to be associated with alterations of endothelial function, which may contribute to the increased arterial resistance characteristic of hypertensive states (Moncada et al., 1991; Lüscher, 1992; Panza et al., 1990; Linder et al., 1990; Zeiher et al., 1993; Watts et al., 1996). However, the responses of arterial smooth muscle to vasoconstrictor agents as well as to endothelium-independent relaxants have usually been found to remain unchanged in hypertensive humans (Falloon & Heagerty, 1994; Cockcroft et al., 1994).
Endothelial function in humans has mainly been evaluated by the use of non-invasive and invasive in vivo techniques, the most widely used methods including the measurement of flow-induced changes in brachial artery diameter by ultrasound (Celermajer et al., 1994; Taddei et al., 1995), infusion of vasoactive agonists to the brachial artery and measurement of changes in flow by plethysmography (Panza et al., 1990; Linder et al., 1990; Taddei et al., 1995), and infusion of agonists into the coronary arteries during coronary angiography (Zeiher et al., 1993). When compared with the in vitro studies, a shortcoming of the experiments in vivo is the limited number of possible pharmacological compounds when investigating the control of arterial tone. Moreover, if functional alterations are observed in vivo, it is often difficult to define whether the changes are located in the endothelium or arterial smooth muscle.
In spite of the intensive research in the field, only relatively few studies have examined in detail the function of the endothelium and arterial smooth muscle in essential hypertension. Therefore, the present study was designed to compare for the first time functional characteristics of a distal branch of the mesenteric artery (outer diameter 0.7–0.9 mm) from hypertensive and normotensive humans. Since only limited data is available concerning the roles of different endothelium-derived mediators in the vascular responses of humans, special attention was paid to the evaluation of the roles of NO, PGI2, EDHF and EDCF in the endothelium-mediated responses. The present findings support the view of an abnormal function of voltage-dependent Ca2+ channels in arterial smooth muscle of hypertensive patients, whereas no changes were observed in the vascular responses induced by endothelium-dependent and -independent vasodilators and classical vasoconstrictor agents between the hypertensive and normotensive subjects.
Methods
Study population
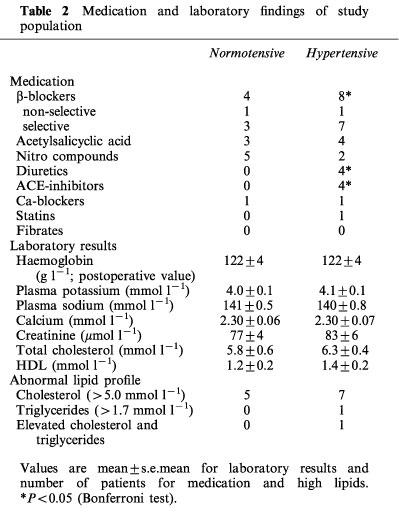
Thirty-nine subjects aged 34–80 years (59.6±2.4 years) were included in the present study. Seventeen subjects had a well established history of chronically elevated blood pressure (>160/95 mmHg before the introduction of antihypertensive medication; duration 15.7±2.2 years). The 22 normotensive control subjects did not differ in age, sex, body mass index (BMI) or laboratory findings when compared with the hypertensive group. The notable differences in the medication between the normotensive and hypertensive groups were as follows: β-adrenoceptor blockers (4 vs 8 users, respectively), angiotensin converting enzyme (ACE) inhibitors (0 vs 4 users), and diuretics (0 vs 4 users). The patient data is given in Tables 1 and 2. All patients were undergoing elective abdominal surgery, during which sections of the distal branch of the superior mesenteric artery (outer diameter 0.7–0.9 mm), just adjacent to the adventitial side of the small intestine, were removed. During the abdominal operations these sections of the mesenteric artery could be obtained because the vessel sample site was removed during intestinal resection or reconstruction. Twenty-one of the patients were operated because of malignant tumours which at the time of the operation did not show significant metastatic spread to the other organs (pancreatic, cholangionic, gastric, colonic, and urinary bladder carcinomas). The rest of the patients were operated because of benign tumours and other non-malignant conditions within the abdominal cavity. The experimental design of the human studies was approved by the Ethical Committee of the Tampere University Hospital and it conformed with the guidelines of the declaration of Helsinki. All patients gave their informed consent to participate in the study.
Table 1.
Characteristics of study population
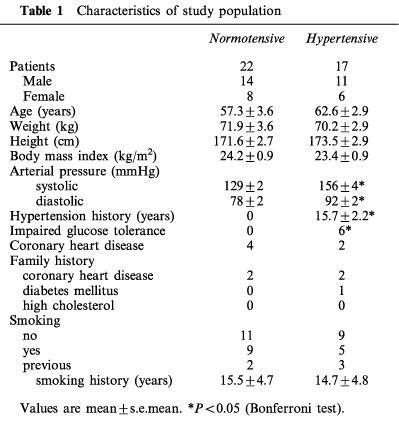
Table 2.
Medication and laboratory findings of study population
Mesenteric arterial responses in vitro
Seven segments (2 mm in length) of a distal section of the mesenteric artery of each subject were cut. In five rings the endothelium was left intact, and from two preparations the vascular endothelium was removed by gently rubbing with a jagged injection needle (Arvola et al., 1992). The rings were placed between stainless steel hooks (diameter 0.20 mm) and suspended in an organ bath chamber (volume 20 ml) in physiological salt solution (PSS; pH 7.4) of the following composition (mM): NaCl 119.0, NaHCO3 25.0, glucose 11.1, CaCl2 1.6, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, and oxygenated with 95% O2 and 5% CO2. The rings were initially equilibrated for 2–3 h at +37°C with a resting force of 1.5 g. In pilot experiments with mesenteric arterial rings from ten patients, these preparations were found to maximally respond to contractile agonists if the preload was adjusted to 1.5 g (resting preloads between 0.5 g and 4 g were tested). The force of contraction was measured with an isometric force-displacement transducer and registered on a polygraph (FT 03 transducer and Model 7 E Polygraph; Grass Instrument Co., Quincy, MA., U.S.A.). The presence of functionally intact endothelium in vascular preparations was confirmed by observing a clear relaxation to 1 μM acetylcholine (ACh) in rings precontracted with 1 μM noradrenaline (NA), and the absence of endothelium by the lack of this response.
Arterial smooth muscle sensitivity to Ca2+ entry blockade by nifedipine
Ca2+ was omitted from the buffer solution, and endothelium-denuded rings were repeatedly contracted with 10 μM NA to empty the cellular Ca2+ stores (Kähönen et al., 1994). The rings were challenged again with 10 μM NA in Ca2+-free buffer, and Ca2+ was returned to the organ bath in increasing concentrations (0.05–2.5 mM). The subsequent contraction was registered. The procedure was repeated 30 min later in the presence of the dihydropyridine Ca2+ entry blocker nifedipine (0.5 nM).
Arterial contractions to NA and serotonin (5-HT)
Concentration-response curves for NA, and 30 min later for 5-HT, were cumulatively determined in endothelium-intact rings. Then the responses to NA and 5-HT were repeated in the presence of diclofenac (3 μM), and in the presence of diclofenac and NG-nitro-L-arginine methyl ester (L-NAME; 0.1 mM). A 30 min stabilization period was allowed between each trial.
Endothelium-independent relaxation and contraction to KCl
The relaxation responses of endothelium-denuded preparations to the NO-donor nitroprusside, non-selective β-adrenoceptor agonist isoprenaline, and ATP-sensitive K+ channel (KATP) opener cromakalim were determined with a 30 min stabilization period between each trial. The relaxations were elicited after full precontraction with NA (1 μM), which resulted in approximately 50% of the maximal contractile response attained in the two groups. The next concentration of relaxing compound was added only after the previous level of relaxation was stable. After 30 min, the concentration-response curve for KCl was cumulatively determined. In solutions containing high concentrations of K+ (20–125 mM), NaCl was replaced with KCl on an equimolar basis.
Potassium-free medium-induced contraction and potassium relaxation
After 30 min the endothelium-denuded preparation was contracted with 125 mM KCl (reference response). Thereafter the rings were exposed to K+-free buffer solution (pH 7.4), which was prepared by substituting KH2PO4 and KCl of normal PSS with NaH2PO4 and NaCl, respectively, on an equimolar basis. The omission of K+ induced gradual contractions in all vascular rings. Once the contraction had reached a plateau, 1 mM K+ was re-added and the subsequent relaxation reflecting the activity of smooth muscle Na+, K+-ATPase was registered (Arvola et al., 1992). Then 30 min later the contractions induced by K+-free buffer solution and relaxations to K+ repletion were repeated in the presence of 1 mM ouabain.
Relaxations to acetylcholine (ACh) and Ca2+ ionophore A23187 after precontraction with NA
Responses to these endothelium-mediated vasodilators were examined in endothelium-intact arterial rings precontracted with NA (1 μM). The responses to ACh and Ca2+ ionophore A23187 were also elicited in the presence of diclofenac (3 μM), in the presence of diclofenac and L-NAME (0.1 mM), and in the presence of diclofenac, L-NAME and apamin (1 μM; inhibitors of cyclo-oxygenase, NO synthase, and small conductance Ca2+-activated K+ channels (KCa), respectively) with a 30 min stabilization period between each trial.
Analysis of results
The contractile responses were expressed in grams and related to tissue dry weight, and the EC50 values for NA and 5-HT in each ring were calculated as percentage of maximal response. The relaxations in response to ACh, Ca2+ ionophore A23187, cromakalim, isoprenaline and nitroprusside were presented as percentage of pre-existing contractile force. The IC50 values for the relaxants were calculated as percentages of 1 μM NA-induced precontraction, and they were analysed with a computer programme and presented as the negative logarithm (pD2), which values were also used in the statistics.
Statistical analysis was carried out by one-way analysis of variance (ANOVA) supported by the Bonferroni test when carrying out pairwise comparisons between the test groups. When appropriate, ANOVA for repeated measurements was applied for data consisting of repeated observations at successive time points. All results are expressed as means with s.e. mean, and differences were considered significant when P<0.05. Unless otherwise indicated the P values in the text refer to ANOVA for repeated measurements.
Drugs
The following drugs were used: acetylcholine chloride, apamin, Ca2+ ionophore A23187, cromakalim, diclofenac, isoprenaline, NG-nitro-L-arginine methyl ester hydrochloride, ouabain, serotonin (Sigma Chemical Co., St. Louis, MO., U.S.A.), L-noradrenaline L-hydrogentartrate, sodium nitroprusside (Fluka Chemie AG, Buchs SG, Switzerland), and nifedipine (Orion Pharmaceutical Co., Espoo, Finland).
Results
Patient characteristics
The characteristics of the study population are shown in Tables 1 and 2. In spite of the use of antihypertensive medication, both systolic and diastolic blood pressures were significantly higher in the hypertensive than the normotensive patients. The study groups did not differ in age, body-mass index, smoking or alcohol consumption, standard laboratory findings, and plasma lipid or glucose levels. Regular medication was also rather similar between the normotensive and hypertensive patients, since the groups only differed in the use of β-adrenoceptor blockers, ACE inhibitors, and diuretics.
Mesenteric arterial responses in vitro
Constrictor responses of endothelium-denuded arterial rings in response to cumulative Ca2+ addition (with NA as agonist), as well as the contractions to KCl, were similar between the study groups. A physiologically relevant low concentration of the Ca2+ entry blocker nifedipine (0.5 nM; Renwick et al., 1988) inhibited the contractions to cumulative Ca2+ addition, and also the responses to KCl, in both groups, but the effect of nifedipine was clearly more pronounced in the hypertensive than the normotensive subjects (P<0.001, Figure 1 and Table 3).
Figure 1.
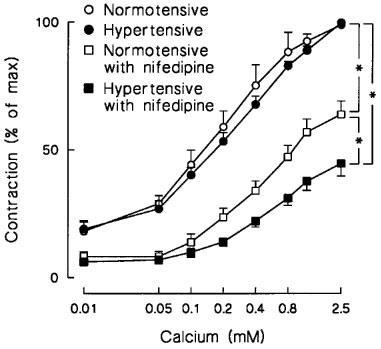
Line graphs show the effect of organ bath Ca2+ concentration on 1 μM noradrenaline-induced contraction in isolated endothelium-denuded mesenteric arterial rings from normotensive and hypertensive subjects. Contractile responses to the addition of Ca2+ were induced in the absence and presence of 0.5 nM nifedipine. Symbols indicate mean±s.e.mean, n=22 in the normotensive and 17 in the hypertensive group; *P<0.001, ANOVA for repeated measurements.
Table 3.
Parameters of contractile responses of isolated mesenteric arterial rings
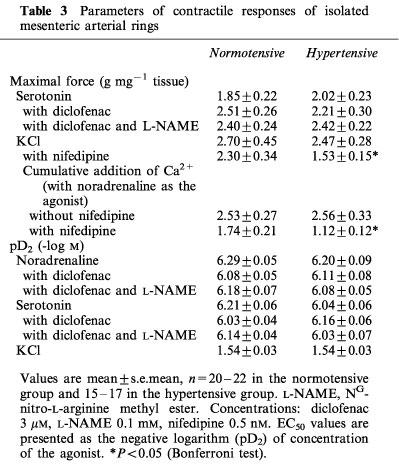
The endothelium-intact vascular rings of hypertensive and normotensive groups showed very similar arterial constrictor sensitivity (i.e. pD2 values), and maximal contractile force generation to NA and 5-HT. The addition of diclofenac (3 μM) and L-NAME (0.1 mM, in the presence of diclofenac) did not significantly affect these responses in the study groups (Table 3 and Figure 2).
Figure 2.
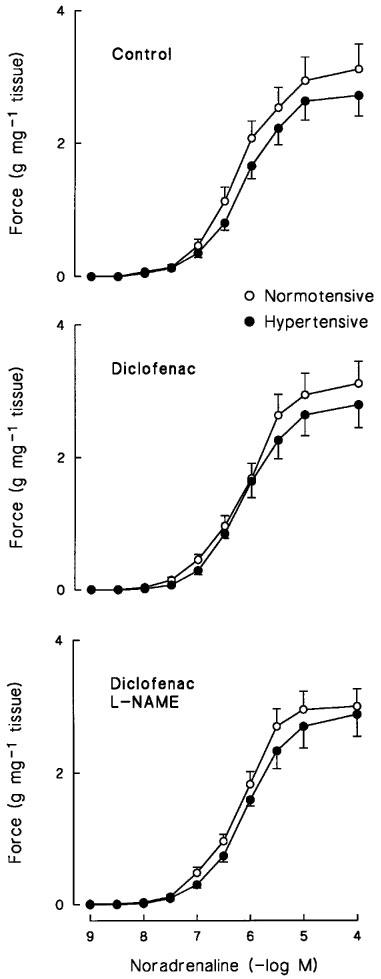
Concentration-response curves of endothelium-intact mesenteric arterial rings to noradrenaline in normotensive and hypertensive subjects. The responses were induced in the absence (top panel) and presence (middle panel) of 3 μM diclofenac, and with diclofenac and 0.1 mM NG-nitro-L-arginine methyl ester (L-NAME) (bottom panel). Symbols indicate mean±s.e.mean, n=20–22 in the normotensive and 15–17 in the hypertensive group.
Vasorelaxations of NA-precontracted arterial rings induced by nitroprusside, isoprenaline and cromakalim, three endothelium-independent vasodilators acting via different mechanisms, were also similar between the hypertensive and normotensive subjects (Table 4 and Figure 3). Moreover, the contractions of endothelium-denuded rings elicited by K+-free solution did not differ between the study groups, and after the return of K+ to the organ bath upon the K+-free precontractions the rate of the subsequent relaxation was comparable between the hypertensive and normotensive groups. The relaxation induced by K+ repletion was also similarly inhibited by the Na+,K+-ATPase inhibitor ouabain (1 mM; Figure 3).
Table 4.
Parameters of relaxation responses of isolated mesenteric arterial rings
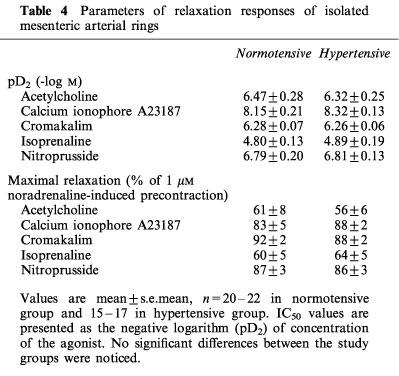
Figure 3.
Relaxations to nitroprusside, isoprenaline and cromakalim in 1 μM noradrenaline-precontracted endothelium-denuded mesenteric arterial rings from normotensive and hypertensive subjects. The relaxations to the readdition of 1 mM K+ were elicited after precontraction with K+-free buffer solution in the absence and presence of 1 mM ouabain. Symbols indicate mean±s.e.mean, n=20–22 in the normotensive and 15–17 in the hypertensive group.
Endothelium-dependent relaxations were evaluated by the use of the Ca2+ ionophore A23187, and the receptor-mediated agonist ACh in endothelium-intact NA-precontracted mesenteric arterial rings. The concentration-response curves of these dilatory responses were similar in both groups (Table 4, Figures 4 and 5). Cyclo-oxygenase inhibition with diclofenac (3μM) had no significant effect on the relaxations to the Ca2+ ionophore A23187 and ACh. In contrast, the NO synthase inhibitor L-NAME (0.1 mM, in the presence of diclofenac) effectively diminished the relaxations induced by the Ca2+ ionophore A23187 and ACh (P<0.001 in both groups), the inhibitory effect of which was comparable in the hypertensive and normotensive vessels. The addition of apamin (1 μM), an inhibitor of small conductance KCa (Nelson, 1993), did not significantly affect the small remaining diclofenac and L-NAME-resistant responses to ACh (P>0.1 in both groups) (Figure 5).
Figure 4.

Relaxations to calcium ionophore A23187 in isolated endothelium-intact mesenteric arterial rings from normotensive and hypertensive subjects. The relaxations were induced after precontraction with 1 μM noradrenaline in the absence and presence of 3 μM diclofenac; and in the presence of diclofenac and 0.1 mM NG-nitro-L-arginine methyl ester (L-NAME). Symbols indicate mean±s.e.mean, n=20–22 in the normotensive and 15–17 in the hypertensive group.
Figure 5.
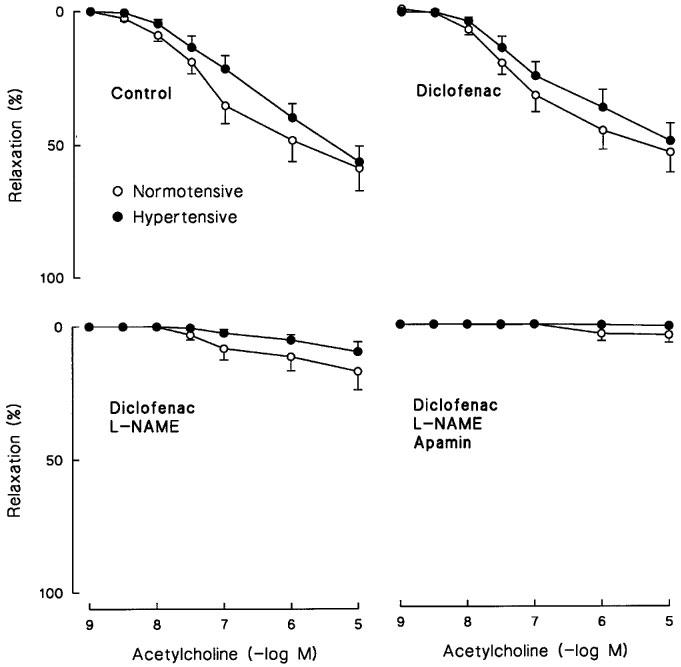
Relaxations to ACh in isolated endothelium-intact mesenteric arterial rings from normotensive and hypertensive subjects. The relaxations were induced after precontraction with 1 μM noradrenaline in the absence and presence of 3 μM diclofenac; in the presence of diclofenac and 0.1 mM NG-nitro-L-arginine methyl ester (L-NAME); and in the presence of diclofenac, L-NAME and 1 μM apamin. Symbols indicate mean±s.e.mean, n=20–22 in the normotensive and 15–17 in the hypertensive group.
Discussion
In the present study we compared the functional properties of a distal segment of the mesenteric artery from normotensive and hypertensive humans and found that: (1) The contractions induced by cumulative addition of Ca2+ with NA as the agonist, and the constrictor responses induced by KCl, were more effectively inhibited by the Ca2+ entry blocker nifedipine in the hypertensive than in the normotensive arteries. (2) Endothelium-dependent relaxations to the Ca2+ ionophore A23187 and ACh, as well as the endothelium-independent relaxations to the NO-donor nitroprusside, the β-adrenoceptor agonist isoprenaline and the KATP channel opener cromakalim did not differ between the hypertensive and normotensive vessels. (3) Vasoconstrictor sensitivity and maximal responses to NA, 5-HT and KCl were comparable in arteries from hypertensive and normotensive humans.
The characteristics of the two patient groups in this investigation were well matched. There were no differences between the hypertensives and normotensives in age, gender, BMI or laboratory results. Furthermore, only four hypertensive patients were on ACE-inhibitors and one was on Ca2+ entry blockade for the treatment of high blood pressure. Since only ACE inhibitors and Ca2+ entry blockers, but no other classes of antihypertensive drugs, have been shown to improve arterial function in hypertensive patients (Frielingsdorf et al., 1996; Thybo et al., 1995; Schiffrin & Deng, 1995), it seems feasible that the medication of these patients did not compromise the results. Furthermore, six patients in the hypertensive group had impaired glucose tolerance, which was controlled by the diet, and no medication for this purpose was in use. Since no differences were observed in the vasodilatory responses between the hypertensive and normotensive patients, the difference in the prevalence of impaired glucose tolerance probably did not affect the present findings. It is noteworthy that the vascular responses of the mesenteric artery within these groups were surprisingly homogeneous and consistent. Therefore, reliable comparisons between the hypertensive and normotensive arteries could be performed in the present study population.
When NA is used as the contractile agonist in Ca2+-free medium, the response primarily reflects Ca2+ release from the sarcoplasmic reticulum, a minor source of the activator Ca2+ being the inner surface of the plasmalemma (Johns et al., 1987). During cumulative Ca2+ re-addition to the organ bath, the further contraction thus results from extracellular Ca2+ entry through the plasmalemma (Karaki & Weiss, 1988). In the present study, the inhibitory effect of the dihydropyridine Ca2+ entry blocker nifedipine on the contractile response to cumulative Ca2+ addition, as well as on the KCl-induced contractions, was more pronounced in the hypertensive than normotensive blood vessels, suggesting higher dependency of Ca2+ influx through dihydropyridine-sensitive channels in the hypertensive group. The blood vessels of hypertensive experimental animals are also more sensitive to effects of dihydropyridine Ca2+ blockers than those of normotensive controls (Arvola et al., 1992; Pörsti, 1992), and the relative proportion of L-type Ca2+ currents has been shown to be higher in the vascular smooth muscle of hypertensive rats, while in the normotensive controls the T-type Ca2+ current predominates (Rusch & Hermsmeyer, 1988). Therefore, the present results suggest that enhanced Ca2+ entry via voltage-dependent L-type channels is also a feature of vascular smooth muscle in essential hypertension. Since the maximal contractile responses in the absence of nifedipine did not differ between the study groups, it can also be argued that some other contractile mechanism, possibly Ca2+ influx through T-type channels, was depressed in the arteries of hypertensive patients.
Ca2+ entry blockers are an established treatment for elevated blood pressure (Kaplan, 1989), but the safety of this class of drugs has been a matter of a debate (Furberg et al., 1995; Pahor et al., 1996). Successful pharmacotherapy of hypertension would require that the compound not only normalizes blood pressure but also counteracts the functional and structural cardiovascular alterations related to hypertension. The present findings provide pharmacological evidence for enhanced voltage-dependent Ca2+ entry in hypertensive arteries, and suggest that Ca2+ entry blockers provide a rational approach to the treatment of hypertension. Nevertheless, it is probably important to avoid fluctuations in the level of the Ca2+ channel blockade in vivo, and the rationale for the use of long-acting pharmacological compounds can be understood. It is noteworthy that recently Thybo et al. (1995) have suggested that Ca2+ handling is abnormal in hypertensive blood vessels, since isolated resistance arteries from patients with essential hypertension showed increased responses to caffeine when compared with normotensive controls.
Vasoconstrictor sensitivity and maximal contractile force generation induced by NA and 5-HT in the absence and presence of diclofenac and L-NAME (cyclo-oxygenase and NO synthase inhibitors, respectively), as well as the contractile responses of endothelium-denuded rings induced by KCl, were similar between the study groups. Thus, the present findings suggest that chronically elevated blood pressure does not affect arterial vasoconstrictor responses in humans. Parallel results have also been obtained in previous studies in vitro, since the sensitivity to various constrictor agonists, including NA, 5-HT and vasopressin, has remained unaltered in isolated arteries of hypertensive patients when compared with normotensive controls (Falloon & Heagerty 1994; Aalkjaer et al., 1987; James et al., 1997).
The endothelium-independent relaxations induced by the NO donor nitroprusside, the β-adrenoceptor agonist isoprenaline and the K+ channel opener cromakalim were similar in the hypertensive and normotensive groups. Previously the responses in vivo to isoprenaline and nitroprusside have also been found to be normal in the brachial artery of hypertensive patients (Cockcroft et al., 1994; Panza et al., 1993). Furthermore, in this study vascular relaxation via Na+,K+-ATPase was evaluated by K+ repletion upon K+-free medium-induced precontractions (Arvola et al., 1992). The return of K+ activates Na+,K+-ATPase, which repolarizes the cell membrane and relaxes smooth muscle (Bonaccorsi et al., 1977). However, K+ relaxation also reflects the general mechanisms of smooth muscle relaxation (Johns et al., 1987), and is only an estimate of vasodilation via Na+,K+-ATPase (Arvola et al., 1992). In the present study, the rate of K+ relaxation was corresponding in the hypertensive and normotensive groups, and it was also effectively inhibited by ouabain, which indicates that Na+,K+-ATPase was involved in the K+ relaxations. Previously, increased activity (Stekiel et al., 1986), unaltered function (Orlov et al., 1992) as well as decreased activity of Na+,K+-ATPase (Arvola et al., 1992) have been reported in experimental hypertension. These contradictory results possibly reflect the fact that the pump's functional, enzymatic and biochemical properties are dissimilarly changed in hypertension (Young et al., 1988), and that the type and duration of hypertension affects these results. Nevertheless, the present findings suggest that Na+,K+-ATPase activity is not changed in mesenteric artery of hypertensive humans. Taken together, all of the above results on vascular relaxation suggest that the endothelium-independent vasodilator capacity of arterial smooth muscle was quite matching in the hypertensive and normotensive arteries.
ACh and other endothelium-dependent vasodilators relax arterial smooth muscle by the release of several factors from the endothelium, the most prominent autacoids being NO, PGI2 and EDHF (Cohen & Vanhoutte, 1995; Moncada et al., 1991). Many studies which have examined either muscarinic-agonist or flow-induced vasodilator responses in the human forearm in vivo have suggested that endothelium-dependent relaxation is impaired in patients with essential hypertension (Panza et al., 1990; Linder et al., 1990; Calver et al., 1992). In addition, the relaxations of epicardial coronary arteries to ACh have been reported to be attenuated in hypertensive patients (Treasure et al., 1992). However, Cockcroft et al. (1994) have found that the endothelium-dependent vasodilator responses were not attenuated in hypertensive patients, and thus endothelial function is not impaired under all conditions in hypertension. Furthermore, essential hypertension has been found to be associated with well preserved endothelial dilator responses in the subcutaneous resistance vessels when compared with normotensive controls (Thybo et al., 1996; James et al., 1997). In the present study, the endothelium-mediated relaxations induced by the Ca2+ ionophore A23187 and ACh did not show any differences between the hypertensive and normotensive groups. The explanation for the discrepancies between the different studies is not known, but could be attributed to the heterogeneity of endothelial dysfunction among hypertensive patients (Lüscher, 1992; Cockcroft et al., 1994). It is noteworthy that in this investigation the Ca2+ ionophore A23187 was a markedly more potent endothelium-mediated vasodilator than ACh. Therefore, if the responses induced by the muscarinic agonist ACh are used as the only indicator of endothelium-mediated vasodilatation in humans, the conclusions should be done with caution, and the use of more than one endothelium-mediated agonist will lead to a more objective interpretation of the findings.
The cyclo-oxygenase inhibition with diclofenac had no significant effect on relaxations induced by the Ca2+ ionophore A23187 and ACh, suggesting that products of the cyclo-oxygenase pathway (i.e. PGI2 and endothelium-derived contractile factors) did not play a role in the endothelium-mediated relaxation in the mesenteric arteries of hypertensive and normotensive humans. In contrast, the addition of the NO synthase inhibitor L-NAME almost completely blocked these responses in both groups, suggesting that NO was the predominant mediator of the endothelium-dependent dilatation, and the role of other relaxing mediators appeared to be marginal. Moreover, we observed minor L-NAME resistant relaxations to Ca2+ ionophore A23187 and ACh in both groups. The endothelium-mediated relaxations which remain resistant to NO synthase and cyclo-oxygenase inhibitions have been found to be mediated by EDHF (Cohen & Vanhoutte, 1995). Functionally this factor is a K+ channel opener, the action of which can be inhibited by the blockade of K+ channels (Cohen & Vanhoutte, 1995). It has been suggested that EDHF relaxes mesenteric arterial smooth muscle mainly via the activation of KCa in rats (Garland & McPherson, 1992; Waldron & Garland 1994). In the present study, the minute L-NAME and diclofenac-resistant relaxations to ACh were not significantly affected by the KCa channel blocker apamin. Thus, these results do not support a role for EDHF in the relaxation of small human mesenteric arteries.
In conclusion, the more pronounced effect of nifedipine on arterial vasoconstrictor responses in the hypertensive than the normotensive subjects indicates abnormal function of voltage-dependent Ca2+ channels in vascular smooth muscle of hypertensive humans. In this investigation the endothelium-dependent and -independent relaxations, as well as the responses to classical vasoconstrictor agents, were almost identical in the mesenteric arteries of normotensive and hypertensive humans. Finally, the distal branch of the mesenteric artery just adjacent to the adventitial side of the small intestine, which can also be obtained as a biopsy sample during abdominal surgery, appears to be a useful preparation for the study of vascular physiology and pathophysiology in humans.
Acknowledgments
This study was supported by the Finnish Cultural Foundation, Pirkanmaa Fund, the Ida Montin Foundation, the Medical Research Fund of Tampere University Hospital, the Paavo Ilmari Ahvenainen Foundation, and Aarne Koskelo Foundation, Finland.
Abbreviations
- ACE
angiotensin converting enzyme
- ACh
acetylcholine
- ANOVA
one-way analysis of variance
- BMI
body mass index
- EDCF
endothelium-derived contractile factor
- EDHF
endothelium-derived hyperpolarizing factor
- 5-HT
serotonin
- KATP
ATP-sensitive K+ channel
- KCa
Ca2+-activated K+ channel
- NA
noradrenaline
- L-NAME
NG-nitro-L-arginine methyl ester
- NO
nitric oxide
- pD2
concentration of agonist producing 50% of maximal response
- PGI2
prostacyclin
- PSS
physiological salt solution
References
- AALKJAER C., HEAGERTY A.M., PETERSEN K.K., SWALES J.D., MULVANY M.J. Evidence for increased media thickness, increased neuronal amine uptake and depressed excitation-contraction coupling in isolated resistance vessels from essential hypertensives. Circ. Res. 1987;2:181–186. doi: 10.1161/01.res.61.2.181. [DOI] [PubMed] [Google Scholar]
- ARVOLA P., PÖRSTI I., VUORINEN P., PEKKI A., VAPAATALO H. Contractions induced by potassium-free solution and potassium relaxation in vascular smooth muscle of hypertensive and normotensive rats. Br. J. Pharmacol. 1992;106:157–165. doi: 10.1111/j.1476-5381.1992.tb14309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONACCORSI A., HERMESMEYER K., APRIGLIANO O., SMITH C.B., BOHR D.F. Mechanism of potassium relaxation of arterial muscle. Blood Vessels. 1977;14:261–276. doi: 10.1159/000158133. [DOI] [PubMed] [Google Scholar]
- CALVER A., COLLIER J., MONCADA S., VALLANCE P. Effect of local intra-arterial NG-monomethyl-L-arginine in patients with hypertension: the nitric oxide dilator mechanism appears abnormal. J. Hypertens. 1992;10:1025–1031. [PubMed] [Google Scholar]
- CELERMAJER D.S., SORENSEN K.E., SPIEGELHALTER D.J., GEORGAKOPOULOS D., ROBINSON J., DEANFIELD J.E. Aging is associated with endothelial dysfunction in healthy men years before the age related decline in women. J. Am. Coll. Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- COCKCROFT J.R., CHOWIENCZYK P.J., BENJAMIN N., RITTER J.M. Preserved endothelium dependent vasodilatation in patients with essential hypertension. N. Engl. J. Med. 1994;330:1036–1040. doi: 10.1056/NEJM199404143301502. [DOI] [PubMed] [Google Scholar]
- COHEN R.A., VANHOUTTE P.M. Endothelium-dependent hyperpolarization beyond nitric oxide and cyclic GMP. Circulation. 1995;92:3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- FALLOON B.J., HEAGERTY A.M. In vitro perfusion studies of human resistance artery function in essential hypertension. Hypertension. 1994;24:16–23. doi: 10.1161/01.hyp.24.1.16. [DOI] [PubMed] [Google Scholar]
- FRIELINGSDORF J., SEILER C., KAUFMANN P., VASSALLI G., SUTER T., HESS O.M. Normalization of abnormal coronary vasomotion by calcium antagonists in patients with hypertension. Circulation. 1996;93:1380–1387. doi: 10.1161/01.cir.93.7.1380. [DOI] [PubMed] [Google Scholar]
- FURBERG C.D., PSATY B.M., MEYER J.V. Nifedipine: Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92:1326–1331. doi: 10.1161/01.cir.92.5.1326. [DOI] [PubMed] [Google Scholar]
- GARLAND C.J., MCPHERSON G.A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br. J. Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES M.A., WATT P.A., POTTER J.F., THURSTON H., SWALES J.D. Endothelial function in subcutaneous resistance arteries from elderly hypertensive and normotensive subjects. Clin. Sci. 1997;92:139–145. doi: 10.1042/cs0920139. [DOI] [PubMed] [Google Scholar]
- JOHNS A., LEIJTEN P., YAMAMOTO H., HWANG K., VAN BREEMEN C. Calcium regulation in vascular smooth muscle contractility. Am. J. Cardiol. 1987;59:18A–23A. doi: 10.1016/0002-9149(87)90171-8. [DOI] [PubMed] [Google Scholar]
- KÄHÖNEN M., ARVOLA P., WU X., PÖRSTI I. Arterial contractions induced by cumulative addition of calcium in hypertensive and normotensive rats: influence of endothelium. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;349:627–636. doi: 10.1007/BF01258469. [DOI] [PubMed] [Google Scholar]
- KAPLAN N.M. Calcium entry blockers in the treatment of hypertension. JAMA. 1989;262:817–823. [PubMed] [Google Scholar]
- KARAKI H., WEISS G.B. Calcium release in smooth muscle. Life Sci. 1988;42:111–122. doi: 10.1016/0024-3205(88)90674-1. [DOI] [PubMed] [Google Scholar]
- LINDER L., KIOWSKI W., BUHLER F.R., LÜSCHER T.F. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation. 1990;82:1762–1767. doi: 10.1161/01.cir.81.6.1762. [DOI] [PubMed] [Google Scholar]
- LÜSCHER T.F. Heterogeneity of endothelial dysfunction in hypertension. Eur. Heart. J. Suppl. D. 1992;13:50–55. doi: 10.1093/eurheartj/13.suppl_d.50. [DOI] [PubMed] [Google Scholar]
- LÜSCHER T.F., VANHOUTTE P.M. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;8:344–348. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- NELSON M. Ca2+-activated potassium channels and ATP-sensitive potassium channels as modulators of vascular tone. Trends Cardiovasc. Med. 1993;3:54–60. doi: 10.1016/1050-1738(93)90037-7. [DOI] [PubMed] [Google Scholar]
- ORLOV S.N., RESINK T.J., BERNHARDT J., BÜHLER F.R. Na+, K+ pump and Na+, K+ co-transport in cultured vascular smooth muscle cells from spontaneously hypertensive and normotensive rats: baseline activity and regulation. J. Hypertens. 1992;10:733–740. [PubMed] [Google Scholar]
- PAHOR M., GURALNIK J.M., SALIVE M.E., CORTI M.-C., CARBONIN P., HAVLIK R.J. Do calcium channel blockers increase the risk of cancer. Am. J. Hypertens. 1996;9:695–699. doi: 10.1016/0895-7061(96)00186-0. [DOI] [PubMed] [Google Scholar]
- PANZA J.A., CASINO P.R., KILCOYNE C.M., QUYYUMI A.A. Role of nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- PANZA J.A., QUYYUMI A.A., BRUSH J.E. , JR, EPSTEIN S.E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N. Engl. J. Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- PÖRSTI I. Arterial smooth muscle contractions in spontaneously hypertensive rats on a high calcium diet. J. Hypertens. 1992;10:255–263. [PubMed] [Google Scholar]
- RENWICK A.G., ROBERTSON D.R., MACKLIN B., CHALLENOR V., WALLER D.G., GEORGE C.F. The pharmocokinetics of oral nifedipine - a population study. Br. J. Clin. Pharmacol. 1988;25:701–708. doi: 10.1111/j.1365-2125.1988.tb05256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSCH N.J., HERMSMEYER K. Calcium currents are altered in the vascular muscle cell membrane of spontaneously hypertensive rats. Circ. Res. 1988;63:997–1002. doi: 10.1161/01.res.63.6.997. [DOI] [PubMed] [Google Scholar]
- SCHIFFRIN E.L., DENG L.-Y. Comparison of effects of angiotensin I-converting enzyme inhibition and β-blockade for 2 years on function of small arteries from hypertensive patients. Hypertension. 1995;25:699–703. doi: 10.1161/01.hyp.25.4.699. [DOI] [PubMed] [Google Scholar]
- STEKIEL W.J., CONTNEY S.J., LOMBARD J.H. Small vessel membrane potential, sympathetic input, and electrogenic pump rate in SHR. Am. J. Physiol. 1986;250:C547–C556. doi: 10.1152/ajpcell.1986.250.4.C547. [DOI] [PubMed] [Google Scholar]
- TADDEI S., VIRDIS A., MATTEI P., GHIADONI L., GENNARI A., FASOLO C.B., SUDANO I., SALVETTI A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- THYBO N.K., MULVANY M.J., JASTRUP B., NIELSEN H., AALKJAER C. Some pharmacological and elastic characteristics of isolated subcutaneous small arteries from patients with essential hypertension. J. Hypertens. 1996;14:993–998. [PubMed] [Google Scholar]
- THYBO N.K., STEPHENS N., COOPER A., AALKJAER C., HEAGERTY A.M., MULVANY M.J. Effect of antihypertensive treatment on small arteries of patients with previously untreated essential hypertension. Hypertension. 1995;25:474–481. doi: 10.1161/01.hyp.25.4.474. [DOI] [PubMed] [Google Scholar]
- TREASURE C.B., MANOUKIAN S.V., KLEIN J.L., VITA J.A., NABEL E.G., RENWICK G.H., SELWYN A.P., ALEXANDER R.W., GANZ P. Epicardial coronary artery responses to acetylcholine are impaired in hypertensive patients. Circ. Res. 1992;71:776–781. doi: 10.1161/01.res.71.4.776. [DOI] [PubMed] [Google Scholar]
- WALDRON G.J., GARLAND C.J. Effect of potassium channel blockers on L-NAME insensitive relaxations in rat small mesenteric artery. Can. J. Physiol. Pharmacol. 1994;72 Suppl. 1:115. [Google Scholar]
- WATTS G.F., O'BRIEN S.F., SILVESTER W., MILLAR J.A. Impaired endothelium-dependent and independent dilatation of forearm resistance arteries in men with diet-treated non-insulin-dependent diabetes: role of dyslipidemia. Clin. Sci. 1996;91:567–573. doi: 10.1042/cs0910567. [DOI] [PubMed] [Google Scholar]
- ZEIHER A.M., DREXTER H., SAURBIER B., JUST H. Endothelium-mediated coronary blood flow modulation in humans: effects of age, atherosclerosis, hypercholesterolemia and hypertension. J. Clin. Invest. 1993;92:652–662. doi: 10.1172/JCI116634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG E.W., BUKOSKI R.D., MCCARRON D.A. Calcium metabolism in experimental hypertension. Proc. Soc. Exp. Biol. Med. 1988;187:123–141. doi: 10.3181/00379727-187-42646. [DOI] [PubMed] [Google Scholar]


