Abstract
Central administration of bombesin inhibits gastric acid production independently of the centrally or peripherally-acting stimuli employed. This study evaluates the role and location of the cerebral nitric oxide (NO) implicated in the inhibitory effect of central bombesin on in vivo rat gastric acid secretion, as induced by distension with 15 cm H2O, insulin (0.75 u.i. kg−1 i.p.) TRH (1.2 μg kg−1, i.c.) or pentagastrin (100 μg kg−1, i.p.).
The acid-inhibitory effect of i.c. bombesin (40 ng kg−1) was prevented by prior administration of L-NAME (80 μg kg−1) in the dorsal motor nucleus of the vagus (DMN). This dose of L-NAME when administered into the nucleus of the tractus solitarious (NTS) did not influence the effects of bombesin. Administration of L-arginine (400 μg kg−1) into the DMN restored the acid-inhibitory effect of i.c. bombesin in animals treated with L-NAME.
Microinjection of bombesin (12 ng kg−1) into the paraventricular nucleus of the hypothalamus (PvN) inhibits acid secretion stimulated by pentagastrin. This inhibitory effect was prevented by a previous injection of L-NAME (80 μg kg−1) into the DMN.
The release of NO in the DMN following i.c. administration of bombesin was confirmed by in vivo electrochemical detection.
Administration by microdialysis in the DMN of the NO-donor SNAP (25 mM in 1.5 μl min−1) into the DMN inhibits pentagastrin-stimulated gastric acid secretion.
The present study suggests that nNOS-containing neurons in the DMN have an inhibitory role in the control of gastric acid responses.
Keywords: Gastric acid, gastrointestinal regulation, brain-gut axis, nitric oxide, central nervous system, central neurotransmitters
Introduction
The dorsal vagal complex (DVC) of the brainstem comprises two interacting nuclei, the nucleus of the tractus solitarious (NTS), which receives primary afferent fibres, and the dorsal motor nucleus of the vagus (DMN) which provides motor input to thoracoabdominal viscera. Both nuclei play a role in the regulation of several peripheral systems and specifically in the gastrointestinal tract. In particular, vagovagal reflexes initiated by mechanical or chemical stimulation of the stomach excite inhibitory neurones in the NTS which project to the DMN and, by modulating its activity, decrease gastric motility and acid secretory responses (Zhang et al., 1998c). Nitric oxide (NO) is a neurotransmitter/neuromodulator in the CNS (Garthwaite et al., 1988). The DMN, especially, contains structures that exhibit immunoreactivity to nNOS, specifically afferent fibres coming from the NTS or the paraventricular nucleus of the hypothalamus (PvN) and neurones which project to extensive regions of the gastrointestinal tract (Berthoud et al., 1991). Previous observations (Barrachina et al., 1995; Martinez Cuesta et al., 1994) suggested a role for this mediator in the central regulation of acid secretory responses. Recently, we have shown that moderate somatic stress inhibits gastric acid secretion, and functional and immunohistochemical evidences suggested that such an inhibitory response is mediated by a neuronal pathway involving NO synthesis in the DMN (Esplugues et al., 1996). Later studies (Krowicki et al., 1997) have also demonstrated the involvement of a similar nitrergic pathway in the control of gastric motor activity.
Bombesin is a tetradecapeptide characterized as a neurotransmitter and neuromodulator in many mammalian tissues. When administered centrally bombesin elicits a broad range of behavioural responses such as grooming and satiety (Ladenheim et al., 1993), and autonomic actions, including cardiovascular (Brown et al., 1984), respiratory (Niewoehner et al., 1983) and thermoregulatory effects (Brown et al., 1988). Likewise central bombesin is a potent inhibitor of gastric functions, including basal and stimulated acid production (Heymann-Mönnikes et al., 1990; Taché et al., 1994) and this peptide has been recently shown to be located in the NTS and the DMN in the brainstem or the PvN of the hypothalamus (Lynn et al., 1996).
The aim of the present study was: (1) to evaluate if the acid inhibitory effects of centrally-administered bombesin are mediated by NO; (2) to characterize whether NO synthesis in the DMN is involved in such a reduction of acid production; (3) to analyse the nature of the acid producing stimuli affected by this cerebral NO and (4) to assess, by administering a NO-donor directly into the dorsal vagal complex, the possible implication of this neurotransmitter in a general acid-inhibitory pathway.
Methods
General preparation
Male Sprague-Dawley albino rats (250–300 g body weight) were deprived of food, but not water, for 18–20 h before the experiment and anaesthetized with urethane anaesthesia (1.5 g kg−1, i.p.). A polyethylene tube was inserted into the trachea to ensure a patent airway, and a jugular vein was cannulated when necessary. Two soft catheters were introduced into the stomach through incisions in the cervical oesophagus and the duodenum, and the gastric lumen was flushed with 50–100 ml of saline to remove any solid content. Thereafter, the stomach lumen was perfused continuously (0.9 ml min−1) via the oesophageal catheter with saline at room temperature. After 1 h of stabilization the gastric effluent was collected periodically at different time intervals, depending on the experimental group, and the H+ output was determined by automatic titration of 8 ml aliquots of the perfusate to pH 7 with 0.01 mM NaOH. A stable acid output for 60 min was considered as the basal rate of acid secretion.
Four different stimulants of acid secretion were used in our studies. Gastric distension was induced by placing the tip of the antroduodenal cannula 15 cm above the perfused stomach, where it was maintained for the remaining 3 h of the experimental period in which acid production was evaluated every 30 min. In other experiments, acid production was stimulated by an intraperitoneal (i.p.) bolus of insulin (0.75 u.i. kg−1; 0.25 ml) or by a microinjection of thyrotropin-releasing hormone (TRH) (1.2 μg kg−1 10 μl per rat) into the cisterna magna, an acid production evaluated every 20 and 10 min respectively, for a period of 1 h in both cases. In the last group of experiments and i.p. bolus of pentagastrin (100 μg kg−1, 0.25 ml) was used and acid secretion evaluated every 10 min for a period of 1 h. In all cases each preparation was used for a single response only, with treatments being allocated randomly between preparations. Values of acid production were expressed as Δμmol H+ 100 g−1 X min−1, with X being the period of time evaluated in each particular experiment.
Microinjection into the DVC and the PvN of the hypothalamus
Microinjections into the two nuclei comprising the dorsal vagal complex, the DMN and the NTS, were performed to identify the cerebral nucleus implicated in the acid inhibitory effects of centrally-administered bombesin. After the general surgical preparation was performed, the animals were placed into a stereotaxic instrument (Stoelting, Mod 51600), their heads were fixed in a nose-down position, and the skin and muscle overlying the occipital bone were retracted. Following the removal of a small segment of this bone a 1 μl Hamilton syringe (26 g), was positioned according to the atlas of stereotaxic coordinates (Paxinos & Watson, 1986). The co-ordinates used for microinjection sites were: DMN (dorsoventral from bone surface, 8.3 mm, anteroposterior from bregma, −13.8 mm; lateral from midline, 0.6 mm) and NTS (dorsoventral from bone surface, 7.9 mm, anteroposterior from bregma, −13.8 mm; lateral from midline, 1 mm). L-NAME (80 μg kg−1), L-arginine (400 μg kg−1), or vehicle were microinjected in a volume of 0.1 μl per rat delivered by pressure injection over 30 s. In preliminary experiments it was shown that basal gastric acid secretion 5.7±1.2 μmol H+, 100 g−1 60 min−1 n=3) was not modified by the administration of 80 μg kg−1 of L-NAME into the DMN (6.2±3.9 μmol H+, 100 g−1 60 min−1 n=3). The syringe was left in place for a further 3 min to allow diffusion of the injected solution. Immediately after the animals received an i.c. microinjection of bombesin (40 ng kg−1, 10 μl per rat) they were removed from the stereotaxic apparatus. The gastric acid secretion was allowed to stabilize for a period of 10 min and it was stimulated thereafter.
In another experimental group a dose of L-NAME (80 μg kg−1 100 nl−1) was administered in the DMN, whereas bombesin (12 ng kg−1, 100 nl−1) was microinjected into the ipsilateral PvN of the hypothalamus (dorsoventral from bone surface 8 mm, anteroposterior from bregma −2.2 mm, lateral from midline 0.6 mm). In all cases acid production was stimulated immediately after the microinjections.
In vivo monitoring of NO
The in vivo release of NO was monitored by electrochemical detection, using electrodes with porphyrinic sensors as previously described (Malinski et al., 1992). Briefly, polymeric films of Tetrakis (3-methoxy-4/hydroxyphenyl porphyrin), containing nickel as the core metal, were deposited by differential normal pulse voltammetry (DNPV) onto carbon fibre microelectrodes (30×200 μm). The porphyrinic surface was then rinsed with water and covered with a cation exchange material (Nafion) to discriminate from NO2 sources. A microprocessor-controlled apparatus (Bioelectrochemical Analyzer, La Laguna, Spain) was used to monitor voltammetric signals. DNPV parameters were as follows: potential range −100 to 1.000 mV, scan rate 20 mV/s, pulse amplitude 40 mV, pulse duration 40 ms, and prepulse duration 50–120 ms. The oxidation potential peak of NO was found at 650 mV. Before in vivo NO determination, each porphyrinic electrode was tested in vitro with two solutions containing known NO concentrations (1 nM and 1 μM) in order to evaluate if voltammetric current (nA) signals were related to NO concentration. To preserve the integrity of the NO microsensor, it was mounted in an assembly carrier device as previously described (Wood et al., 1994).
Once the surgical preparation was completed, rats were positioned in the stereotaxic apparatus, where they remained throughout the experimental period, and the NO-electrode was positioned into the DMN. A reference (Ag-AgCl) electrode was placed between the skull and the dura and an auxiliary electrode, which was kept wet with saline-soaked pads, was placed on the skull. Data were collected and stored on-line on a floppy disk and analysed continuously by a computerized direct-fit program (Wood et al., 1994). Preliminary experiments showed that once basal voltammetric signals were obtained, the maximum peak that follows NO released were achieved within the first 5 min that followed the administration of bombesin, and thus results are expressed as ΔpA 5 min−1. Following a period of 40 min in which basal acid secretion remained constant, rats received an i.c. administration of bombesin (40 ng kg−1, 10 μl) or vehicle (PBS). Thereafter, animals received an i.p. bolus of pentagastrin (100 μg kg−1, 0.25 ml) and gastric acid secretion was evaluated for 1 h at 10 min intervals.
Microdialysis studies
In these experimental groups, once the general surgical preparation was completed, rats were placed in the stereotaxic frame and L-shaped probes were positioned into the DVC and attached to the skull using dental cement. The microdialysis probes (i.d. 0.22 mm; o.d. 0.31 mm) were prepared from hollow fibres of polyacrilonitrile/sodium methalyl sulphonate copolymer (AN69, HOSPAL Bologna, Italy) and covered with epoxy glue to obtain a dialysis membrane of 0.6 mm (Wallace et al., 1995). The probes were connected to an infusion pump (Harvard Apparatus) by polyethylene tubing (i.d. 0.28 mm) and continuously perfused (3 μl min−1) with artificial cerebrospinal fluid (aCSF, in mM) NaCl 140; KCL 3.5; CaCl2 1.26; MgCl 1; buffered at pH 7.4 with 2 mM phosphate buffer. The microdialysis perfusated from the initial 30 min sampling period was discarded. A dead space of 30 μl existed between syringes and dialysis tips, which resulted in a delay of 10 min between the changing of solutions and the access of new microdialysis perfusates into the DVC.
Once basal gastric acid secretion had remained stable for 30 min, the animals received a continuous infusion (i.v) of pentagastrin (8 μg kg−1 h−1) and acid production was evaluated every 10 min. When a stable plateau of acid secretion had been achieved the microdialysis perfusate (aCSF) was replaced with a similar solution containing SNAP (25 mM) which was perfused for a further 30 min.
Histological examinations
To confirm the position of the microinjection, or the location of either the microdialysis cannulae or the electrode, at the end of each experimental period rats were perfused through the heart with 200 ml of 0.9% saline in 1 mM sodium phosphate buffer (pH 7.4, room temperature). This rinse solution was followed by 500 ml of fixative containing 4% paraformaldehyde in 0.1 M phosphate buffer (4C, pH 7.4). Thereafter, the brains were removed, kept for 3 h immersed in the 4% paraformaldehyde solution and washed and stored overnight in 0.1 M phosphate buffer containing 30% saccharose. Coronal sections (40 μm) were mounted, stained in Thionine Nissl, and examined microscopically. The location sites were identified by the visualization of the tip of the tract and marked on plates reproduced from the atlas of Paxinos and Watson and only the data from those animals in which the position was correct (80–90%) were considered in the experimental analysis.
Drugs
Urethane, bombesin, TRH, L-NAME, and L-arginine were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Human insulin (Insulatard®, Laboratorios Novo) and pentagastrin (Peptavlon®, ICI) were used as commercially-available preparations. Unless otherwise mentioned, all drugs were dissolved in saline immediately before use and when administered i.c. the volume was 10 μl per rat.
Statistical analysis
All data are expressed as the mean±s.e.mean. Comparisons between groups were performed by ANOVA followed by a Tukey test. Significance between voltammetric measurements were evaluated by Mann-Whitney. In all cases a probability of P<0.05 or less was considered as significant.
Results
Effects of bombesin on distension-stimulated gastric acid secretion
As shown in Figure 1, distension of the stomach in control rats with an intragastric pressure of 15 cm H2O produced a progressive increase in acid secretion that reached its maximum after 2 h. The total stimulated acid output in this period was 53±19 μmol H+ 100 g−1 120 min−1 (n=7). The i.c. injection of bombesin (40 ng kg−1) significantly (P<0.001) decreased acid secretion to 3.8±2.1 μmol H+ 100 g−1 120 min−1 (n=6). When administered i.v. this dose (40 ng kg−1) of bombesin did not modify acid output stimulated by distension. Prior administration of L-NAME (80 μg kg−1) into the DMN prevented (39±8.2 μmol H+ 100 g−1 120 min−1, n=10) the acid inhibitory effects of i.c. bombesin (40 ng kg−1). In contrast when administered into the NTS, microinjection of L-NAME (80 μg kg−1) did not influence the inhibitory effects of i.c. bombesin (2.8±2.7 μmol H+ 100 g−1 120 min−1, n=5). In animals receiving L-NAME in the DMN and bombesin i.c., the values of gastric acid secretion (39±8.2 μmol H+ 100 g−1 120 min−1, n=10) were not significantly different from those observed in control animals (53±19 μmol H+ 100 g−1 120 min−1, n=7). This dose of L-NAME (80 μg kg−1), if administered i.c., did not modify the acid inhibitory effects of i.c. bombesin (40 ng kg−1). In animals receiving L-NAME (80 μg kg−1) by microinjection, the co-administration of L-arginine (400 μg kg−1) into the DMN re-established the inhibition by bombesin (40 ng kg−1) of distension-stimulated acid secretion (3.8±1.3 μmol H+ 100 g−1 120 min−1, n=6).
Figure 1.
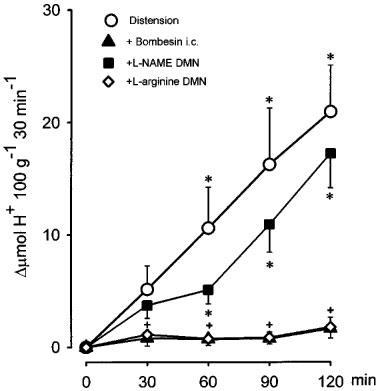
Inhibition by bombesin (40 ng kg−1, i.c.) of acid secretion induced by gastric distension (15 cm H2O) in the perfused stomach of the anaesthetized rat. Microinjection of L-NAME (80 μg kg−1) into the DMN prevented the acid-inhibitory effects of bombesin, an action that was absent following the co-administration of L-arginine (400 μg kg−1). Each point represents mean±s.e.mean of at least six animals and significant difference from the bombesin group is shown by *P<0.05, and from the L-NAME group by +P<0.05.
Effects of bombesin on insulin-stimulated gastric acid secretion
As shown in Figure 2 the i.p. administration of insulin (0.75 U.I. kg−1) in control rats produced a progressive increase in acid secretion that reached its maximum after 60 min. The total stimulated acid output in this period was 24±5.7 μmol H+ 100 g−1 60 min−1 (n=14). The i.c injection of bombesin (40 ng kg−1) abolished insulin-stimulated acid secretion (0.9±0.2 μmol H+ 100 g−1 60 min−1, n=10) (P<0.001). Prior administration of L-NAME (80 μg kg−1) into the DMN prevented (30±1.2 μmol H+ 100 g−1 60 min−1, n=10) the acid inhibitory effects of i.c. bombesin (40 ng kg−1) and the values the gastric secretion reached, in these conditions, were not significantly different from the control group. In animals receiving a microinjection of L-NAME (80 μg kg−1), co-administration of L-arginine (400 μg kg−1) into the DMN re-established the inhibition by bombesin (40 μg kg−1) of insulin-stimulated acid secretion (5±2.4 μmol H+ 100 g−1 60 min−1, n=5).
Figure 2.
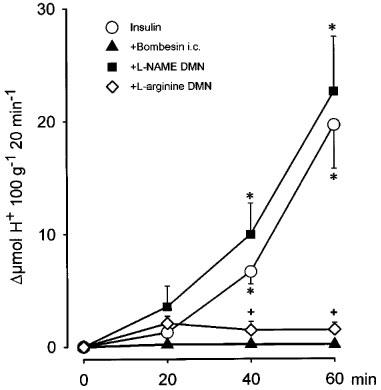
Acid secretion stimulated by insulin (0.75 U.I. kg−1, i.v.) was abolished by bombesin (40 ng kg−1, i.c). Microinjection of L-NAME (80 μg kg−1) into the DMN prevented the acid-inhibitory effects of bombesin, an action that was absent following the co-administration of L-arginine (400 μg kg−1). Each point represents mean±s.e.mean of at least six animals. Significance of difference from the bombesin group is shown by *P<0.05, and from the L-arginine treated group by +P<0.05.
Effects of bombesin on TRH-stimulated gastric acid secretion
As shown in Figure 3 the i.c. injection of TRH (300 ng kg−1) of control rats produced a progressive increase in acid secretion that reached its maximum after 20 min. The total stimulated acid output in this period was 8.8±1.8 μmol H+ 100 g−1 20 min−1, n=9). The i.c. injection of bombesin (40 ng kg−1) significantly (P<0.05) decreased TRH-stimulated acid secretion (2.3±0.6 μmol H+ 100 g−1 30 min−1, n=9), whereas prior administration of L-NAME (80 μg kg−1) into the DMN prevented the acid inhibitory effects of i.c. bombesin (7.4±1.5 μmol H+ 100 g−1 30 min−1, n=11), leading to a stimulation of gastric secretion non significantly different from the control group.
Figure 3.
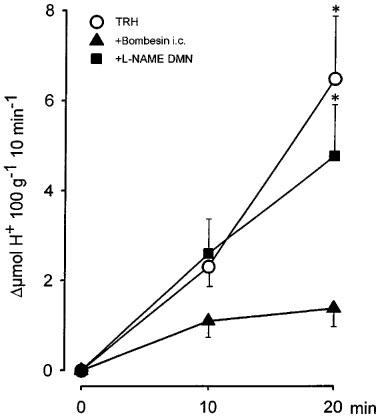
Acid secretion stimulated by TRH (300 ng kg−1, i.c.) in the perfused stomach of the rat was abolished by central bombesin (40 ng kg−1, i.c.). Microinjection of L-NAME (80 μg kg−1) into the DMN prevented the acid-inhibitory effects of bombesin. Each point represents mean±s.e.mean of at least six animals and significant difference from the bombesin group is shown by *P<0.05.
Effects of bombesin on pentagastrin-stimulated gastric acid secretion
As shown in Figure 4, the administration of a bolus injection of pentagastrin (100 μg kg−1, i.p) in control rats produced a progressive increase in acid secretion that reached the maximal H+ production after 20 min. The total stimulated acid output in this period was 5.2±1.2 μmol H+ 100 g−1 20 min−1, n=8. The i.c. injection of bombesin (40 ng kg−1) significantly (P<0.01) decreased pentagastrin-stimulated acid secretion (1±0.2, μmol H+ 100 g−1 20 min−1, n=7). Prior administration of L-NAME (80 μg kg−1) into the DMN prevented the acid inhibitory effects of i.c. bombesin (5.7±0.9 μmol H+ 100 g−1 20 min−1, n=7). In contrast, microinjection of L-NAME (80 μg kg−1) into the NTS did not influence the acid inhibitory effects of i.c. bombesin (2±0.7 μmol H+ 100 g−1 20 min−1, n=5). In animals receiving L-NAME in the DMN and bombesin i.c., the values of gastric acid secretion were not significantly different from those observed in control animals. In our experiments pentagastrin-stimulated acid responses were not influenced by vagotomy (4.6±2 μmol H+ 100 g−1 20 min−1, n=3). Furthermore, when administered in vagotomized animals (Figure 5) 40 ng kg−1 of bombesin (i.c.) did not modify acid output stimulated by pentagastrin, 4.6±0.9 μmol H+ 100 g−1 20 min−1 in controls (n=5) and 5±1.2 μmol H+ 100 g−1 20 min−1 in vagotomized rats (n=8).
Figure 4.
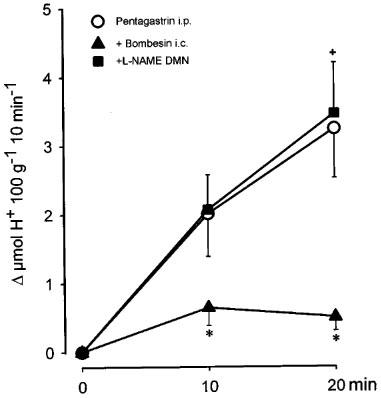
Acid secretion stimulated by pentagastrin (100 μg kg−1, i.p., 0.25 ml) in the perfused stomach of the rat was abolished by central bombesin (40 ng kg−1, i.c). Microinjection of L-NAME (80 μg kg−1) into the DMN, prevented the acid-inhibitory effects of bombesin. Each point represents mean±s.e.mean of at least six animals and significant difference from the control group is shown by *P<0.05 and from the bombesin group by +P<0.05.
Figure 5.
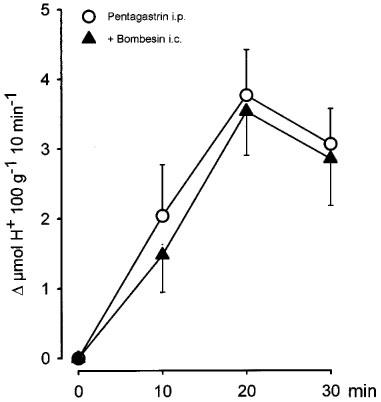
Central administration of bombesin (40 ng kg−1, i.c.) did not modify the acid response stimulated by pentagastrin (100 μg kg−1, i.p., 0.25 ml) in vagotomized rats. Each point represents mean±s.e.mean of at least five animals.
In a further group of experiments shown in Figure 6, microinjection of bombesin (12 ng kg−1) into the PvN significantly (P<0.001) reduced pentagastrin-stimulated gastric acid production (100 μg kg−1, i.p) by 61.5±25%. However the acid-secretory effects of pentagastrin were maintained if L-NAME (80 μg kg−1) was microinjected into the DMN prior to the administration of bombesin into the PvN.
Figure 6.
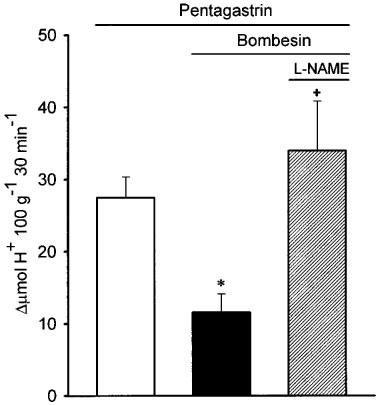
Acid secretion stimulated by pentagastrin (100 μg kg−1, i.p., 0.25 ml) in the perfused stomach of the rat was reduced by the microinjection of bombesin in the PvN of the hypothalamus (12 ng kg−1). Microinjection of L-NAME (80 μg kg−1) into the DMN prevented the acid-inhibitory effects of bombesin injected in the PvN. Each bar represents mean±s.e.mean of at least five animals and significant difference from the control group is shown by *P<0.05 and from the bombesin group by +P<0.05.
In vivo detection of NO
Preliminary in vitro experiments showed that with each electrode the intensity of the voltammetric currents registered were directly correlated to NO concentrations. Central (i.c.) administration of PBS modified neither gastric acid production induced by pentagastrin (10±0.9 μmol H+ 100 g−1 30 min−1, n=4) nor the voltammetric register of NO oxidation (−3±2 ΔpA 5 min−1; n=4). Central administration of bombesin (40 ng kg−1, i.c.) induced both a significant (P<0.005) inhibition of gastric acid production (1.7±0.7 μmol H+ 100 g−1 30 min−1, n=5) and a significant (P<0.01) increase in the current induced by oxidation of NO (106±19 ΔpA 5 min−1; n=5), (Fig. 7).
Figure 7.
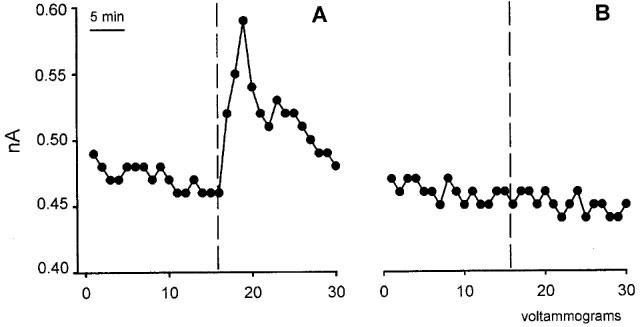
Examples of electrochemical detection of NO in the DMN after i.c. administration of bombesin (40 ng kg−1, i.c.) (A) or vehicle (B). Each point (voltammogram) represents the current generated by NO oxidation at 650 mV. The vertical dotted line indicates the voltammogram during which the i.c. administration was performed.
Microdialysis studies
As shown in Figure 8, the perfusion of SNAP into the DVC induced a rapid diminution of acid production stimulated by the i.v. infusion of pentagastrin (8 μg kg−1 h−1), with the maximum inhibitory effect (−56±8.8%; n=4) being reached 20 min later. Perfusion of aCSF in control rats did not influence pentagastrin-stimulated acid production which remained constant throughout the 30 min experimental period.
Figure 8.
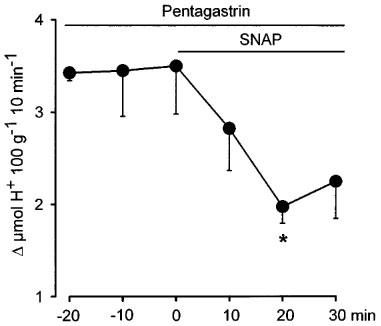
Administration into the dorsal vagal complex by microdialysis of a continuous infusion (1.5 μl min−1) of artificial cerebral spinal fluid containing the NO-donor SNAP (25 mM) decreases acid secretion stimulated by the i.v. perfusion of pentagastrin (8 μg kg−1 h−1). Each point represents mean±s.e.mean of at least five animals and significant difference from the basal level of acid secretion is shown by *P<0.05.
Discussion
The present study shows that central administration of bombesin inhibits acid responses induced by various stimulants of gastric secretion. The various stimuli used exhibited substantial differences either in the mechanisms for acid production or the time needed to elicit such a response. However, in all cases their inhibition by bombesin involves a neuronal pathway in which NO released in the DMN plays a key role. Distension of the stomach wall activates mechanoreceptors of vagal primary afferents which carry their signals to the NTS, where they are integrated and trigger an inhibitory response by the DMN. (Fogel et al., 1996; Zhang et al., 1998a, 1998b). The present study demonstrates that microinjection of L-NAME into the DMN reversed the acid inhibitory effects of i.c. bombesin at doses that had no such an effect when administered i.v. The low doses of L-NAME microinjected had no effect on acid secretion when administered i.c. and did not affect systemic arterial blood pressure when administered either i.c. or i.v. thus excluding the possibility that systemic peripheral actions of this compound could be responsible for the reversal of acid inhibition. Administration of the precursor of NO synthesis, L-arginine into the DMN restored the inhibitory effect of i.c. bombesin in animals treated with L-NAME, again implying that the synthesis of NO in the DMN is needed for the acid inhibitory effects of centrally administered bombesin. Although both DMN and NTS contain neurones and fibres stained for NOS inmunoreactivity (Krowicki et al., 1997), in our experiments microinjection of L-NAME into the NTS failed to affect the inhibitory effects of bombesin. This would suggest that the synthesis of NO involved in the abolition of acid responses by centrally administered bombesin could be specifically located within the DMN and not within the NTS. However this statement should be taken with some reservation because due to the large size of the NTS and the fact that microinjections were aimed to the central area of this nucleus, it could be possible that NTS neurons located in the close vicinity of the DMN (Rinaman et al., 1990; Shapiro et al., 1985), for instance those of the medial subnucleus, could have been influenced by microinjections targeted to the DMN and spared from the effects of those directed to the NTS.
In further experiments, acid secretion was also induced by two other central stimuli of acid production, i.p. insulin and i.c. administration of TRH. The hypoglycaemic status generated by insulin is sensed by gastric-type glucose sensitive neurons contained in the lateral hypothalamic area which project to the medulla oblongata (Shiraishi, 1980). Simultaneously, peripheral glucose sensors send information through vagal afferents to the NTS which functions as a relay centre between these afferents and the hypothalamus (Shiraishi, 1988). There is some controversy as to how these two mechanisms interact, but it is well established that their final response is the stimulus of gastric secretion via activation of the DMN (Shiraishi, 1980; Shiraishi et al., 1985). TRH administered i.c. or directly into the DMN induces a potent and vagally-mediated increase of gastric acid production (Ishikawa et al., 1988). It is thus important that, despite the differences between the central pathways involved in the acid secretory actions of insulin and TRH, bombesin inhibited both. Furthermore, NO released into the DMN was involved again in such an inhibitory action as shown by the findings that the microinjection of L-NAME in this nucleus reversed the inhibitory effects of i.c. bombesin, and that co-administration of L-arginine with L-NAME in the DMN restored the inhibitory effects of central bombesin.
Pentagastrin is an analogue of the endogenous peptide gastrin which induces the production of acid by direct stimulation of parietal oxyntic cell and by generating the release of histamine by ECL cells (Forte et al., 1987). Thus, the fact that central bombesin also inhibited pentagastrin-induced acid secretion suggested a central control of peripherally-stimulated acid production via the vagus, as demonstrated when vagotomy restored the acid secretory effects of pentagastrin in animals treated with i.c. bombesin. This idea is in keeping with the previous observations that i.c. administration of nmolar concentrations of bombesin decrease the discharge of gastric vagal efferents (Yoshida-Yoneda et al., 1993) and that vagotomy abolishes the reduction in intragastric pressure provoked by microinjection of L-arginine into the DVC (Krowicki et al., 1997).
Centrally administered bombesin may not only act in the DVC but also in every area of the CNS sensitive to bombesinergic neurons (Gunion et al., 1987; Lynn et al., 1996). A recent study combining retrograde tracing from the DVC with immunohistochemistry for bombesin has shown that despite the various locations in the brain of bombesin-like neurons, only those in the parvicellular area of the PvN of the hypothalamus project to the DVC (Costello et al., 1991). Further expanding the concept that there is a nitrergic link between the PvN and the DMN, we now show that when bombesin is administered in the PvN, its acid inhibitory effects requires the synthesis of NO in the DMN. Similarly, NO in the DMN has been shown to be involved also in another pathway which modulates the gastrointestinal motility (Krowicki et al., 1996). It is interesting to note that although bombesin can influence gastric function and feeding at sites of action in the forebrain as well as the DVC, the latter alone is sufficient to mediate these responses (Flynn, 1992; Gunion et al., 1987). This implies that the DVC acts as the final integrating regulator of the central effects of mediators such as bombesin on gastro-autonomic functions.
The pharmacological evidence that NO synthesis in the DMN mediates the central acid-inhibitory effects of bombesin were confirmed by in vivo and in situ monitoring of NO release. Our experiments with electrochemical detection showed an immediate increase in NO in the DMN following the i.c administration of an acid-inhibitory dose of i.c. bombesin. Furthermore, the finding that administration by microdialysis in the DVC of the NO donor SNAP can inhibit pentagastrin-stimulated acid secretion provides direct evidence that activation of a common L-arginine-NO pathway in the CNS, specifically in the DVC, is a mechanism involved in the control of gastric acid secretion. It is also important to point out that this central inhibitory control of acid production is unrelated to the nature of the stimuli triggering the production of H+, and thus influences both central or peripherally acid stimuli. Anatomical (Lynn et al., 1997; Rinaman et al., 1990; Shapiro et al., 1985) and electrophysiological (Tache et al., 1988; Yosida-Yoneda et al., 1993) evidence suggests that the actions of bombesin result from either direct inhibition of vagal motoneurons or by stimulation of interneurons that provide an inhibitory effect on vagal motoneurons. From the present study we cannot determine the exact mechanism of action elicited by bombesin although our results imply the involvement of interneurons releasing the NO needed for the acid inhibitory action exerted by this peptide.
In conclusion, the present study suggests that nNOS-containing neurons in the DMN have a leading inhibitory role in the control of acid responses and it is implicit that any impairment of this descendent inhibitory mechanism could be in the basis of some gastrointestinal disorders related to gastric secretion.
Acknowledgments
We want to thank Dr Jose Luis Gonzalez-Mora for his most valuable help in the in vivo measurement of NO, Dr Luis Granero for his technical advice in the microdyalisis studies, and Amparo Canet for her technical support. The present study has been supported by grants SAF95-0472 and SAF98-0118. Belén Beltrán is the recipient of a grant from Conselleria d'Educació i Ciència (Generalitat Valenciana).
Abbreviations
- CNS
central nervous system
- DMN
dorsal motor nucleus of the vagus
- DNPV
differential normal pulse voltammetry
- DVC
dorsal vagal complex
- L-NAME
Ng-nitro-L-arginine methyl esther
- NTS
nucleus of the tractus solitarious
- PvN
paraventricular nucleus
- TRH
thyrotropin releasing hormone
References
- BARRACHINA M.D., WHITTLE B.J.R., MONCADA S., ESPLUGUES J.V. Cerebral nitric oxide mediates endotoxin inhibition of rat acid secretion stimulated by gastric distension. Br. J. Pharmacol. 1995;114:8–12. doi: 10.1111/j.1476-5381.1995.tb14898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTHOUD H.R., CARLSON N.R., POWLEY T.L. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am. J. Physiol. 1991;260:R200–R207. doi: 10.1152/ajpregu.1991.260.1.R200. [DOI] [PubMed] [Google Scholar]
- BROWN M.R., CARVER K., FISHER L.A. Bombesin: Central nervous system actions to affect the autonomic nervous system. Ann. N.Y. Acad. Sci. 1988;547:174–182. doi: 10.1111/j.1749-6632.1988.tb23885.x. [DOI] [PubMed] [Google Scholar]
- BROWN M.R., FISHER L.A. Brain peptide regulation of adrenal epinephrine secretion. Am. J. Physiol. 1984;247:E41–E46. doi: 10.1152/ajpendo.1984.247.1.E41. [DOI] [PubMed] [Google Scholar]
- COSTELLO J.F., BROWN M.R., GRAY T.S. Bombesin immunoreactive neurons in the hypothalamic paraventricular nucleus innervate the dorsal vagal complex in the rat. Brain Res. 1991;542:77–82. doi: 10.1016/0006-8993(91)91000-q. [DOI] [PubMed] [Google Scholar]
- ESPLUGUES J.V., BARRACHINA M.D., BELTRAN B., CALATAYUD S., WHITTLE B.J.R., MONCADA S. Inhibition of gastric acid secretion by stress: a protective reflex mediated by cerebral nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14839–14844. doi: 10.1073/pnas.93.25.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLYNN F.W. Caudal brainstem systems mediate effects of bombesin-like peptides on intake in rats. Am. J. Physiol. 1992;262:R39–R45. doi: 10.1152/ajpregu.1992.262.1.R39. [DOI] [PubMed] [Google Scholar]
- FOGEL R., ZHANG X., RENEHAN W. Relationships between the morphology and function of gastric and intestinal distension sensitive neurons in the dorsal motor nucleus of the vagus. J. Comp. Neurol. 1996;364:78–91. doi: 10.1002/(SICI)1096-9861(19960101)364:1<78::AID-CNE7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- FORTE J.G., WOLOSIN J.M.HCl secretion by the gastric oxyntic cell Physiology of the gastrointestinal tract. 1987Raven Press, New York; 853–863.In: Jhonson, L.R. (ed.) [Google Scholar]
- GARTHWAITE J., CHARLES S.L., CHESS-WILLIAMS R. Endothelium derived relaxing factor release on activation of NMDA receptors suggests a role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- GUNION M., TACHÉ Y. Fore and hindbrain mediation of gastric hypoacidity after intracerebral bombesin. Am. J. Physiol. 1987;252:G675–G684. doi: 10.1152/ajpgi.1987.252.5.G675. [DOI] [PubMed] [Google Scholar]
- HEYMANN-MÖNNIKES I., LIVINGSTON Y., TACHÉ Y., SIERRA A., WEINER H., GARRIK T. Bombesin microinjected into the dorsal vagal complex inhibits TRH-stimulated gastric contractility in rats. Brain Res. 1990;533:309–314. doi: 10.1016/0006-8993(90)91354-j. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA T., YANG G., TACHÉ Y. Medullary sites of action of the TRH analogue, RX 77368, for stimulation of gastric acid secretion in the rat. Gastroenterology. 1988;95:1470–1476. doi: 10.1016/s0016-5085(88)80065-9. [DOI] [PubMed] [Google Scholar]
- KROWICKI Z.K., HORNBY P.J. The inhibitory effect of substance P on gastric motor function in the nucleus raphe obscurus is mediated via nitric oxide in the dorsal vagal complex. J. Auton. Nerv. Syst. 1996;58:177–180. doi: 10.1016/0165-1838(95)00133-6. [DOI] [PubMed] [Google Scholar]
- KROWICKI Z.K., SHARKEY K.A., SERRON S.C., NATHAN N.A., HORNBY P.J. Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J. Comp. Neurol. 1997;377:49–69. doi: 10.1002/(sici)1096-9861(19970106)377:1<49::aid-cne6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- LADENHEIM E.E., RITTER R.C. Caudal hindbrain participation in the suppression of feeding by central and peripheral bombesin. Am. J. Physiol. 1993;264:R1229–R1234. doi: 10.1152/ajpregu.1993.264.6.R1229. [DOI] [PubMed] [Google Scholar]
- LYNN R.B., BECHTOLD L.S., MISELIS R.R. Ultrastructure of bombesin-like immunoreactive nerve terminals in the nucleus of the solitary tract and the dorsal motor nucleus. J. Auton. Nerv. Syst. 1997;62:174–182. doi: 10.1016/s0165-1838(96)00125-7. [DOI] [PubMed] [Google Scholar]
- LYNN R.B., HYDE T.M., COOPERMAN R.R., MISELIS R.R. Distribution of Bombesin-Like immunoreactivity in the nucleus of the solitary tract and dorsal motor nucleus of the rat and human: Colocalization with tyrosine hydroxylase. J. Comp. Neurol. 1996;369:552–570. doi: 10.1002/(SICI)1096-9861(19960610)369:4<552::AID-CNE6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- MALINSKI T., TAHA Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature. 1992;358:676–678. doi: 10.1038/358676a0. [DOI] [PubMed] [Google Scholar]
- MARTINEZ CUESTA M.A., BARRACHINA M.D., WHITTLE B.J.R., PIQUE J.M., ESPLUGUES J.V. Involvement of neuronal processes and nitric oxide in the inhibition by endotoxin of pentagastrin-stimulated gastric acid secretion. Naunyn Schmied. Arch. Pharmacol. 1994;349:523–527. doi: 10.1007/BF00169142. [DOI] [PubMed] [Google Scholar]
- NIEWOEHNER D.E., LEVINE A.S., MORLEY J.E. Central effects of neuropeptides on ventilation in the rat. Peptides. 1983;4:277–281. doi: 10.1016/0196-9781(83)90132-8. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C.The rat brain in stereotaxic coordenates 1986Academic, New York; 2nd edition [Google Scholar]
- RINAMAN L., MISELIS R.R. Thyrotropin-releasing hormone-immunoreactive nerve terminals synapse on the dendrites of gastric vagal motoneurons in the rat. J. Comp. Neurol. 1990;294:235–251. doi: 10.1002/cne.902940208. [DOI] [PubMed] [Google Scholar]
- SHAPIRO R.E., MISELIS R.R. The central organization of the vagus nerve innervating the stomach of the rat. J. Comp. Neurol. 1985;238:473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- SHIRAISHI T. Effect of lateral hypothalamic stimulation on medulla oblongata and gastric vagal neural responses. Brain Res. Bull. 1980;5:245–250. doi: 10.1016/0361-9230(80)90165-3. [DOI] [PubMed] [Google Scholar]
- SHIRAISHI T. Hypothalamic control of gastric acid secretion. Brain Res. Bull. 1988;20:791–797. doi: 10.1016/0361-9230(88)90093-7. [DOI] [PubMed] [Google Scholar]
- SHIRAISHI T., KAWASHIMA M., OOMURA Y. Endogenous sugar acid control of hypothalamic neuron activity and gastric acid secretion in rats. Brain Res. Bull. 1985;14:431–438. doi: 10.1016/0361-9230(85)90021-8. [DOI] [PubMed] [Google Scholar]
- TACHE Y., ISHIKAWA T., GUNION M., RAYBOULD H.E. Central nervous system action of bombesin to influence gastric secretion and ulceration. Ann. N.Y. Acad. Sci. 1988;547:183–193. doi: 10.1111/j.1749-6632.1988.tb23886.x. [DOI] [PubMed] [Google Scholar]
- TACHÉ Y., MARKI W., RIVIER J., VALE W., BROWN M. Central nervous system inhibition of gastric secretion in the rat by gastrin-releasing peptide, a mammalian bombesin. Gastroenterology. 1994;81:298–302. [PubMed] [Google Scholar]
- WALLACE J.L., MCKNIGHT W., DEL SOLDATO P., BAYDOUN A.R., CIRINO G. Anti-thrombotic effects of a nitric oxide-releasing, gastric-sparing aspirin derivative. J. Clin. Invest. 1995;96:2711–2718. doi: 10.1172/JCI118338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD J., GARTHWAITE J. Models of the diffusional spread of nitric oxide: implications for neural oxide signalling and its pharmacological properties. Neuropharmacology. 1994;33:1235–1244. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]
- YOSHIDA-YONEDA E., WEI J.Y., TACHÉ Y. Bombesin acts in the brain to decrease gastric vagal efferent discharge in rats. Peptides. 1993;14:339–343. doi: 10.1016/0196-9781(93)90050-q. [DOI] [PubMed] [Google Scholar]
- ZHANG X., FOGEL R., RENEHAN W.E. Relationships between the morphology and function of gastric and intestine sensitive neurons in the nucleus of the solitary tract. J. Comp. Neurol. 1998a;363:37–52. doi: 10.1002/cne.903630105. [DOI] [PubMed] [Google Scholar]
- ZHANG X., FOGEL R., RENEHAN W.E. Physiology and morphology of neurons in the dorsal motor nucleus of the vagus and the nucleus of the solitary tract that are sensitive to distension of the small intestine. J. Comp. Neurol. 1998b;323:432–448. doi: 10.1002/cne.903230310. [DOI] [PubMed] [Google Scholar]
- ZHANG X., RENEHAN W.E., FOGEL R. Neurons in the vagal complex of the rat respond to mechanical and chemical stimulation of the GI tract. Am. J. Physiol. 1998c;274:G331–G341. doi: 10.1152/ajpgi.1998.274.2.G331. [DOI] [PubMed] [Google Scholar]


