Abstract
Sigma (σ) receptors have recently been cloned, though their endogenous ligand(s) remain unidentified. However, some neuroactive steroids, such as progesterone, have a high affinity for these receptors. Some σ ligands, such as DTG, (+)-pentazocine and DHEA, act as σ ‘agonists' by potentiating the neuronal response to NMDA. Others, such as haloperidol, NE-100 and progesterone, act as σ ‘antagonists' by reversing the potentiations induced by σ ‘agonists'.
We compared the effects of σ ‘agonists' in four series of female rats: in controls, at day 18 of pregnancy, at day 5 post-partum, and in ovariectomized rats following a 3-week treatment with a high dose of progesterone.
In pregnant rats and following a 3-week treatment with progesterone, 10 fold higher doses of DTG, (+)-pentazocine and DHEA were required to elicit a selective potentiation of the NMDA response comparable to that obtained in control females. Conversely, at day 5 post-partum and following the 3-week treatment with a progesterone and after a 5-day washout, the potentiation of the NMDA response induced by the σ ‘agonist' DTG was greater than in control females.
The present data suggest that endogenous progesterone acts as an ‘antagonist' at σ receptors. The resulting changes in the function of σ receptors during pregnancy and post-partum may be implicated in emotional phenomena occurring during these periods.
Keywords: NMDA, progesterone, dehydroepiandrosterone (DHEA), (+)-pentazocine, DTG, pregnancy, post partum, neurosteroids
Introduction
Steroids contribute to maintaining cell integrity, regulate membrane permeability and play a crucial role during cell division and growth (for review, see: Majewska, 1987; Paul & Purdy, 1992). Steroid hormones derive from cholesterol; they are highly lipophilic, which ensures their penetration of biological membranes, including the blood–brain barrier. Neuroactive steroids (Paul & Purdy, 1992) produce a diversity of both rapid and delayed neuroendocrine and behavioral effects (Majewska, 1987).
In 1976, Martin et al. (1976) postulated the existence of σ receptors to account for the psychotomimetic effects of certain benzomorphans. Nearly 20 years later, a σ receptor was cloned by Moebius et al. (1993). Much of the interest in σ receptors resulted from the early hypothesis that they may be involved in the pathophysiology of human psychoses (Snyder & Largent, 1989). The finding that progesterone and testosterone act as competitive inhibitors of the binding of N-Allyl-normetazocine ([3H]-(+)SKF-10,047, the prototypical σ ‘agonist') (Su et al., 1988; McCann et al., 1994) raised the possibility that progesterone may function as an endogenous ligand for the σ receptors.
Previous studies have demonstrated that low doses of selective σ ligands, such as di(2-tolyl)guanidine (DTG) (Weber et al., 1986) and (+)-pentazocine (Steinfels et al., 1988), potentiate selectively the neuronal response of rat CA3 dorsal hippocampus pyramidal neurons to microionophoretic applications of N-methyl-D-aspartate (NMDA) (Monnet et al., 1990; 1992; Walker & Hunter, 1994; Bergeron et al., 1995). This effect is reversed by other σ ligands such as N-di-propyl- 2-(4-methoxy-3(2-phenylethoxy)phenyl)-ethylamine monohydrochloride (NE-100) (Okuyama et al., 1993; Chaki et al., 1994) and haloperidol, but not by spiperone which has a binding profile similar to that of haloperidol, except for its low affinity for σ receptors (Tam & Cook, 1984).
We have reported that progesterone and testosterone per se have no effect on NMDA-induced neuronal activation (Bergeron et al., 1996). However, when these two neuroactive steroids are administered at low doses (20 μg kg−1, i.v.), they suppress the potentiation of the NMDA response induced by selective σ ‘agonists' such as DTG. We have also reported that DHEA potentiates selectively the neuronal response to NMDA (Bergeron et al., 1996; Debonnel et al., 1996a). This potentiation is reversed by progesterone, testosterone, NE-100 and haloperidol. Furthermore, 2 weeks following an ovariectomy, the potentiation of the NMDA response induced by DTG was significantly greater than in control female rats, suggesting that σ receptors might be tonically inhibited by endogenous progesterone (Bergeron et al., 1996; Debonnel et al., 1996a).
During pregnancy, plasma levels of progesterone increase by 6 fold, reaching their maximum at day 18, after which they decrease very rapidly down to normal levels after delivery (3 days later) (Pepe & Rothchild, 1974). The present experiments were thus undertaken to assess if hormonal variations during pregnancy could impact on the role of σ receptors in modulating neuronal responses to NMDA. Therefore, the effects of σ ‘agonists' were compared in control female rats, in pregnant rats, in post-partum rats and in ovariectomized rats, treated for 3 weeks with a high dose of progesterone, and following either 2 or 5 day washouts.
Methods
The experiments were carried out in vivo, in the CA3 region of the rat dorsal hippocampus, where the responsiveness of pyramidal neurons to microionophoretic applications of NMDA, Quisqualate (QUIS) and Acetylcholine (ACh) was assessed using extracellular unitary recording. Anatomical and electrophysiological studies have suggested that the different subtypes of σ receptors are heterogeneously distributed in various areas of the brain, including the CA1 and CA3 regions of the hippocampus, the caudate and accumbens nuclei, or the ventral tegmental area and the substantia nigra (Okuyama et al., 1993; Gronier & Debonnel, 1999; McCann et al., 1994; Monnet et al., 1994; Debonnel et al., 1996b). The CA3 area was therefore chosen since the effects of neuroactive steroids and of the σ ligands tested in the present study had already been assessed in this brain region (Bergeron et al., 1996; Debonnel et al., 1996a).
Preparation of animals
Control female Sprague-Dawley rats were obtained from Charles River, (St-Constant, Québec, Canada) 2–3 days before the electrophysiological experiments. Pregnant females were also obtained 2–3 days before the experiments which were carried out at day 18. Similarly, post-partum female rats were obtained 2–3 days before the period at which the experiments were conducted. Finally, ovariectomized rats were obtained 2 weeks after the surgery, at which time they were implanted with a subcutaneous osmotic minipump (Alza). These rats were treated for 21 days with progesterone (1000 μg kg−1 day−1) or saline. This dose was chosen following the report of the group of Baulieu that a sub-chronic treatment with a dose of 500 μg kg−1 day−1 of progesterone induced plasma levels about 50% lower than those observed in pregnant rats (day 19) (Courpéchot et al., 1993). This long-term treatment was followed by a washout period of 2 or 5 days, at which time the electrophysiological experiments were carried out. Rats were housed one per cage, with free access to food and water. They were maintained at constant temperature (25°C) under a 12 : 12 h light:dark cycle. Animal care was according to the Canadian and McGill University regulations.
For the electrophysiological experiments, rats were anaesthetized with urethane (1.25 g kg−1, i.p.) and mounted on a stereotaxic apparatus. Body temperature was maintained at 37°C throughout the experiments. Extracellular recordings were obtained in the CA3 region, at a depth of 3.5–4.5 mm below the cortical surface in an area defined stereotaxically as 4.2±0.2 mm anterior to lambda and 4.2±0.2 mm lateral to midline (Paxinos & Watson, 1986).
Drugs
The following substances were used: DTG (Aldrich Chemicals, Milwaukee, WI, U.S.A.); NMDA and ACh (Sigma Chemical Co. Ltd, Milwaukee, WI, U.S.A.); QUIS (Tocris Neuramin, Buckburst Hill, Essex, U.K.); haloperidol (McNeil Laboratories, Stouffville, Ontario, Canada); spiperone, (+)-pentazocine, progesterone and DHEA (RBI, Natick, MA, U.S.A.). (+) N-cyclopropylmethyl - N-methyl - 1,4-diphenyl-1-ethyl-butyl-2-N-1-ylamine-hydrochloride (JO-1784) was a generous gift from François Roman (Institut de Recherche Jouveinal, Fresnes, France), NE-100 was a generous gift from Shigcru Okuyama (Taisho Pharmaceutical, Japan).
Preparation of micropipettes
Microionophoretic applications and extracellular unitary recordings were performed with five-barrelled glass micropipettes prepared in a conventional manner (Haigler & Aghajanian, 1974). Three of the side barrels used for microionophoresis were filled with NMDA (10 mM in 200 mM NaCl, pH: 8), QUIS (1.5 mM in 400 mM NaCl, pH: 8) and ACh (20 mM in 200 mM NaCl, pH: 4). The remaining side barrel, used for automatic current balancing, and the central barrel, used for extracellular unitary recordings, were filled with a solution of 2 M of NaCl.
Recording from CA3 dorsal hippocampus pyramidal neurons
Pyramidal neurons were identified by their long duration (0.8–1.5 ms) and large amplitude (0.5–2 mV) action potentials, and by the presence of characteristic complex spike discharges alternating with simple spike activity (Kandel & Spencer, 1961). Neuronal firing activity was monitored on an oscilloscope after signal magnification by a high input-impedance amplifier. Action potentials were detected by a differential amplitude discriminator generating square pulses, which were stored in an on-line computer, and to a paper chart recorder generating integrated firing rate histograms. Alternate microionophoretic applications of 50 s of each excitatory substance (NMDA, QUIS and ACh), separated by 50 s retention periods, were carried out continuously during the duration of the recording. The duration of the microionophoretic applications and the intensity of the currents used were also stored in the computer. The effects of these applications on pyramidal neuron firing activity were expressed as the number of spikes generated nanocoulomb−1 (1 nanoCoulomb (nC) being the charge generated by 1 nA applied for 1 s). After a neuron was isolated, it was recorded for a period of at least 20 min before any drug was injected. The recording was stored on computer without interruption for the whole duration of the experiment. Five to six applications of each excitatory substance were carried out before the σ compounds were injected or applied microionophoretically. The effects of neurosteroids could be assessed only following their intravenous administration, due to their poor water solubility which prevented their use with microionophoretic technics. Effects of the drugs studied appeared within a 10-min period after their intravenous injection. The data were calculated when the maximal effect of the drug was observed (within the first 20 min).
Experimental series
Neuroactive steroids were prepared in a solution of 40% polyethylene glycol. The σ ligands were prepared in physiological saline and were administered via a lateral tail vein. Only one dose of each drug was administered to one rat while recording from one neuron.
In the first series of experiments, DTG, (+)-pentazocine or DHEA were injected intravenously to pregnant rats at day 18.
In the second series of experiments, the effects of intravenous administration of DTG and DHEA were assessed at post-partum days 5, 10 and 15.
In the third series of experiments, the effects of intravenous administration of DTG and DHEA were assessed in rats treated with progesterone for 21 days after a 2-day or 5-day washout. The effects of microionophoretic applications of DTG, JO-1784 and (+)-pentazocine were also measured in these series of rats. Finally, the effect of the intravenous administration of DTG was assessed following the same long-term treatment with progesterone and a 5-day washout period.
Calculations
The computer calculated the effect of each 50-s microionophoretic application of an excitatory substance as the total number of spikes generated nC−1. Each value was calculated by the computer as the mean of the effect of three consecutive applications of the same excitatory substance.
Statistical analyses
All results are expressed as means±s.e.mean of the number of spikes generated nC−1 of NMDA, QUIS or ACh. Statistical significance was assessed using the Student t-test for the degree of potentiation of the NMDA response by the σ ‘agonists.' Variance analyses were used to assess the reduction of the degree of potentiation of the NMDA response induced by progesterone, haloperidol or NE-100. Covariance analyses were used to compare the degree of the potentiation of the NMDA response induced by DTG (1 μg kg−1, i.v.) or by DHEA (100 μg kg−1, i.v.) in control, pregnant and post-partum female rats. Statistical differences smaller than 0.01 were considered as significant.
Results
In all experiments, σ ligands modified neither the spontaneous firing rate of CA3 pyramidal neurons, nor their responses to quisqualate (QUIS) and acetylcholine (ACh) as previously reported (Bergeron et al., 1996). Moreover, the baseline responses to NMDA, QUIS and ACh were altered neither during pregnancy or the post-partum period nor after a 21-day treatment with progesterone (1000 μg kg−1 day−1).
In control female rats, the dose of 1 μg kg−1, i.v. of DTG induced a 3 fold increase of the NMDA response (Figure 1A), whereas the same dose of DTG was completely ineffective at day 18 of pregnancy (Figure 1B). In this latter group of rats, the administration of a dose of 10 μg kg−1, i.v. of DTG induced only a 2 fold increase of the NMDA response (Figures 1B and 2). A dose of (+)-pentazocine 10 fold higher than that used in non-pregnant rats was also required to obtain a similar degree of potentiation of the NMDA response in pregnant rats (Figure 3). These potentiations of the NMDA response, obtained in pregnant rats with a dose of 10 μg kg−1, i.v. of DTG or 100 μg kg−1, i.v. of (+)-pentazocine, were both suppressed by haloperidol (20 μg kg−1, i.v.; Figure 1A), by progesterone (20 μg kg−1, i.v.), but not by spiperone (20 μg kg−1, i.v.; Figure 1A). We have previously reported that, in control rats, following the intravenous administration of doses of DTG higher than 3 μg kg−1, and upon microionophoretic applications of NMDA, the neuronal activity appears to spread to numerous neurons, resulting in an epileptoid activity which renders it impossible to record any unitary activity which is replaced by a ‘field potential' (Bergeron et al., 1995). In pregnant rats, this phenomenon was not observed, even following doses of DTG as high as 10 μg kg−1, i.v.
Figure 1.
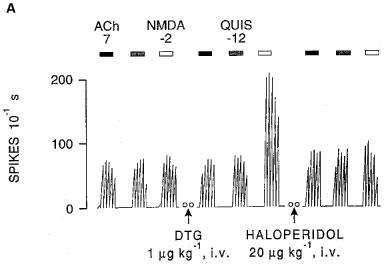
Integrated firing rate histograms from extracellular unitary recordings of CA3 dorsal hippocampus pyramidal neurons showing the effects of microionophoretic applications of NMDA, QUIS and ACh. (A) Recording obtained from a control rat before and after the intravenous administration of DTG, and after that of haloperidol. (B) Recording obtained from a pregnant rat at day 18, before and after the intravenous administration of two doses of DTG, followed by spiperone and haloperidol. Bars indicate the duration of applications for which currents are given in nA. Open circles (oo) indicate a 5-min interruption of the histogram. Time reference applies to both histograms.
Figure 2.
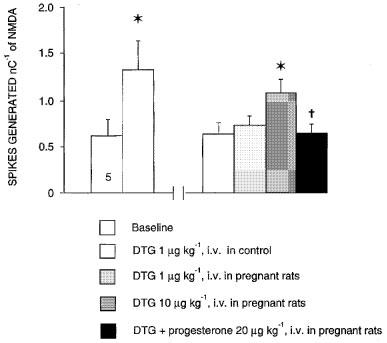
Effects of sigma ligand DTG on the neuronal response to NMDA in control and in 18-day pregnant rats. The responsiveness of CA3 dorsal hippocampus neurons to microionophoretic applications of NMDA is expressed as the number of spikes generated nC−1 (mean±s.e.mean) before and after the intravenous administration of 1 and 10 μg kg−1 of DTG and following the intravenous administration of 20 μg kg−1 of progesterone. The number at the bottom of the first column indicates the number of neurons tested (one neuron per rat in this and subsequent bar chart histograms). *P<0.01, using the Student's t-test, †P<0.01, using variance analysis.
Figure 3.
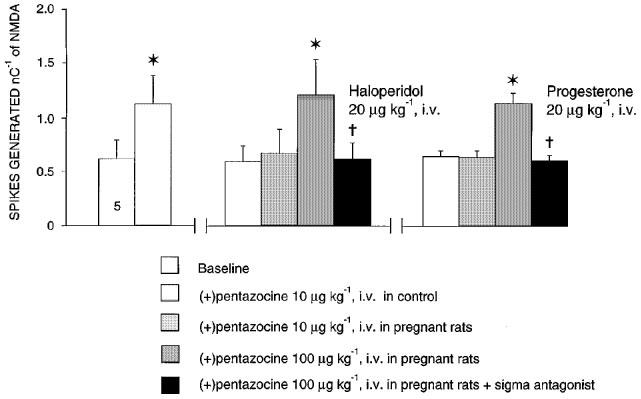
Effects of sigma ligand (+)-pentazocine on the neuronal response to NMDA in control and in 18-day pregnant rats. The responsiveness of CA3 dorsal hippocampus neurons to microionophoretic applications of NMDA is expressed as the number of spikes generated nC−1 (mean±s.e.mean) before and after the intravenous administration of 10 and 100 μg kg−1 of (+)-pentazocine following the intravenous administration of 20 μg kg−1 of haloperidol or 20 μg kg−1 of progesterone. *P<0.01, using the Student's t-test, †P<0.01, using variance analysis.
In keeping with previous results (Bergeron et al., 1996), in control female rats, DHEA induced a selective potentiation of the neuronal response to NMDA at doses as low as 100 μg kg−1, i.v. In pregnant rats, this dose of 100 μg kg−1, i.v. of DHEA was totally ineffective in inducing any potentiation of the NMDA response. A much higher dose (1000 μg kg−1, i.v.) induced a small but significant potentiation of the NMDA response, which was suppressed by the subsequent administration of NE-100 (Figure 4).
Figure 4.
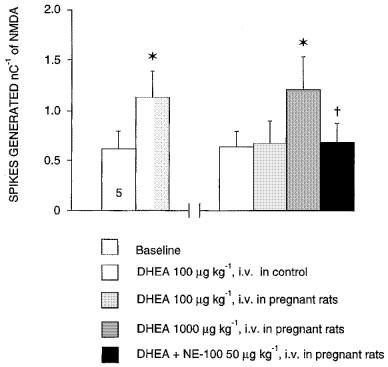
Effects of neuroactive steroids DHEA on the neuronal response to NMDA in control and in 18-day pregnant rats. The responsiveness of CA3 dorsal hippocampus neurons to microionophoretic applications of NMDA is expressed as the number of spikes generated nC−1 (mean±s.e.mean) before and after the intravenous administration of 100 and 1000 μg kg−1 of DHEA following the intravenous administration of 50 μg kg−1 of NE-100. *P<0.01, using the Student's t-test, †P<0.01, using variance analysis.
The effects of DTG, (+)-pentazocine and DHEA were also assessed at day 5 of the post-partum period (Figure 5). At that time, the degree of the potentiation of the NMDA response induced by DTG (1 μg kg−1, i.v.), by (+)-pentazocine (10 μg kg−1, i.v.) and by DHEA (100 μg kg−1, i.v.) was significantly higher than that observed in control female rats (Figure 5). At Days 10 and 15 of the post-partum period, the degree of potentiation of the NMDA response had returned to control values (Figure 5).
Figure 5.

Effect of sigma ligand DTG on the NMDA response in control rats and during the post-partum period. The responsiveness of CA3 dorsal hippocampus neurons to microionophoretic applications of NMDA is expressed as the number of spikes generated nC−1 (mean±s.e.mean) before and after the intravenous administration of 1 μg kg−1 of DTG in control female rats, and in rats at days 5, 10 and 15 post-partum. *P<0.01 using paired Student's t-test, †P<0.001 comparing the effect of DTG at day 5 of the post-partum period to that in control females, with analysis of covariance, using the NMDA response before DTG as the regressor.
In order to assess the potential role of progesterone in these modifications of the neuronal response to sigma ligands, rats obtained 2 weeks after the ovariectomy were treated for 3 weeks with progesterone (1000 μg kg−1 day−1).
Following a 21-day treatment with progesterone (1000 μg kg−1 day−1) and after a 2-day washout, neither the intravenous administration of low doses of DTG, JO-1784 or (+)-pentazocine (data not shown) nor the microionophoretic application of these σ ligands induced any potentiation of the NMDA response (Figure 6A, B and C). Moreover, following a 21-day treatment with progesterone (1000 μg kg−1 day−1) and after a 2-day washout, the intravenous administration of a low dose of DHEA failed to potentiate the NMDA response (Figure 6D).
Figure 6.
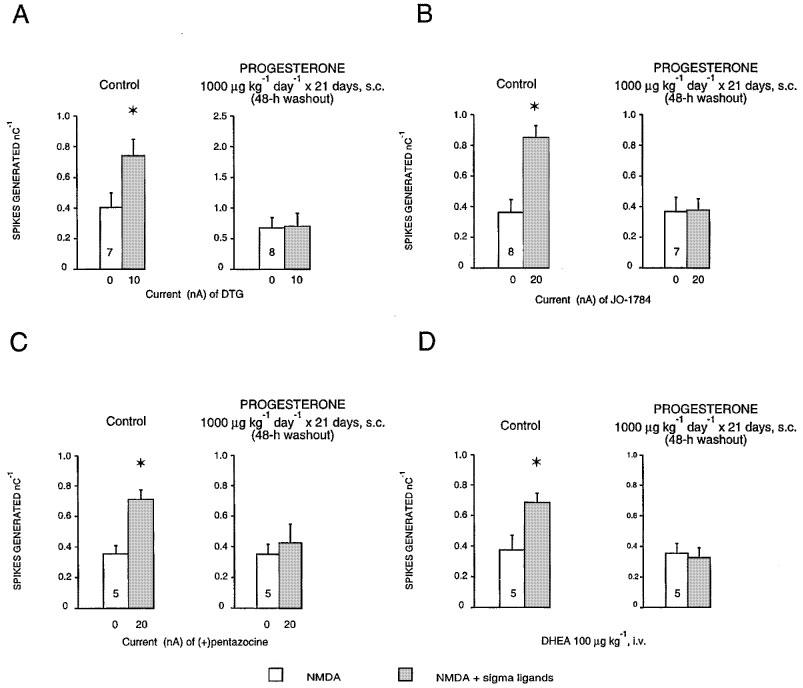
Responsiveness expressed as the number of spikes generated per nanocoulomb (mean±s.e.mean) of CA3 dorsal hippocampus neurones to microionophoretic applications of NMDA before and after the microionophoretic applications of DTG (A), JO-1784 (B), (+)-pentazocine (C) and the i.v. administration of DHEA (D), in control and in rats treated with 1000 μg kg−1 day−1 of progesterone for 21 days after a 2-day washout. The number at the bottom of the first column indicates the number of neurons tested. *P<0.01, using the Student's t-test.
Following a 21-day treatment with progesterone (1000 μg kg−1 day−1) and after a 5-day washout, the intravenous administration of DTG induced a potentiation of the NMDA response similar to that obtained in the control rats. This potentiation was suppressed by the subsequent administration of haloperidol (20 μg kg−1, i.v.) (Figure 7).
Figure 7.

Responsiveness expressed as the number of spikes generated per nanocoulomb (mean±s.e.mean) of CA3 dorsal hippocampus neurones to microionophoretic applications of NMDA before, after the intravenous administration of DTG, and after the intravenous administration of haloperidol in control rats (A), or in treated rats with 1000 μg kg−1 of progesterone for 21 days after a 5-day washout (B).
Discussion
In agreement with several reports from different laboratories, our results suggest a functional interaction between neuroactive steroids, σ ligands and NMDA receptors (Monnet et al., 1990; Walker & Hunter, 1994; Bergeron et al., 1995; Nabeshima & Okuyama, 1994; Maurice et al., 1996; 1997; Su et al., 1988; 1989; McCann & Su, 1992). Moreover, the present data confirm that, in control rats, low doses of DTG, (+)-pentazocine and DHEA potentiate selectively the neuronal response to microionophoretic applications of NMDA on pyramidal neurons in the CA3 region of the rat dorsal hippocampus. Our results also show that, in late pregnancy, the potentiation of the NMDA response induced by the neuroactive steroid DHEA and by selective σ ‘agonists' is markedly reduced (Figures 1, 2, 3, 4): ten times higher doses of these σ ‘agonists' were required to produce a similar degree of potentiation of the NMDA response. This potentiation was reversed by the σ ‘antagonists' NE-100 and haloperidol, and also by progesterone, but not by the non-sigma receptor D2 antagonist spiperone. Early during post-partum, the degree of potentiation of the NMDA response, induced by low doses of DTG, (+)-pentazocine and DHEA, was significantly increased. The degree of potentiation of the NMDA response was similar to that obtained in control rats at days 10 and 15 of post-partum (Figure 5). Similarly, a 3-week treatment with progesterone (1000 μg kg−1 day−1) also reduced markedly the effects of the σ ligands DTG, (+) pentazocine, JO-1784 and of DHEA (Figures 6 and 7).
In the last decade, the search for an endogenous ligand at the σ receptors has been an area of intense research. Su and co-workers (Su et al., 1988; McCann et al., 1994) have shown that several steroids, particularly progesterone and testosterone, can displace [3H]-(+)SKF-10,047 and [3H]-haloperidol binding to σ receptors. They have hypothesized that progesterone might be an endogenous ligand of σ receptors (Su et al., 1988; McCann et al., 1994). Schwarz and co-workers challenged this hypothesis and argued that the concentration of endogenous progesterone was insufficient to occupy the σ receptors in the brain, even in late pregnancy (Schwarz et al., 1989). However, the present observations suggest that the lack of potentiating effects by low doses of σ ‘agonists' in late pregnancy is related to the occupation of σ receptors in the CNS by progesterone. First, it is known that progesterone levels in the periphery increase 6 fold in late pregnancy and that CNS levels parallel the peripheral ones (Pepe & Rothchild, 1974). Second, we have previously demonstrated that, in control rats, progesterone is a very potent ‘antagonist' at σ receptors, as indicated by the observation that low doses of this neuroactive steroid prevent and suppress the potentiation of the NMDA response induced by selective σ ‘agonists' (Bergeron et al., 1996). Third, the present results indicate that, in late pregnancy, ten-times higher doses of DTG, (+)-pentazocine and DHEA are required to induce a selective potentiation of the NMDA response. Fourth, the potentiation of the NMDA response obtained with higher doses of σ ‘agonists' is reversed by progesterone, NE-100 and haloperidol, but not by spiperone. Fifth, this notion is further supported by the fact that early in post-partum, the potentiation of the NMDA response induced by low doses of σ ‘agonists' is even greater than in control female rats. Finally, our results are in keeping with a report showing a reduced number of (+)-[3H]SKF-10,047 binding sites, both in pregnant rats and following the administration of a high dose of progesterone (Maurice et al., 1996).
We have previously reported that, in control rats, epileptoid activity is consistently induced by microionophoretic applications of NMDA following the intravenous administration of doses of DTG higher than 3 μg kg−1 or by the combined administration of σ1 and σ2 selective ligands (Bergeron et al., 1995; Couture & Debonnel, 1998). In our paradigm, DTG is thus far the only σ ligand producing this phenomenon. In the series of experiments undertaken in late pregnancy, doses of DTG as high as 10 μg kg−1, i.v. did not induce this phenomenon. Certain derivatives of progesterone have CNS depressant properties since they decrease brain excitability and increase seizure threshold (Backstrom et al., 1985). The failure of 10 μg kg−1, i.v. of DTG to promote NMDA-induced epileptoid activity in late pregnancy is probably due to the ‘antagonistic' action of progesterone at σ receptors. Moreover, the fact that the baseline NMDA responses were unchanged in pregnancy and post-partum strongly suggests that the phenomenon observed here is related to σ receptors, and not to an alteration of the function of the NMDA receptor complex itself.
The abrupt fall in progesterone concentration during post-partum appears to induce an enhanced responsiveness to the administration of σ ligands, since the doses of 1 μg kg−1, i.v. of DTG, 10 μg kg−1, i.v. of (+)-pentazocine and 100 μg kg−1, i.v. of DHEA produced a greater potentiation of the NMDA response than in control female rats (Figure 5). This potentiation of the NMDA response was back to normal at days 10 and 15 of post-partum (Figure 5), suggesting that the supersensitivity of the σ receptors, induced by the rapid drop of progesterone levels after delivery, is transient.
We have previously reported that long-term treatments with σ ‘antagonists' produce a desensitization, whereas long-term treatments with σ ‘agonists' induce a supersensitivity of the σ receptors (Bergeron et al., 1997). As illustrated in Figures 6 and 7, no potentiation of the NMDA response was induced by the intravenous administration of DHEA, nor by microionophoretic applications of DTG, JO-1784 or (+)-pentazocine in rats treated with progesterone for 21 days at the dose of 1000 μg kg−1 day−1 after a 2-day washout. These results might appear surprising since early in post-partum we observed a hypersensitivity of the σ receptors. This might be explained by the higher levels of progesterone in the group of rats treated with the minipumps, compared to the group of rats in post-partum. There is also a possibility that the lack of potentiation of the NMDA response by the acute administration of σ ‘agonists' could have been due to residual progesterone despite a 2-day washout. When the electrophysiological experiments were carried out in rats treated with progesterone at the same dose (1000 μg kg−1 day−1 for 21 days) following a 5-day washout, the potentiation of the NMDA response by σ ‘agonists' was back to normal (Figure 7). This contrasts with the observation of a greater effect of σ ligands on the NMDA response in the post-partum period (at day 5). Thus, the supersensitivity of σ receptors observed during post-partum (at day 5) might not be due solely to the rapid fall of progesterone levels but also to the alternations of the levels of one of the numerous neuroactive steroids.
For many years, the potential role of σ receptors in some psychiatric disorders, including depression, has been evoked. More recently, clinical data have suggested an antidepressant effect of a selective σ ligand (Pande et al., 1998). The alterations of σ receptor function during pregnancy and post-partum might thus be associated with some of the psychological changes often occurring in women during these periods. This certainly warrants further investigation.
Acknowledgments
This work was supported by the Medical Research Council of Canada (Grant MA-6444 to C. de Montigny) and by the Fonds de la Recherche en Santé du Québec (F.R.S.Q.). G. Debonnel was in receipt of a Scholarship from the F.R.S.Q. and R. Bergeron was in receipt of a Fellowship from the F.R.S.Q. We would like to thank C. Bouchard for the preparation of the Figures, Y. Simard for technical advice, L. Martin, H. Cameron and G. Corpadean for secretarial assistance.
Abbreviations
- ACh
Acetylcholine
- DHEA
Dehydroepiandrosterone
- DTG
Di(2-tolyl)guanine
- JO-1784
(+)N-cyclopropylmethyl-N-methyl-1,4-diphenyl-1-ethyl-butyl-2-N-1-ylamine-hydrochloride
- nC
nanoCoulomb
- NE-100
N-di-propyl-2-(4-methoxy-3(2-phenylethoxy)phenyl)-ethylamine monohydrochloride
- NMDA
N-methyl-D-aspartate
- QUIS
Quisqualate
- (+)SKF-10,047
N-allyl-normetazocine
References
- BACKSTROM T., BIXO M., HAMMARBACK S. Ovarian steroid hormones. Effects on mood, behaviour and brain excitability. Acta Obstet. Gynecologica Scand. 1985;130:19–24. doi: 10.3109/00016348509157142. [DOI] [PubMed] [Google Scholar]
- BERGERON R., DE MONTIGNY C., DEBONNEL G. Biphasic effects of sigma ligands on the neuronal response to N-methyl-D-aspartate. Naunyn Schmiedebergs Arch. Pharmacol. 1995;351:252–260. doi: 10.1007/BF00233244. [DOI] [PubMed] [Google Scholar]
- BERGERON R., DE MONTIGNY C., DEBONNEL G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: Effects mediated via sigma receptors. J. Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGERON R., DE MONTIGNY C., DEBONNEL G. Effect of short-term and long-term treatments with σ ligands on the N-methyl-D-aspartate response in the CA3 region of the rat dorsal hippocampus. Br. J. Pharmacol. 1997;120:1351–1359. doi: 10.1038/sj.bjp.0701042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAKI S., TANAKA M., MURAMATSU M., OTOMO S. NE-100, a novel potent σ ligand, preferentially binds to σ1 binding sites in guinea pig brain. Eur. J. Pharmacol. 1994;251:R1–R2. doi: 10.1016/0014-2999(94)90453-7. [DOI] [PubMed] [Google Scholar]
- COURPÉCHOT J., YOUNG J., CALVEL M., WEHREEY C., VELTZ J.N., TOUYER G., MOUREN M., PRRASAD V.V.K., BANNER C., SJOVALL J., BAULIEU E.E., ROBEL P. Neurosteroids: 3a-hydroxy-5a-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- COUTURE S., DEBONNEL G. Modulation of the neuronal response to N-methyl-D-Aspartate by selective sigma2 ligands. Synapse. 1998;29:62–71. doi: 10.1002/(SICI)1098-2396(199805)29:1<62::AID-SYN5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- DEBONNEL G., BERGERON R., DE MONTIGNY C. Potentiation by dehydroepiandrosterone of the neuronal response to N-methyl-D-aspartate in the CA3 region of the rat dorsal hippocampus: An effect mediated via sigma receptors. J. Endocrinol. 1996a;150:S33–S42. [PubMed] [Google Scholar]
- DEBONNEL G., BERGERON R., MONNET F.P., DE MONTIGNY C. Differential effects of sigma ligands on the N-methyl-D-aspartate response in the CA1 and CA3 regions of the dorsal hippocampus: Effect of mossy fiber lesioning. Neuroscience. 1996b;71:977–987. doi: 10.1016/0306-4522(96)80001-7. [DOI] [PubMed] [Google Scholar]
- GRONIER B., DEBONNEL G. Involvement of σ receptors in the modulation of the glutamatergic/NMDA neurotransmission in the dopaminergic systems. Eur. J. Pharmacol. 1999;368:183–196. doi: 10.1016/s0014-2999(99)00025-4. [DOI] [PubMed] [Google Scholar]
- HAIGLER H.J., AGHAJANIAN G.K. Lysergic acid diethylamide and serotonin: A comparison of effects on serotonergic neurons receiving a serotonergic input. J. Pharmacol. Exp. Ther. 1974;168:688–699. [PubMed] [Google Scholar]
- KANDEL E.R., SPENCER W.A. Electrophysiology of hippocampal neurons. II. After potentials and repetitive firing. J. Neurophysiol. 1961;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- MAJEWSKA M.D. Steroids and brain activity. Essential dialogue between body and mind. Biochem. Pharmacol. 1987;36:3781–3788. doi: 10.1016/0006-2952(87)90437-0. [DOI] [PubMed] [Google Scholar]
- MARTIN W.R., EADES C.G., THOMPSON J.A., HUPPLER R.E., GILBERT P.E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- MAURICE T., JUNIEN J.L., PRIVAT A. Dehydroepiandrosterone sulfate attenuates dizocilpine-induced learning impairment in mice via σ1-receptors. Behav. Brain Res. 1997;83:159–164. doi: 10.1016/s0166-4328(97)86061-5. [DOI] [PubMed] [Google Scholar]
- MAURICE T., ROMAN F.J., PRIVAT A. Modulation by neurosteroids of the in vivo (+)-[3H]SKF-10,047 binding to σ1-receptors in the mouse forebrain. J. Neurosci. Res. 1996;46:734–743. doi: 10.1002/(SICI)1097-4547(19961215)46:6<734::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- MCCANN D.J., SU T.-P. Tris inhibits (+)-[3H]SKF-10,047 binding to σ receptors. Neurosci. Lett. 1992;141:239–242. doi: 10.1016/0304-3940(92)90903-k. [DOI] [PubMed] [Google Scholar]
- MCCANN D.J., WEISSMAN A.D., SU T.-P. Sigma-1 and sigma-2 sites in rat brain: Comparison of regional, ontogenetic, and subcellular patterns. Synapse. 1994;17:182–189. doi: 10.1002/syn.890170307. [DOI] [PubMed] [Google Scholar]
- MOEBIUS F.F., BURROWS G.G., HANNER M., SCHMID E., STRIESSNIG J., GLOSSMANN H. Identification of a 27-kDa high affinity phenylalkylamine- binding polypeptide as the σ1 binding site by photoaffinity labeling and ligand-directed antibodies. Mol. Pharmacol. 1993;44:966–971. [PubMed] [Google Scholar]
- MONNET F.P., DEBONNEL G., BERGERON R., GRONIER B., DE MONTIGNY C. The effects of sigma ligands and of neuropeptide Y on N-methyl-D-aspartate-induced neuronal activation of CA3 dorsal hippocampus neurones are differentially affected by pertussis toxin. Br. J. Pharmacol. 1994;112:709–715. doi: 10.1111/j.1476-5381.1994.tb13134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONNET F.P., DEBONNEL G., DE MONTIGNY C. In vivo electrophysiological evidence for a selective modulation of N-methyl-D-aspartate-induced neuronal activation in rat CA3 dorsal hippocampus by sigma ligands. J. Pharmacol. Exp. Ther. 1992;261:123–130. [PubMed] [Google Scholar]
- MONNET F.P., DEBONNEL G., JUNIEN J.L., DE MONTIGNY C. N-methyl-D-aspartate-induced neuronal activation is selectively modulated by sigma receptors. Eur. J. Pharmacol. 1990;179:441–445. doi: 10.1016/0014-2999(90)90186-a. [DOI] [PubMed] [Google Scholar]
- NABESHIMA T., OKUYAMA S. Physiological function of sigma receptors: Central pharmacological effects of sigma ligands. Jap. J. Pharmacol. 1994;14:51–76. [PubMed] [Google Scholar]
- OKUYAMA S., IMAGAWA Y., OGAWA S., ARAKI H., AJIMA A., TANAKA M., MURAMATSU M., NAKAZATO A., YAMAGUCHI K., YOSHIDA M., OTOMO S. NE-100, a novel sigma receptor ligand: In vivo tests. Life Sci. 1993;53:PL285–PL290. doi: 10.1016/0024-3205(93)90588-t. [DOI] [PubMed] [Google Scholar]
- PANDE A., GENEVE J., SCHERRER B. Igmesine, a novel sigma ligand, has antidepressant properties. Int. J. Neuropsychopharmacol. 1998;1 Sup. 1:S8. [Google Scholar]
- PAUL S.M., PURDY R.H. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- PAXINOS G., WATSON C.The Rat Brain in Stereotaxic Coordinates 1986San Diego, CA: Academic Press; 2 Ed [Google Scholar]
- PEPE G.J., ROTHCHILD I. A comparative study of serum progesterone levels in pregnancy and in various types of pseudopregnancy in the rat. Endocrinology. 1974;95:275–279. doi: 10.1210/endo-95-1-275. [DOI] [PubMed] [Google Scholar]
- SCHWARZ S., POHL P., ZHOU G.Z. Steroid binding at sigma-opioid receptors. Science. 1989;246:1635–1637. doi: 10.1126/science.2556797. [DOI] [PubMed] [Google Scholar]
- SNYDER S.H., LARGENT B.L. Receptor mechanisms in antipsychotic drug action: Focus on receptors. J. Neuropsychiatry. 1989;1:7–15. doi: 10.1176/jnp.1.1.7. [DOI] [PubMed] [Google Scholar]
- STEINFELS G.F., ALBERICI G.P., TAM S.W., COOK L. Biochemical, behavioral, and electrophysiologic actions of the selective sigma receptor ligand (+)pentazocine. Neuropsychopharmacology. 1988;1:321–327. [PubMed] [Google Scholar]
- SU T.P., LONDON E.D., JAFFE J.H. Steroid binding at σ receptors suggests a link between endocrine, nervous and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- SU T.P., LONDON E.D., JAFFE J.H. Steroid binding at sigma-opioid receptors. Science. 1989;246:1637–1638. doi: 10.1126/science.246.4937.1637. [DOI] [PubMed] [Google Scholar]
- TAM S.W., COOK L. Sigma opiates and certain antipsychotic drugs mutually inhibit (+)-[3H]SKF 10047 and [3H]haloperidol binding in guinea pig brain membranes. Proc. Natl. Acad. Sci. USA. 1984;81:5618–5621. doi: 10.1073/pnas.81.17.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER J.M., HUNTER W.S. Role of sigma receptors in motor and limbic system function. Neuropsychopharmacology. 1994;10:837s–837s. [Google Scholar]
- WEBER E., SONDERS M., QUARUM M., MCLEAN S., POU S., KEANA J.F.W. 1,3-Di(2-[5-3H]tolyl)guanidine: A selective ligand that labels sigma-type receptors for psychotomimetic opiates and antipsychotic drugs. Proc. Natl. Acad. Sci. USA. 1986;83:8784–8788. doi: 10.1073/pnas.83.22.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]


