Abstract
Bovine pulmonary artery endothelium (CPAE) expresses phospholipase C (PLC)-linked P2Y1 and P2Y2 receptors, for them 2-methylthio-ATP (2MeSATP) and UTP are respective agonists. Here, we have investigated the particular protein kinase C (PKC) isoform(s) responsible for the inhibition of P2Y1 and P2Y2 receptor-evoked inositol phosphate (IP) formation by phorbol 12-myristate 13-acetate (PMA).
Although short-term (20 min) pretreatment of cells with PMA attenuated 2MeSATP- and UTP-induced phosphoinositide (PI) breakdown, this inhibition was lost after 15 h. Preincubation with PMA for 24 h, on the contrary, potentiated 2MeSATP and UTP responses. The IP formation stimulated by NaF was unaltered by PMA pretreatment.
Western blot analysis showed that treatment of CPAE with PMA resulted in a rapid translocation of PKC isoform βI, ε and μ, but not λ, from the cytosol to the membrane fraction.
Pretreatment of the selective PKC inhibitor Ro 31-8220 attenuated the inhibitory effect of PMA on IP formation. Go 6976 (an inhibitor of conventional PKCα, β and γ) and LY 379196 (a selective PKCβ inhibitor) also dose-dependently inhibited the PMA-mediated desensitization.
Transfection of PKCβ-specific antisense oligonucleotide reduced PKCβI protein level and inhibited PMA-mediated PI reduction.
RT–PCR analysis showed that PMA treatment for 4–24 h up-regulated P2Y1 and P2Y2 receptors at the mRNA levels.
These results suggest that PKCβI may exert a negative feedback regulation on endothelial P2Y1 and P2Y2 receptor-mediated PI turnover. The down-regulation of PKCβI and enhanced P2Y receptor expression together might contribute to the late PI enhancing effect of PMA.
Keywords: UTP, 2MeSATP, P2 receptors, PKCβI, PI turnover, receptor up-regulation, endothelium
Introduction
Several nucleotides, such as ATP and UTP, exert a widespread influence on cellular function by acting on a variety of cell surface P2 receptors. ATP is a neurotransmitter which can mediate synaptic transmission in the central and peripheral nervous systems (Edwards et al., 1992; Evans et al., 1992). In addition to its release during neurotransmission, ATP is thought to accumulate at micromolar concentration in the extracellular fluid at sites of inflammation and tissue injury (Born & Kratzer, 1984; Osipchuk & Cahalan, 1992). Recent study has shown that ATP and UTP can be released from platelets (Boarder & Hourani, 1998) and cells upon mechanical stimulation (Lazarowski et al, 1997). So far two large families of P2 receptors activated by ATP and other nucleotides have been cloned (Boarder et al., 1995; Communi & Boeynaems, 1997; Fredholm et al., 1997; North & Barnard, 1997; Boarder & Hourani, 1998). Ionotropic P2X receptors consisting of at least seven subtypes, P2X1–P2X7, are ATP-gated cation channels. Metabotropic P2Y receptors form a distinct subset of G protein-coupled receptors; most of which coupling to phospholipase C (PLC).
It is well established that ATP and other nucleotides released from platelets and vascular smooth muscle cells act on endothelial P2 receptors and lead to the synthesis and release of prostacyclin and nitric oxide (NO) (Boeynaems & Pearson, 1990; Brown et al., 1996; Patel et al., 1996; Boarder & Hourani, 1998; Li et al., 1998), thereby resulting in a relaxant and antiproliferative influence on vascular smooth muscle cells, and attenuating further platelet activation (Boarder & Hourani, 1998). In addition to acute effects on prostacyclin and NO synthesis, it was shown that ATP can increase in endothelial cells several biochemical responses that are associated with a mitogenic action (Darbon et al., 1986; Boutherin-Falson et al., 1990; Pirotton et al., 1990; Van Daele et al., 1992). Subsequent studies further characterized the co-presence of P2Y1 and P2Y2 receptors in endothelial cells, which activated by 2-methylthio-ATP (2MeSATP) and UTP, respectively (Communi et al., 1995; Chen et al., 1996). Engagement of both P2Y receptors leads to the activation of PLC, which subsequently cleaves phosphatidylinositol bisphosphate into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 and DAG are two secondary messengers respectively responsible for the increase in intracellular Ca2+ ([Ca2+]i) and activation of protein kinase C (PKC).
Several studies have demonstrated that activation of PKC inhibits G protein-coupled receptor-mediated hydrolysis of phosphoinositide (PI) turnover (Lin & Chuang, 1992; Nishizuka, 1992) and subsequent increases in [Ca2+]i (Levesque et al., 1997), thus PKC activation represents the negative-regulatory arm of receptor mediated-IP3 generation (Communi et al., 1995; Chen et al., 1996). PKC consists of at least 12 isoforms that vary in their requirements for lipid or Ca2+ for activation and are differentially expressed in various cell types (Mochly-Rosen & Gordon 1998). The recent development of some isozyme-specific inhibitors (Martiny-Baron et al., 1993; Lin & Chen, 1998), and the use of technology such as RNA antisense to block expression of individual isozymes (Xu & Ware, 1995) is beginning to allow for the assignment of specific functions of each PKC isozyme. In this study, we investigated the time-dependent effect of PKC activation on P2Y receptor-induced PI turnover in endothelial cells and explored the involvement of specific PKC isoform in this PKC-dependent regulatory process.
Methods
Cell culture
Bovine pulmonary artery endothelium (CPAE) cells obtained from the American Type Culture Collection were grown as monolayer cultures in minimum essential medium (MEM) supplemented with 10% foetal bovine serum at 37°C, in a humidified atmosphere of 95% air/5% CO2. Cells were passaged with 0.25% trypsin/1 mM EDTA.
Measurement of PI turnover
PI hydrolysis was measured by the accumulation of inositol phosphates (IP) in the presence of 10 mM LiCl, as described previously (Lin & Chuang, 1992). Confluent cells on 35-mm Petri dishes were labelled with [3H]-myoinositol (2.5 μCi dish−1) in the growth medium for 24 h, washed with physiological saline solution (in mM): NaCl 118, KCl 4.7, CaCl2 1.8, MgCl2 1.2, KH2PO4 1.2, glucose 11 and HEPES 20, pH 7.4, containing 10 mM LiCl and incubated at 37°C for 20 min. After this preincubation, the indicated drugs were added and incubation continued for another 30 min. The reaction was terminated by aspiration of the reaction solution and addition of ice-cold methanol. The cells were scraped off and the [3H]-IP was isolated with an AG-1X8 column (formate form, 100–200 mesh), eluated with 0.2 N ammonium formate/0.1 N formic acid and counted by β-counter. The agonist-elicited [3H]-IP accumulation was expressed as percentages of the basal [3H]-IP level (per cent of control).
Western blotting analysis
CPAE cells treated with stimuli as indicated were washed twice in ice-cold PBS and immediately placed on ice to stop the reactions. The cells were scraped from the plates in buffer A (in mM): Tris-HCl 20, EGTA 0.5, EDTA 2, DDT 2, p-methylsulphonyl fluoride 0.5 and 10 μg ml−1 leupeptin, pH 7.5, transferred to microfuge tubes and sonicated. For PKC translocation, the lysates were separated into cytosolic and membrane fractions by centrifugation at 40,000×g for 45 min. Samples of equal amount of protein (50–100 μg) were subjected to SDS–PAGE on 9% polyacrylamide gels, then transferred onto a nitrocellulose membrane, which was then incubated in TBST buffer (NaCl 150 mM, Tris 20 mM, 0.1% Tween 20, pH 7.4) containing 1% milk for overnight at 4°C, washed with TBST buffer, and incubated with PKC isoform-specific primary antibodies for 1.5 h. After further washing, the blots were incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibody for 1 h. After further washing, the blots were processed for visualization using ECL, following the manufacturer's instructions. Densitometrical analyses were performed on a Molecular Dynamics densitometer.
Antisense oligonucleotide synthesis and treatment of cells with oligonucleotides
Phosphorothioate oligodeoxynucleotides were synthesized on a PS 250 CRUACHEM DNA synthesizer, using the cyanoethyl phosphoroamidate method, and purifed by gel filtration. The PKCβ antisense and sense sequences were derived from the published coding sequences of the PKCβ isoform DNA (Ohno et al., 1987). Sequences of antisense PKCβ nucleotide used were 5′-GGA CCC CCC CCG TCG ATG-3′ and sense PKCβ nucleotide used were 5′-CAT CGA CGG GGG GGG TCC-3′. CPAE cells were cultured in 35-mm dishes with growth medium, after 24 h, the medium was changed to serum-free Opti MEM containing Lipofectamine™ (Gibco Laboratories). Oligonucleotides were then added at the required concentration and well-mixed with medium by swirling the dish. The cells were incubated at 37°C for 4 h, washed once with MEM to remove Lipofectamine™, and then the cells were incubated with growth medium for 48 h. Immunoreactivity of PKCβ and PI turnover were performed as mentioned above.
Reverse transcription-polymerase chain reaction (RT–PCR)
To amplify endothelial P2Y receptor subtypes, the specific primers were synthesized. The purinoceptor primers used were: P2Y1 receptor sense (661–680), 5′-ACG ACT GTG GCC ATG TTC TG-3′ and antisense (1051–1070), 5′-ATT TCT TCA CTC TTG GAT TG-3′; P2Y2 receptor sense (31–50), 5′-ACC ATC AAT GGC ACC TGG GA-3′ and antisense (374–393), 5′-CCG GTG CAC GCT GAT GCA GG-3′; P2Y4 receptor sense, 5′-CAC CGA TAC CTG GGT ATC TG-3′ and antisense, 5′-CAG ACA GCA AAG ACA GTC AG-3′ (Webb et al., 1996); P2Y6 receptor sense (315–334), 5′-GCT TCC TCT TCT ATG CCA AC-3′ and antisense (779–798), 5′-GTA GGC TGT CTT GGT GAT GT-3′; P2Y11 receptor sense (94–113), 5′-CTG GTG GTT GAG TTC CTG GT-3′ and antisense (308–327), 5′-GTT GCA GGT GAA GAG GAA GC-3′. β-actin mRNA levels were used as internal controls. The β-actin primers used were: sense (613–632), 5′-GAC TAC CTC ATG AAG ATC CT-3′ and antisense (1103–1122), 5′-CCA CAT CTG CTG GAA GGT GG-3′. Confluent cells, grown in 10 cm Petri dishes, were treated with PMA for different periods, then harvested. The total RNA was purified using RNAzol reagent, and RT–PCR carried out using a RNA PCR kit (Gibco), according to the manufacturer's instructions, using 10 μg of total RNA as a template. Equal amounts (1 μg of cDNA) of each RT product were PCR-amplified with Taq polymerase in 35 cycles consisting of 40 s at 95°C, 40 s at 48°C (for P2Y1 receptor), 54°C (for P2Y2 receptor), 55°C (for P2Y4 and P2Y6 receptors), 57°C (for P2Y11 receptor) or 53°C (for β-actin) and 2 min at 72°C. The amplified cDNA was run on 1% agarose gels and visualized by ethidium bromide. The time-dependent effect of P2Y receptor expression after phorbol 12-myristate 13-acetate (PMA) treatment was reflected by the changes in the amount of PCR products deriving from P2Y receptor mRNA. The RT samples were also used to generate β-actin PCR products and their amount was considered as internal control.
Chemicals
MEM, foetal bovine serum, and 0.25% trypsin/1 mM EDTA were obtained from Gibco BRL (Grand Island, NY, U.S.A.). [3H]-myoinositol (20 Ci mmol−1) was purchased from New England Nuclear (Boston, MA, U.S.A.). 2MeSATP was from RBI (Natick, MA, U.S.A.). Go 6976 (12-(2-cyanoethyl)-6,7,12,13 -tetrahydro -13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole) and Ro 31-8220 {3-[1-[3-(Amidinothio)propyl - 1H-indol-3-yl] -3 -(1-methyl-1H-indol-3-yl)-maleimide-methane sulphonate} were purchased from Calbiochem (La Jolla, CA, U.S.A.). LY 379196 was a generous gift from Eli Lilly (Indianapolis, IN, U.S.A.). Monoclonal antibodies of PKC ε, λ, and μ were purchased from Transduction Laboratories (Lexington, KY, U.S.A.) and polyclonal antibody of PKCβI was from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Horseradish peroxidase-conjugated goat anti-mouse and sheep anti-rabbit antibodies were purchased from Amersham Pharmacia Biotech. All the materials for SDS–PAGE were obtained from Bio-Rad Laboratories (Hercules, CA, U.S.A.). Other chemicals were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.).
Statistical analysis
Each experiment was performed in duplicate, and data represent the mean±s.e.mean from at least three independent experiments. P<0.05 was considered significant by evaluation of the data with analysis of Variance (ANOVA) and/or Dunnetts test. The error bar was omitted when it was within the symbol representing the mean value.
Results
Effects of PMA on 2MeSATP- and UTP-induced PI responses
CPAE cells stimulated with 2MeSATP and UTP, each at 100 μM, for 30 min resulted in increases in IP accumulation by 766±52% (n=6) and 700±50% (n=6) of control, respectively. The basal level of IP accumulation was 56±4 c.p.m. dish−1 (n=18). As shown in Figure 1, the time course for modulation of PI response to 2MeSATP or UTP by PMA (1 μM) was clearly biphasic. Short-term (10 min) pretreatment of cells with PMA (1 μM) inhibited the PI responses to 2MeSATP and UTP by 56±4% (n=3) and 32±1% (n=3), respectively. Extending the period of pretreatment to 2 h, the inhibitory effects of PMA exhibited the greatest degree, with 62 and 36% inhibition respectively for 2MeSATP and UTP responses. At 4 and 8 h after pretreatment, the inhibitory effect of PMA was a little diminished. However, with an extended PMA preincubation time of 24 h, a marked potentiation of the PI response occurred, with 149±8% (n=3) and 137±8% (n=3) of control for 2MeSATP and UTP, respectively. PMA alone had no effect on the basal level of IP accumulation at any treatment time (data not shown). To explore whether the downstream signalling of Gq protein activation is the site of PMA action, its effect on PI turnover induced by NaF, a direct activator of GTP-binding proteins, was further investigated. We found that NaF-induced PI hydrolysis was unaffected by 10 min or 24 h treatment with PMA at concentrations up to 1 μM (data not shown).
Figure 1.
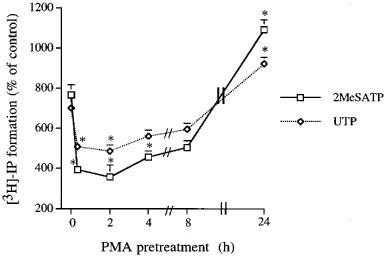
Time-dependent effects of PMA on 2MeSATP- and UTP-induced [3H]-IP formation in CPAE cells. Cells were pretreated with PMA (1 μM) for different periods of time before challenge with 100 μM 2MeSATP (□) or 100 μM UTP (⋄). Results are expressed as the mean±s.e.mean of three independent experiments. *Using ANOVA followed by Dunnetts test, P<0.05 was considered significant as compared to the 2MeSATP- or UTP-induced PI turnover without PMA pretreatment.
PMA-induced PKC isoform translocation
In our previous study, we identified CPAE cells for the presence of PKC isoforms βI, ε, λ, and μ. Moreover, we found that, when CPAE cells treated with 1 μM PMA up to 24 h, the immunoreactivities of βI and ε were time-dependently down-regulated, while those of λ and μ were unchanged (Chen et al., 1999). Here to determine which PKC isoform(s) rapidly activated by PMA responsible for its inhibitory effect on P2 receptor-mediated PI turnover, short-term treatment with PMA was carried out. As shown in Figure 2, translocation of PKCβI, ε, and μ, but not λ, from the cytosol to the membrane fraction occurred within 10 min addition of PMA. The membrane immunoreactivities of PKCβI, ε, and μ were respectively increased by 134±8, 76±1 and 58±7% (n=3) after 10 min treatment with PMA. These results together with the rapid attenuation of PI responses of P2 receptor agonists suggest the involvement of at least one of these three PKC isoforms in the desensitization of P2 receptor signalling.
Figure 2.
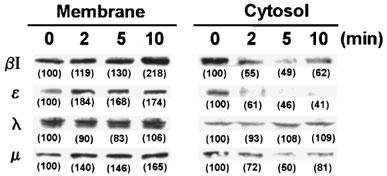
Effect of PMA treatment on the translocation of PKCβI, ε, λ and μ in CPAE cells. Cells were treated with 1 μM PMA for different time (2, 5, and 10 min) and then fractionated into the cytosolic and membrane fractions. Equal amount of protein was separated by 9% SDS–PAGE, transferred to nitrocellulose membrane, and immunoblotted with antibodies specific for PKCβI, ε, λ and μ, as described in ‘Methods'. The values in the parentheses indicate the changes of PKC immunoreactivities which were expressed as percentages of control. The results are representative of three experiments.
PKCβ-dependent inhibition of IP response
We next examined the effects of three PKC inhibitors on PMA-induced PI inhibition. When CAPE cells were preincubated with a non-selective PKC inhibitor, Ro 31-8220 (1 μM) (Beltman et al., 1996), the inhibitory effects of PMA on 2MeSATP- and UTP-stimulated PI responses were abolished (data not shown). Go 6976, a selective inhibitor of conventional PKCα, β, and γ (Martiny-Baron et al., 1993) also reversed the inhibitory effects of PMA on P2Y1 and P2Y2 receptor responses in a concentration-dependent manner (Figure 3a and b). At 1 μM, Go 6976 not only abolished the PI inhibition caused by PMA, but also induced small increases (11±4% (n=4) and 17±9% (n=4), respectively) in control PI responses of 2MeSATP and UTP (Figure 3a and b).
Figure 3.
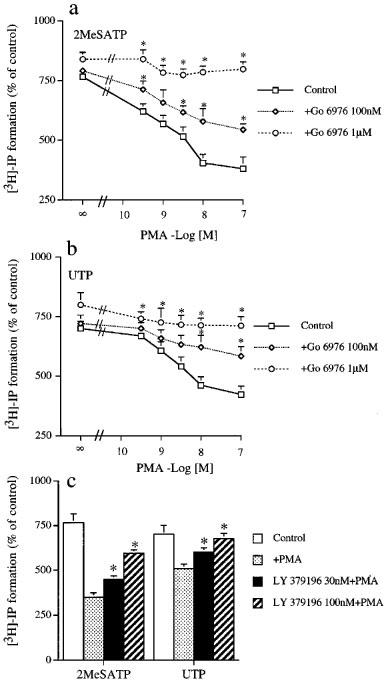
Concentration-dependent effects of Go 6976 and LY 379196 on PMA-induced inhibition of 2MeSATP- and UTP-evoked PI turnover in CPAE cells. In (a) and (b), cells were incubated with vehicle (DMSO) or Go 6976 (100 nM–1 μM) for 20 min followed by addition of PMA at concentrations within 0.3–100 nM for another 30 min. Subsequent to these treatments, 2MeSATP (100 μM) (a) or UTP (100 μM) (b) was added to trigger the accumulation of [3H]-IP. In (c), cells were pretreated with 30 or 100 nM LY 379196 for 20 min followed by addition of 1 μM PMA for another 30 min. Then 2MeSATP (100 μM) or UTP (100 μM) was added to induce PI turnover. The data represent the mean±s.e.mean of three independent experiments performed in duplicate. For (a) and (b), using two-way ANOVA, Go 6976 caused a significant difference from control (P<0.0001). When significance was reached, using Dunnetts test, *P<0.05 that are significantly different from PMA response in the absence of Go 6976. For (c), using ANOVA followed by Dunnetts test, *P<0.05 was considered significant as compared to the PMA response in the absence of PKC inhibitors.
To confirm PKCβI indeed involved in 2MeSATP- and UTP-induced PI turnover, we tested a selective PKCβ inhibitor, LY 379196 (Lin & Chen, 1998). We found that LY 379196 (30 and 100 nM) pretreatment also resulted in a concentration-dependent antagonism on PMA-induced inhibition of P2Y1 and P2Y2 receptor-mediated PI responses. Upon treating at the highest concentration (100 nM) possessing the selectivity for PKCβ but not other PKC isoforms, our results indicated that partial and complete reverse of PMA effect were respectively observed for 2MeSATP and UTP actions (Figure 3c).
To strongly support the inhibitory role of PKCβI, we directly treated cells with PKCβI antisense oligonucleotide. After 48 h treatment with 1.5 μg PKCβI antisense oligonucleotide, the protein amount of PKCβI was reduced by 65±10% (n=3), while those of PKC ε, λ, and μ were unaffected. Control cells treated with sense oligonucleotide expressed the same amount of these four PKC isoforms (Figure 4a). Under these conditions unaffecting the control PI responses of P2Y1 and P2Y2 receptor agonists, PMA-induced PI inhibition was diminished by approximately 39±5% (n=3) and 48±8% (n=3) respectively for 2MeSATP and UTP responses. On the other hand, sense oligonucleotide did not alter the [3H]-IP accumulation induced by 2MeSATP and UTP (100 μM) (Figure 4b). These results indicate that PKCβI transduces the negative feedback on P2Y1 and P2Y2 receptor-coupled PI hydrolysis in CPAE cells.
Figure 4.
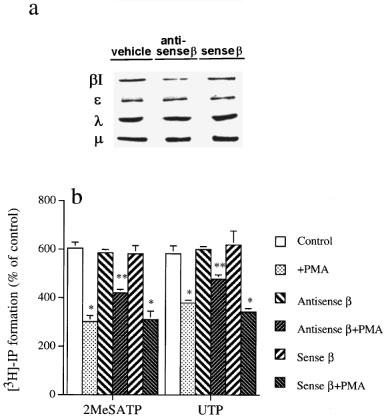
Antisense oligonucleotide inhibits immunoreactive PKCβI protein expression and PMA-induced reduction of 2MeSATP- and UTP-induced PI turnover. (a) Cells were treated with vehicle, antisense or sense PKCβ oligonucleotide (1.5 μg) for 4 h, then incubated in replaced growth medium for another 48 h as described under ‘Methods'. The immunoreactivities of PKC isoforms were analysed by immunoblots and the results shown are representative of three experiments. (b) After cells were processed as described in (a), PMA (1 μM) was incubated for 20 min followed by the stimulation with 2MeSATP (100 μM) or UTP (100 μM) for 30 min. The accumulated [3H]-IP was measured. The data represent the mean±s.e.mean of three independent experiments performed in duplicate. Using ANOVA followed by Dunnetts test, *significantly different (P<0.05) from the control response. **Significantly different (P<0.05) from the inhibitory PMA response without oligonucleotide pretreatment.
Regulation of P2Y1 and P2Y2 receptor mRNA expression by PMA
As Figure 1, showing the potentiation on 2MeSATP and UTP responses by 24 h treatment with PMA, we explored the effects of PMA on P2Y1 and P2Y2 receptor expression. Using RT–PCR analysis, we found the presence of P2Y1 and P2Y2 receptor mRNA in CPAE cells. On stimulation with PMA (100 nM), both receptor mRNA levels were up-regulated after 4 h treatment, and were sustainedly increased over 24 h treatment (Figure 5). These results suggest the increased P2Y1 and P2Y2 receptor mRNA contributing to the PMA-elicited enhancement on PI turnover. Besides P2Y1 and P2Y2 receptors, we also tested whether CPAE cells express other three P2Y receptor subtypes, namely P2Y4, P2Y6 and P2Y11 receptors. P2Y4 and P2Y6 receptors are two UTP-responsive P2Y receptors and have been detected in human umbilical vein endothelial cells (Jin et al., 1998), while P2Y11 receptor is responsive to 2MeSATP stimulation. In RT–PCR experiments using rat alveolar macrophages as positive controls for these three receptor expression, we cannot detect their mRNA signals in CPAE cells (data not shown).
Figure 5.
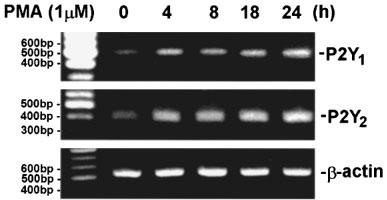
RT–PCR analysis of P2Y1 and P2Y2 mRNA levels in PMA-treated cells. CPAE cells were treated with 100 nM PMA for 4, 8, 18 or 24 h followed by extraction of total RNA and analysis of mRNA levels of P2Y1, P2Y2 receptor and β-actin. RT–PCR technique was performed as described in ‘Methods'. β-actin levels normalized the amount of cDNA template used in each PCR reaction. The results are representative of three experiments.
Discussion
To date, at least 12 PKC isoforms have been characterized at the molecular level. It is suggested that individual PKC isoform might have differential intracellular location and distinct functions (Nishizuka, 1995; Mochly-Rosen & Gordon, 1998). The observation that phorbol esters are well known inhibitors on agonist-induced PI turnover in many types of cells (for reviews see Nishizuka, 1984; Chuang, 1989) strongly suggests PKC functioning as a negative-feedback regulator of cellular function. At present, the PKC isoforms involved in the inhibition of PI turnover triggered by various PLC-linked receptors, however, is cell- and receptor-dependent. In endothelial cells, we and others have previously confirmed that PMA, a potent activator of PKC, can inhibit P2Y1- or P2Y2-receptor mediated IP accumulation and [Ca2+]i increase (Chen et al., 1996; 1999; Patel et al., 1996). In this study, we have determined the PKC isoform involved in the regulation of P2Y receptor-elicited PI signalling. We showed that PMA affects 2MeSATP and UTP responses in a time-dependent manner. Short-term treatment with PMA results in a marked attenuation of P2Y1 and P2Y2 receptor signalling, however, with an extended PMA preincubation time within 24 h, the inhibitory effect of PMA diminishes and is substituted by a potentiation. With respect to the early phase of inhibition, we for the first time provide evidence to show the involvement of PKCβI in P2Y receptor desensitization. On the other hand, the late phase of potentiation by PMA is partially due to the up-regulation of P2Y receptor gene expression. In contrast to the inhibition of UTP response in CPAE by short-term treatment with PMA, as we observed here and previously (Chen et al., 1996), no effect of PKC activation on P2Y2 receptor response was reported in bovine aortic endothelial cells (Purkiss et al., 1994). In this regard, we presently cannot provide evidence to explain this discrepancy observed in these two cell types, but the G protein species coupling to P2Y2 receptors might be different. The Gq/G11 and Gi2 proteins were respectively proposed to transduce P2Y2 receptors of CPAE and aortic endothelial cells to PLC activation (Communi et al., 1995; Chen et al., 1996).
In an attempt to assess the significance of particular PKC isoform as a signalling mechanism for PMA action, we utilized pharmacological and biochemical approaches to elucidate this notion. Firstly, preincubation of CPAE cells with a non-selective PKC inhibitor, Ro 31-8220 (Beltman et al., 1996), completely reversed the PMA-induced desensitization on P2Y receptor signalling. These results confirmed the action of PMA being through PKC activation. Secondly, Go 6976, a very effective and selective inhibitor of conventional PKC isoforms (α, β, and γ) (Martiny-Baron et al., 1993), substantially antagonized the ability of PMA to inhibit PI responses of P2Y1 and P2Y2 receptor stimulation. This result together with our previous findings showing the presence of four PKC isoforms, i.e. βI, ε, λ and μ, in CPAE cells (Chen et al., 1999) suggest that Ca2+-sensitive conventional PKCβI mediates the PKC-dependent desensitization of P2Y receptor response. Additional support for this suggestion comes from the results of experiments with LY 379196, which is a selective inhibitor of PKCβI and βII (Gillig, personal communication; Lin & Chen, 1998). Thirdly, short-term (2–10 min) treatment of cells with PMA indeed resulted in a rapid translocation to the cell membrane of PKCβI, ε, and μ, but not λ, supporting the activation of DAG-sensitive PKC isoforms by PMA. Fourthly, it has been widely reported that individual PKC isoform shows different patterns of down-regulation on prolonged exposure to PMA. In CPAE cells, we have previously shown that PKCβI and ε can be time-dependently down-regulated by the treatment with PMA for 2–24 h (Chen et al., 1999). Consistent with this kinetic effect on PKCβI changes is the accompanied recovery of PMA-induced desensitization. Fifthly, to directly evaluate the suggested role of PKCβI in PI inhibition, we used antisense oligonucleotide to selectively reduce PKCβI protein expression. We found that the selective attenuation of PKCβI protein reduced the inhibitory extent elicited by PMA. Taken together these results all indicate that PKCβI is involved in the inhibition of P2Y receptor-coupled PI signalling in CPAE cells. With respect to the regulatory role of other PKC isoforms in P2Y receptor signalling, it is interesting to mention that we and others have shown the important role of PKCε in potentiating P2Y receptor-stimulated arachidonic acid and prostacyclin release from endothelial cells (Patel et al., 1996; Chen et al., 1999).
Long-term phorbol ester treatment in some cases has been shown to sensitize the PI signalling pathway to several receptor agonists (Lin & Chuang, 1992; Hepler et al., 1998). In addition to the down-regulation of inhibitory PKCβI accounts for the long-term potentiation effect of PMA, the effects of PMA on P2Y receptor expression need to be considered, as several studies have shown the changes of receptor expression after PMA stimulation. For example, the mRNA expression for P2Y2 receptor was down-regulated in PMA-induced differentiated myeloid leukocytes (Martin et al., 1997). Here, we for the first time found the up-regulation of P2Y1 and P2Y2 receptor gene expression by PKC activation. Therefore, we conclude that the down-regulation of PKCβI together with the up-regulated expression of P2Y1 and P2Y2 receptors are both responsible for the PI enhancing effect of PMA.
In conclusion, we have demonstrated a biphasic effect of PKC activation on P2Y1 and P2Y2 receptor-induced PI signalling. The initial inhibition by PMA depends on the activation of negative regulator PKCβI, while the late recovery and eventually potentiation rely on the enhanced P2Y receptor expression and PKCβI down-regulation.
Acknowledgments
This research was supported by the grant from the National Science Council of Taiwan (NSC 88-2314-B002-108).
Abbreviations
- [Ca2+]i
intracellular calcium
- CPAE
bovine pulmonary artery endothelium
- hDAG
diacylglycerol
- Go 6976
12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-indolo(2,3-a)pyrrolo(3,4-c)carbazole
- IP
inositol phosphate
- IP3
inositol trisphosphate
- MEM
minimum essential medium
- 2MeSATP
2-methylthio-ATP
- NO
nitric oxide
- PI
phosphoinositide
- PKC
protein kinase C
- PLC
phospholipase C
- PMA
phorbol 12-myristate 13-acetate
- Ro 31-8220
1-[3-(amidinothio) propyl-1H-indoyl-3-yl]-3-(1-methyl-1H-indoyl-3-yl)-maleimide-methane sulphate
- RT–PCR
reverse transcription-polymerase chain reaction
References
- BELTMAN J., MCCORMICK F., COOK S.J. The selective protein kinase C inhibitor, Ro 31-8220, inhibits mitogen-activated protein kinase phosphatase-1 (MKP-1) expression, induced c-Jun expression, and activates Jun N-terminal kinase. J. Biol. Chem. 1996;171:27018–27024. doi: 10.1074/jbc.271.43.27018. [DOI] [PubMed] [Google Scholar]
- BOARDER M.R., HOURANI S.M. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol. Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BOARDER M.R., WEISMAN G.A., TURNER J.T., WILKINSON G.F. G protein-coupled P2 purinoceptors: from molecular biology to functional responses. Trends Pharmacol. Sci. 1995;16:133–139. doi: 10.1016/s0165-6147(00)89001-x. [DOI] [PubMed] [Google Scholar]
- BOEYNAEMS J.-M., PEARSON J.D. P2 purinoceptors on vascular endothelial cells: physiological significance and transduction mechanisms. Trends Pharmacol. Sci. 1990;11:34–37. doi: 10.1016/0165-6147(90)90039-b. [DOI] [PubMed] [Google Scholar]
- BORN G.V.R., KRATZER M.A.A. Source and concentration of extracellular adenosine triphosphate during haemostasis in rats, rabbits and man. J. Physiol. Lond. 1984;354:419–429. doi: 10.1113/jphysiol.1984.sp015385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUTHERIN-FALSON O., REUSE S., DUMONT J.E., BOEYNAEMS J.-M. Increased levels of c-fos and c-myc mRNA in ATP-stimulated endothelial cells. Biochem. Biophys. Res. Commun. 1990;172:306–312. doi: 10.1016/s0006-291x(05)80210-4. [DOI] [PubMed] [Google Scholar]
- BROWN C.A., PATEL V., WILKINSON G., BOARDER M.R. P2 purinoceptor-stimulated conversion of arginine to citrulline in bovine endothelial cells is reduced by inhibition of protein kinase C. Biochem. Pharmacol. 1996;52:1849–1854. doi: 10.1016/s0006-2952(96)00550-3. [DOI] [PubMed] [Google Scholar]
- CHEN B.C., LEE C.M., LIN W.W. Characterization of signaling pathway of P2Y and P2U purinoceptors in bovine pulmonary artery endothelial cells. J. Cardiovasc. Pharmacol. 1996;28:192–199. doi: 10.1097/00005344-199608000-00003. [DOI] [PubMed] [Google Scholar]
- CHEN B.C., LIN L.L., LIN W.W.PKCε-dependent pathway of ERK activation by P2Y1 and P2Y2 purinoceptors that activate cPLA2 in endothelial cells Eu. J. Pharmacol. 1999(in press) [DOI] [PubMed]
- CHUANG D.-M. Neurotransmitter receptors and phosphoinositide turnover. Ann. Rev. Pharmacol. Toxicol. 1989;29:71–110. doi: 10.1146/annurev.pa.29.040189.000443. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., BOEYNAEMS J.-M. Receptors responsive to extracellular pyrimidine nucleotides. Trends Pharmacol. Sci. 1997;18:83–86. doi: 10.1016/s0165-6147(96)01035-8. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., RASPE E., PIROTTON S., BOEYNAEMS J.-M. Coexpression of P2Y and P2U receptors on aortic endothelial cells. Comparison of cell localization and signaling pathways. Circ. Res. 1995;76:191–198. doi: 10.1161/01.res.76.2.191. [DOI] [PubMed] [Google Scholar]
- DARBON J.M., TOURNIER J.F., TANBER J.P., BAYARD F. Possible role of protein phosphorylation in the mitogenic effect of high density lipoproteins on cultured vascular endothelial cells. J. Biol. Chem. 1986;261:8002–8008. [PubMed] [Google Scholar]
- EDWARDS F.A., GIBB A.J., COLQUHOUN D. ATP receptor mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- EVANS R.J., DERKACH V., SURPRENANT A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992;357:503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACHIO M.P., BURNSTOCK G., DUBYAK G.R., HARDEN T.K., JACOBSON K.A., SCHWABE U., WILLIAMS M. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol. Sci. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPLER J.R., EARP H.S., HARDEN T.K. Long-term phorbol ester treatment down-regulates protein kinase C and sensitizes the phosphoinositide signaling pathway to hormone and growth factor stimulation: evidence for a role of protein kinase C in agonist-induced desensitization. J. Biol. Chem. 1998;263:7610–7619. [PubMed] [Google Scholar]
- JIN J., RAO DASARI V., SISTARE F.D., KUNAPULI S.P. Distribution of P2Y receptor subtypes on haematopoietic cells. Br. J. Pharmacol. 1998;123:789–794. doi: 10.1038/sj.bjp.0701665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., HOMOLYA L., BOUCHER R.C., HARDEN T.K. Direct demonstration of mechanically induced release of cellular UTP and its implication for uride nucleotide receptor activation. J. Biol. Chem. 1997;272:24348–24354. doi: 10.1074/jbc.272.39.24348. [DOI] [PubMed] [Google Scholar]
- LEVESQUE L., DEAN N.M., SASMOR D.H., CROOKE S.T. Antisense oligonucleotides targeting human protein kinase C-α inhibit phorbol ester-induced reduction of bradykinin-evoked calcium mobilization in A549 cells. Mol. Pharmacol. 1997;51:209–216. doi: 10.1124/mol.51.2.209. [DOI] [PubMed] [Google Scholar]
- LI H., OEHRLEIN S.A., WALLEERATH T., IHRIG-BIEDERT I., WOHLFART P., ULSHFER T., JESSEN T., HERGET T., FORSTERMANN U., KLEINERT H. Activation of protein kinaseCα and/or ε enhances transcription of the human endothelial nitric oxide synthase gene. Mol. Pharmacol. 1998;53:630–637. doi: 10.1124/mol.53.4.630. [DOI] [PubMed] [Google Scholar]
- LIN W.W., CHEN B.C. Distinct PKC isoforms mediated the activation of cPLA2 and adenylyl cyclase by phorbol ester in RAW 264.7 macrophages. Br. J. Pharmacol. 1998;125:1601–1609. doi: 10.1038/sj.bjp.0702219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN W.W., CHUANG D.M. Regulation of bradykinin-induced phosphoinositide turnover in cultured cerebellar astrocytes: possible role of protein kinase C. Neurochem. Int. 1992;21:573–579. doi: 10.1016/0197-0186(92)90090-e. [DOI] [PubMed] [Google Scholar]
- MARTIN K.A., KERTESY S.B., DUBYAK G.B. Down-regulation of P2U-purinergic nucleotide receptor messenger RNA expression during in vitro differentiarion of human myeloid leukocytes by phorbol esters or inflammatory activators. Mol. Pharmacol. 1997;51:97–108. doi: 10.1124/mol.51.1.97. [DOI] [PubMed] [Google Scholar]
- MARTINY-BARON G., KAZANIETZ M.G., MISCHAK H., BLUMBERG P.M., KOCHS G., HUG H., MARME D., SCHACHTELE C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- MOCHLY-ROSEN D., GORDON A.D. Anchoring proteins for protein kinase C: a means for isozyme selectivity. FASEB J. 1998;12:35–42. [PubMed] [Google Scholar]
- NISHIZUKA Y. The role of protein kinase C in cell surface signal transduction and tumor promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- NISHIZUKA Y. Intracellulr signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- NISHIZUKA Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- NORTH R.A., BARNARD E.A. Nucleotide receptors. Curr. Opin. Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- OHNO S., KAWASAKI H., IMAJOH S., SUZUKI K. Tissue-specific expression of three distinct types of rabbit protein kinase C. Nature. 1987;325:161–166. doi: 10.1038/325161a0. [DOI] [PubMed] [Google Scholar]
- OSIPCHUK Y., CAHALAN M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- PATEL V., BROWN C., BOARDER M.R. Protein kinase C isoforms in bovine aortic endothelial cells: role in regulation of P2Y- and P2U-purinoceptor-stimulated prostacyclin release. Br. J. Pharmacol. 1996;118:123–130. doi: 10.1111/j.1476-5381.1996.tb15374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIROTTON S., LECOMTE M., ROBAYE B., DEMOLLE D., VAN COEVORDEN A., NAIRN A.C., BOEYNAEMS J.M. P2-purinergic receptors on vascular endothelial cells: Transduction mechanisms. Ann. NY Acad. Sci. 1990;603:480–483. [Google Scholar]
- PURKISS J.R., WILKINSON G.F., BOARDER M.R. Different regulation of inositol 1,4,5-trisphosphate by co-existing P2Y-purinoceptors and nucleotide receptors on bovine aortic endothelial cells. Br. J. Pharmacol. 1994;111:723–728. doi: 10.1111/j.1476-5381.1994.tb14797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DAELE P., VAN COEVORDEN A., ROGER P.P., BOEYNAEMS J.-M. Effects of adenosine nucleotides on the proliferation of aortic endothelial cells. Circ. Res. 1992;70:82–90. doi: 10.1161/01.res.70.1.82. [DOI] [PubMed] [Google Scholar]
- WEBB T.E., BOLUYT M.O., BARNARD E.A. Molecular biology of P2Y purinoceptors: expression in rat heart. J. Auto. Pharmacol. 1996;16:303–307. doi: 10.1111/j.1474-8673.1996.tb00040.x. [DOI] [PubMed] [Google Scholar]
- XU Y., WARE A. Selective inhibition of thrombin receptor-mediated Ca2+ entry by protein kinase C β. J. Biol. Chem. 1995;270:23887–23890. doi: 10.1074/jbc.270.41.23887. [DOI] [PubMed] [Google Scholar]


