Abstract
Thymidylate synthase (TS), the key enzyme in de novo synthesis of thymidine, is an important target for antitumour chemotherapy. It was hypothesized that antisense oligonucleotide down-regulation of TS mRNA would decrease TS levels and enhance the cytotoxicity of inhibitors of TS, including the pyrimidine analogues 5-fluorouracil (5-FU) and 5-fluorodeoxyuridine (5-FUdR), and the folate analogue Tomudex (ICI D1694; N-(5-[N-(3,4-dihydro-2-methyl-4-oxoquinazolin-6-ylmethyl)-N-methylamino]-2-theonyl-L-glutamic acid).
2′-Methoxyethoxylated, phosphorothioated 20-mer oligodeoxynucleotides (ODNs), complementary to various sequences in TS mRNA, were synthesized, along with control oligomers consisting of the same, respective bases in randomized order, against which all the biological effects were compared. Following a 6-h transfection of HeLa cells using polycationic liposome at 3 μg ml−1, ODN 83 (50 nM), complementary to a region in the 3′-untranslated region of the TS mRNA, decreased TS mRNA levels by approximately 70% within 24 h. ODN 83 also decreased TS enzyme activity, as measured by binding of TS to radiolabelled 5-fluorodeoxyuridine monophosphate. In addition to inhibiting proliferation by up to approximately 40%, ODN 83 enhanced the cytotoxicity of Tomudex or 5-FU, added 1 day following transfection, by 50–60%. ODN 83 also enhanced sensitivity to 5-FUdR by 70%, but did not affect the toxicity of cisplatin, chlorambucil, melphalan, doxorubicin, ionizing radiation, paclitaxel, or irinotecan.
These data indicate that antisense ODN down-regulation of TS can inhibit human tumour cell proliferation and enhance the efficacy of TS-targeted drugs.
Keywords: Antisense; thymidylate synthase; drug resistance, 5-fluorouracil; 5-fluorodeoxyuridine; Tomudex
Introduction
Thymidylate synthase (5,10-methylenetetrahydrofolate:dUMP C-methyltransferase; EC 2.1.1.45) (TS) is a highly conserved homodimer of 35 kDa subunits which catalyzes the synthesis of thymidylate from deoxyuridylate and 5,10-methylenetetrahydrofolate (Me-FH4) (Chu & Allegra, 1996a; Danenberg, 1977). The activity and expression of TS are tightly controlled throughout the cell cycle, particularly at the translational level (Johnson, 1994). The TS protein itself binds to the TS mRNA both at the translational start site (TSS) and in the coding region, inhibiting translational processing of the message (Chu et al., 1991; 1993b). TS can also bind to the mRNA of at least nine other important gene products, including those of p53 (Chu et al., 1996b) and c-myc (Chu et al., 1994). Therefore, manipulating the level of the TS protein could induce a cascade of consequential effects on cell growth.
Because of its importance in DNA precursor synthesis and repair, TS has proved to be an important target for anticancer chemotherapy. Direct inhibitors of TS include the nucleoside analogue 5-fluorodeoxyuridine monophosphate (5-FdUMP) [a derivative of 5-fluorouracil (5-FU) and 5-fluorodeoxyuridine (5-FUdR) produced within mammalian cells], and folate analogues such as N-(5-[N-(3,4-dihydro-2-methyl-4-oxoquinazolin-6-ylmethyl-N-methylamino]-2-thenoyl)-L-glutamic acid, (Tomudex, ICI D1694) (Jackman et al., 1991). In drug-selected cell lines, a common mechanism of resistance to both these classes of drugs is increased cellular expression of TS (Zhang et al., 1992). However, in drug-sensitive cells, induction of increased TS expression is also a potential impediment to antitumor activity. 5-FdUMP, as well as Me-FH4, relieve the repression of TS mRNA translation by the TS protein (Chu & Allegra, 1996a; Chu et al., 1991), resulting in a transient increase in TS protein levels, a phenomenon observed in vitro (Chu et al., 1990; 1991; 1993a; Keyomarsi et al., 1993; Van der Wilt et al., 1992), in animals (Van der Wilt et al., 1992), and in patients (Peters et al., 1994; Swain et al., 1989). This induction of TS synthesis [upwards of 3–5 fold (Chu et al., 1990; 1993a; Van der Wilt et al., 1992)] would serve to at least partially circumvent the cytotoxic effect of the drug (Berne et al., 1986). In view of the importance of regulation of mRNA in TS production, a protocol was designed to reduce the cellular levels of TS mRNA through the use of specific antisense oligodeoxynucleotides (ODNs). By choosing TS mRNA as a target to reduce the synthesis of TS protein, it is potentially possible to: (a) inhibit cellular proliferation, a process dependent on thymidylate availability; (b) enhance the cytotoxicity of TS inhibitors by decreasing the amount of protein target against which they act; and (c) inhibit TS mRNA translation and suppress the overexpression of TS in drug-resistant cells.
A 422-base TS-antisense expression vector was shown to down-regulate TS enzyme by inhibiting translation, thus enhancing toxicity of 5-FUdR (Ju et al., 1998). However, 18-base phosphodiester ODNs antisense to various parts of the TS mRNA only transiently down-regulated TS, and after 24 h led to a 2 fold increase in TS with a resultant resistance to 5-FUdR (Ju et al., 1998). However, preliminary results from this laboratory using stable phosphorothioester ODNs suggested that down-regulation of TS was possible. The transfection efficiency of ODNs would also be expected to be much higher than that of an expression vector, enabling sensitization of many more (if not all) cells.
The use of antisense ODNs has proven to be a specific method to down-regulate a number of desired targets (Bennett, 1998; Citro et al., 1998; Dean et al., 1994a; 1996). Antisense ODNs are most commonly directed against short, specific mRNA sequences, resulting in a variety of biochemical consequences. When antisense ODNs associate with mRNA the complex becomes a substrate for RNase H, resulting in cleavage of the mRNA (Bennett, 1998; Binder et al., 1994; Stein et al., 1988). In addition, ODNs associated with the mRNA TSS prevent the normal processing of mRNA by ribosomes, at least in vitro (Bennett, 1998). However, the 3′, untranslated region (UTR) of mRNA is often a superior target for down-regulation compared with the 5′-region and/or the TSS (Stein & Cheng, 1993). Phosphorothioated ODNs are more stable than their phosphodiester counterparts, and are degraded less rapidly by S1 and P1 nucleases (Dean et al., 1996; Shaw et al., 1991; Stein & Cheng, 1993; Stein et al., 1988). Because antisense ODNs can be made highly specific to a target of choice, they provide a promising approach to modulation of many cellular targets and biochemical processes. We report here that a 20-base ODN (ODN 83), complementary to a sequence in the TS mRNA 3′ UTR, transiently down-regulated the level of TS mRNA and protein in HeLa cells, inhibited cell proliferation, and enhanced the cytotoxicity of TS-directed chemotherapy drugs.
Materials and methods
Oligonucleotides
Fully phosphorothioated 20-base oligonucleotides were synthesized by ISIS Pharmaceuticals (Carlsbad, CA, U.S.A.), as described (Dean et al., 1996). The six nucleotides on either end of the oligomer were methoxyethoxylated in the 2′-position, enhancing hybridization as well as resistance to exonucleation (Dean et al., 1994a,1994b). The middle eight nucleotides were not methoxyethoxylated to allow RNase H endonucleation and degradation of mRNA hybridized to the oligomer (Dean et al., 1994b). ODN 83 is complementary to TS mRNA, starting from a position 136 bases downstream of the translational stop site (5′-GCCAGTGGCAACATCCTTAA-3′). ODN 32 is a randomized sequence of ODN 83 (5′-ATGCGCCAACGGTTCCTAAA-3′), with the same base constituents in random order. A search of available mRNA sequences using the NCBI BLAST search tool revealed that ODN 83 had sequences of ten or more complementary bases to only human TS mRNA, while ODN 32 had sequences of ten or more complementary bases to no known mRNAs.
Radioisotopes
[6-3H]5-dUMP (specific activity 18.6 Ci mmol−1) was purchased from Moravek Biochemicals (Brea, CA, U.S.A.). This isotope was 99.98% pure upon initial production, with a degradation rate of 0.5–1% per month at −20°C, and was used within 3 months of manufacture. [α32P]-dCTP (specific activity 3000 Ci mmol−1) was purchased from Amersham Pharmacia Biotech (Oakville, Ontario, Canada).
Chemotherapy reagents
Tomudex was generously provided by Zeneca Pharmaceuticals, Inc. (Macclesfield, Cheshire, U.K.). Doxorubicin was a gift from Adria Laboratories (Mississauga, Canada). 5-FUdR was purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). 5-FU and irinotecan (CPT-11) (Pharmacia & Upjohn, Inc.), chlorambucil and melphalan (Glaxo Wellcome, Inc.), and cisplatin and paclitaxel (Bristol-Myers Squibb Co.) were purchased from the London Regional Cancer Centre pharmacy.
Other supplies
Cell culture chemicals and nutrients were obtained from Canadian Life Technologies (GIBCO/BRL) (Burlington, ON, Canada). All other chemicals were obtained from commercial sources. Plasticware was purchased from VWR Canlab (Mississauga, ON, Canada) and Fisher Scientific (Uniondale, ON, Canada).
Cell culture
Cell lines
Human cervical carcinoma HeLa cells were maintained in Dulbecco's modified Eagle's medium (D-MEM) plus 10% foetal bovine serum and penicillin (50 units ml−1)/streptomycin (50 μg ml−1) (growth medium). Cultures were incubated in a humidified atmosphere of 5% CO2 at 37°C. Rapidly proliferating cells were utilized for establishing cultures of experimental cells, which were allowed to plate overnight prior to manipulation.
Transfection of ODNs
Transfection was performed using Lipofectamine® (LFA) (GIBCO/BRL), a polycationic liposome formulation. Cells to be used for proliferation experiments were plated at a starting cell number of between 0.6 and 1×105 cells per 25-cm2 tissue culture flask, and LFA was used at 3 μg ml−1. For cells in 75-cm2 flasks, to be harvested and extracted for assay of mRNA or TS content, the starting cell number was approximately 8–10×105, and the LFA concentration was 4 μg ml−1. Prior to transfection, adherent HeLa cells were washed once with phosphate-buffered saline (0.15 M NaCl+ 0.67 mM KH2PO4, pH 7.4) (PBS) and then treated with antisense or scrambled control ODN (50 nM) in the appropriate concentration of LFA in serum-free D-MEM, at 37°C for 6.0 h. The cells were then washed once with PBS and cultured in the presence of growth medium. They were harvested for RNA isolation, TS protein binding assay, and cell number measurement (by numeration with a particle counter [Coulter Electronics, Hialeah, FL, U.S.A.]) at 1–6 days following ODN transfection. Proliferation was assessed as the increase in cell number at various times, as a percentage of the increase in cells treated with LFA in the absence of ODNs.
Cytotoxicity assays
Cells treated with both ODNs and cytotoxic agents were first exposed to anti-TS ODN or control ODN, and then to cytotoxic agent 24 h later. Drug exposure was initiated by addition of 0.2-volume of growth medium containing the agent at six times the final concentration. At the time of addition of drug or treatment with ionizing radiation, and after 4 days of incubation, cell numbers were determined in three separate flasks by enumerating with a particle counter or, alternatively, by lysing cells in 0.1% sodium dodecyl sulphate and measuring absorbance at 260 nm by ultraviolet spectrophotometry: absorbance was proportional to, and an indicator of, cell number. The proliferation of drug-treated cells (fold-increase in cell number) was calculated in two ways: (1) as a percentage of that of the control cells treated with LFA plus ODN only, to observe the effect of ODN on drug sensitivity isolated from the effect of ODN plus LFA alone on proliferation (Figure 5), and (2) as a percentage of that of control cells untreated with ODN, LFA, or cytotoxic drug, to observe the combined effect of ODN as a single agent on cell proliferation plus its effect on sensitivity to cytotoxic drugs. IC50 and IC90 values (concentrations of drug that inhibited proliferation by 50 and 90%, respectively) were determined by interpolation of plotted data.
Figure 5.
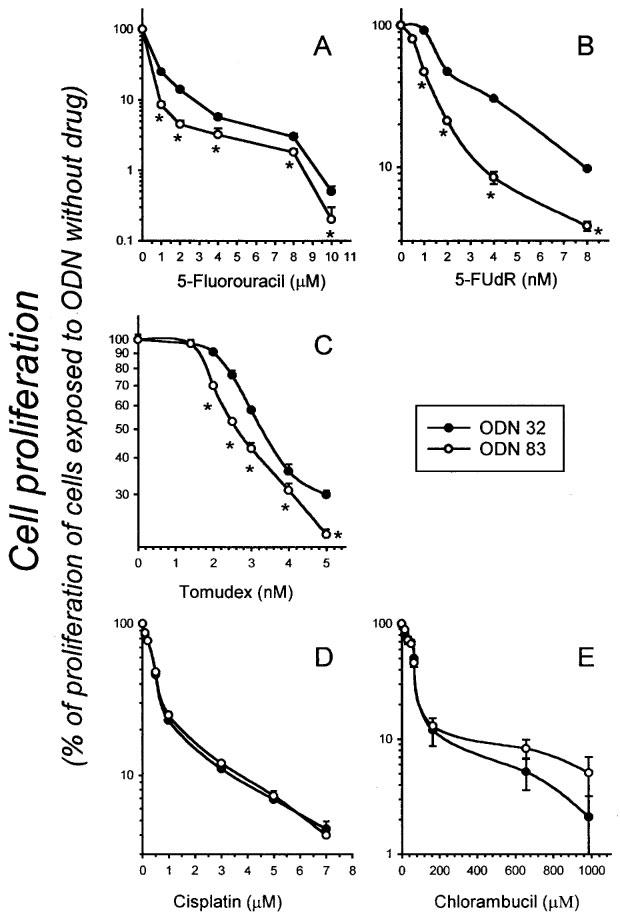
Antisense TS ODN 83 sensitizes HeLa cells to the toxic effects of 5-FU, 5-FUdR, and Tomudex, but not cisplatin or chlorambucil. HeLa cells were transfected with ODN 83 or control ODN 32, and then continuously exposed to various concentrations of 5-FU (A), 5-FUdR (B), Tomudex (C), cisplatin (D) or chlorambucil (E) for 4 days, beginning 24 h after transfection, as described in Materials and methods. Data points indicate the number of cells (mean±s.e. of four independent cultures) at the 4 day time point, as a percentage of the number of cells in cultures treated with ODN 83 or 32 (as appropriate) but unexposed to drug. Where error bars are not apparent, they are obscured by the symbol. Asterisks (*) indicate significant differences (P⩽0.02, Student t-test).
Ionizing radiation treatment
HeLa cells, in sealed tissue culture flasks containing growth medium under normal atmospheric oxygen, were treated with γ radiation using a 60Co source (Theratron Eldorado 6) at a dose rate of 160 cGy min−1. Exposure times were from 0 to 10 min. Control cells were left in the exposure chamber for 10 min with the radiation source sealed off. Flasks were returned to CO2 incubators immediately following radiation.
Reverse transcriptase–polymerase chain reaction (RT–PCR) to measure TS mRNA
Measurement of TS and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) mRNA in the same ODN-transfected cell populations at 1–4 days post-transfection required a method that would allow accurate quantitation of mRNAs isolated from the small numbers of cells available 1–2 days after transfection. Therefore, RNA was isolated from transfected cells using Trizol® (GIBCO/BRL). Complementary DNA was synthesized from 1 μg of total RNA using 200 U of Moloney Murine Leukaemia Virus reverse transcriptase (GIBCO/BRL) (in mM): Tris-HCl (pH 8.3) 50, KCl 75, MgCl2 3, mixed dNTP 1, 100 pmol random primers and 10 mM dithiothreitol at 37°C for 1 h. The enzyme was inactivated at 95°C for 5 min. The resulting cDNAs (in a volume of 2.5 μl) were amplified by PCR using 1.25 U of Taq DNA polymerase in 50 μl of (mM) Tris-HCl (pH 8.4) 20, KCl 50, MgCl2 2, mixed dNTP 0.2, and 50 pmol of primers specific for TS and GAPDH cDNAs. TS and GAPDH cDNAs were amplified together in the same reaction tube so that the level of housekeeping GAPDH cDNA could be used to determine the relative level of TS mRNA. Twenty-four to 27 cycles of PCR amplification (94°C, 45 s; 55°C, 30 s; 72°C, 90 s) produced fragments of 208 bp and 752 bp using primer sets for TS (forward 5′-CACACTTTGGGAGATGCACA-3′; reverse 5′-CTTTGAAAGCACCCTAAACAGCCAT-3′) and GAPDH (forward 5′-TATTGGGCGCCTGGTCACCA-3′; reverse 5′-CCACCTTCTTGATGTCATCA-3′), respectively. PCR products were separated on a 1.2% agarose gel, and transferred to Hybond nylon membrane (Amersham Pharmacia Biotech) by Southern blotting (Sambrook et al., 1989). Blots were hybridized (Church & Gilbert, 1984) to [α-32P]-dCTP random primer-labelled probe: pcHTS-1 [a generous gift from Dr K. Takeishi, University of Shizuoka, Shizuoka, Japan (Takeishi et al., 1985)]; or a cDNA insert recognizing GAPDH (Denhardt, 1992). Hybridization signals were quantitated using a PhosphorImager and ImageQuant (Molecular Dynamics, Sunnyvale, CA, U.S.A.).
TS binding assay
Cellular content of TS was assayed by binding of [6-3H]-5-FdUMP, as described previously (Spears & Gustavsson, 1988). This method labelled total TS unless the cells were pretreated with 5-FU or 5-FUdR (Chu et al., 1990), and correlated well with an in situ activity assay (Ju et al., 1998). The assay was performed using cells that were treated with antisense ODN 83 or the scrambled control ODN 32. Briefly, cells were harvested by scraping into PBS and resuspending the subsequent pellet in 100 mM KH2PO4 (pH 7.4). Cells were disrupted by freezing and thawing, followed by sonication. The total protein concentration was determined using Coomassie staining (BioRad reagent) (Bradford, 1976) in order to express results as pmol 5-FdUMP bound per mg total protein. FdUMP binding was assessed in lysates from cells transfected with ODN 83 or ODN 32, extracted at the times indicated post-transfection. Extracts from three sets of transfections were assayed a total of five times, and pairs (e.g., ODN 83 versus ODN 32 on day 1) were always assessed together under the same reaction conditions. On each occasion, the incubation vessel contained 50 μg of total protein, 75 μM Me-FH4, 100 mM mercaptoethanol, 50 mM KH2PO4 (pH 7.4), and 15 nM [6-3H]-5-FdUMP in a final volume of 200 μl. After 30 min at 37°C, the incubation was stopped by addition of 5 volumes of albumin-coated, acidified charcoal. After 10 min (room temperature), this slurry was centrifuged (3000×g, 30 min, 22°C), and the supernatant re-centrifuged to completely remove particulate matter. Two aliquots of 300 μl each were removed from the final, clarified supernatant for scintillation counting.
Statistical analysis
Data for cell proliferation after treatment with ODNs alone, or in combination with cytotoxic drugs, are presented as the mean±standard error or standard deviation as determined by Student t-test. For determinations of FdUMP binding, differences between paired samples from cells transfected with different ODNs were assessed using a paired t-test. This controlled for differences in experimental conditions on each of the five occasions that FdUMP binding was assessed. In all cases, significance was chosen a priori to be indicated by differences at a confidence level of P⩽0.02.
Results
Growth inhibition by ODN 83
The primary objective of the study was to determine whether an antisense ODN targeted against TS could alter human tumour cell proliferation and/or enhance the cytotoxicity of TS-directed drugs. As a necessary preliminary investigation, various combinations of differing cell density, LFA concentration, and ODN concentration were evaluated to determine optimal conditions. The selected conditions induced minimal non-specific toxicity due to the transfection conditions alone (LFA plus scrambled control antisense ODN 32, 50 nM). These transfection conditions allowed us to observe the effect of ODN 83 alone, or ODN 83 plus cytotoxic drug, on cell proliferation. The optimal conditions to measure ODN-induced alterations in HeLa cell proliferation were 50 nM ODN in 3 μg ml−1 LFA, at a starting cell density of between 0.6 and 1×105 cells per 25-cm2 flask. Under these conditions, transient transfection of a β-galactosidase expression vector revealed that greater than 50% of cells successfully took up vector and synthesized active β-galactosidase enzyme. Because the modified, single-stranded ODNs used in this study enter cells more effectively than large, double-stranded DNA vectors (Dean et al., 1996), this is likely a low estimate of transfection efficiency of ODNs 83 and 32.
HeLa cells treated with antisense TS ODN 83 grew significantly more slowly than cells transfected with scrambled control ODN 32. Compared to cells treated with LFA alone, scrambled control ODN 32 had no significant effect on cell growth at concentrations as high as 100 nM, at two different LFA concentrations (Figure 1A and B). In contrast, antisense TS ODN 83 reduced proliferation by a maximum of 20% at 2 μg ml−1 LFA (Figure 1A), and nearly 100% at 4 μg ml−1 LFA (Figure 1B). Because 4 μg ml−1 LFA alone affected cell viability non-specifically (data not shown), we assessed the effect of 50 nM ODN 83 or 32 plus 3 μg ml−1 LFA on cell proliferation. Compared to untreated cells, control ODN 32 plus LFA had no effect, while antisense TS ODN 83 decreased proliferation by approximately 50% at 5 days post transfection (Figure 1C, representative data from one of 16 experiments). Growth inhibition was most evident in the first 48 h following ODN 83 transfection, with growth rate returning to the same level seen in cells transfected with control ODN 32 in the 48–144 h period following transfection (Figure 2). This early inhibition translated to a significant difference in cell number at the end of 5 or 6 days. There was no evidence of enhanced cell death (decreased cell number) in the ODN 83-treated cells compared with cells treated with control ODN 32.
Figure 1.
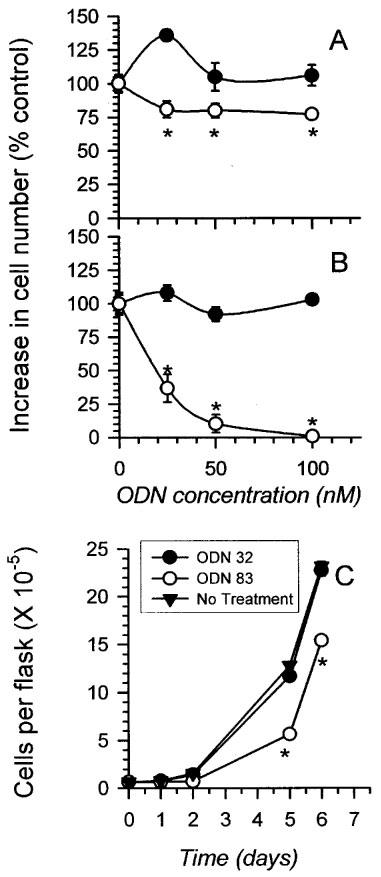
Antisense TS ODN 83 inhibits HeLa cell proliferation. (A) Cells were transfected with 0, 25, 50 or 100 nM antisense TS ODN 83 or scrambled control ODN 32 in the presence of 2 μg ml−1 LFA, and counted 4 days later as described in Materials and methods. The mean number of cells in three independent growth chambers±s.e. is plotted. Where error bars are not apparent, they are smaller than the symbol. Asterisks (*) indicate data significantly different from that obtained from cells treated with LFA alone, or scrambled control ODN 32 plus LFA (P⩽0.02, Student t-test). (B) Cells were transfected as for A, except that 4 μg ml−1 LFA transfection reagent was used. (C) Cells were transfected with 50 nM antisense TS ODN 83 or 50 nM scrambled control ODN 32 in the presence of 3 μg ml−1 LFA. They were counted 1,2,5 and 6 days later. Control cells were plated without exposure to LFA or DNA. As in A and B, data points indicate the mean cell number in three independent growth chambers±s.e. Error bars are smaller than the symbols in every case.
Figure 2.
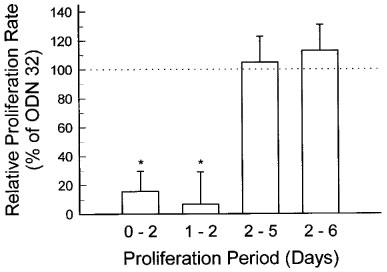
Antisense TS ODN 83 suppresses HeLa cell growth after transfection, followed by recovery to control proliferation rate after 48 h. HeLa cells were transfected with 50 nM antisense TS ODN 83 or 50 nM scrambled control ODN 32 as described in the legend to Figure 1C. Values derived from cells transfected with ODN 32 were normalized to 100%. Each bar indicates the difference from that value induced by treatment with ODN 83 (mean±s.d. of four independent experiments). Asterisks (*) indicate data significantly different from cells treated with scrambled control ODN 32 (P⩽0.02, Student t-test).
Effect of ODN 83 on TS mRNA and protein
To determine whether inhibition of proliferation was due to a direct effect of the antisense oligomer on its intended target, TS mRNA levels (relative to GAPDH mRNA) were measured by RT–PCR at various times following HeLa cell transfection with ODNs 83 or 32. A representative blot of PCR products from one independent experiment of two performed (Figure 3) revealed that TS mRNA levels were lower in cells transfected with ODN 83 than in cells transfected with control ODN 32. Antisense ODN 83 treatment reduced the TS:GAPDH ratio, at 24 h, to 30% of that seen in cells transfected with control ODN 32, rising to 57% at 48 h, and 64% at 96 h following transfection (as measured by phosphorimage analysis of Southern-blotted PCR products). The rise in TS mRNA levels over time was in agreement with the recovery in proliferation seen over time following transfection with ODN 83 (Figure 2). Therefore, transfection of HeLa cells with antisense TS ODN 83 decreased TS mRNA levels, relative to GAPDH mRNA, at 1–4 days following ODN transfection.
Figure 3.
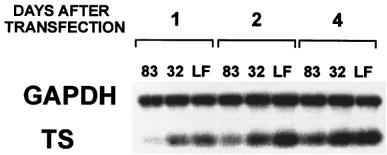
Treatment of HeLa cells with ODN 83 leads to decreased TS mRNA levels. HeLa cells were transfected with ODN 83 or scrambled control ODN 32, or treated with LFA alone (LF) as described in Material and methods. Cells were harvested at 1, 2, and 4 days post-transfection and total cellular RNA isolated, reverse-transcribed, and TS and GAPDH cDNA amplified by 24 PCR cycles, in the same reaction vessel. TS (208 b.p.) and GAPDH (752 b.p.) RT–PCR products were confirmed by Southern blotting and hybridization to specific radioactively-labelled probes.
Antisense down-regulation of TS mRNA was reflected in decreased TS protein levels. As measured by [3H]-5-FdUMP binding, transfection with ODN 83 reduced TS protein to approximately 25% of the level seen in control cells within 24 h, followed by a gradual recovery in TS levels up to approximately 80% of control levels by 4 days post-transfection (Figure 4). The FdUMP-binding assay was chosen in preference to another method (tritium release assay) to assess TS activity, as the latter assay yielded inconsistent results in cells with known high and low TS activity (results not shown). The FdUMP-binding assay employed here potentially measures enzymatically inactive TS, thereby, if anything, underestimating the effect of the ODN.
Figure 4.
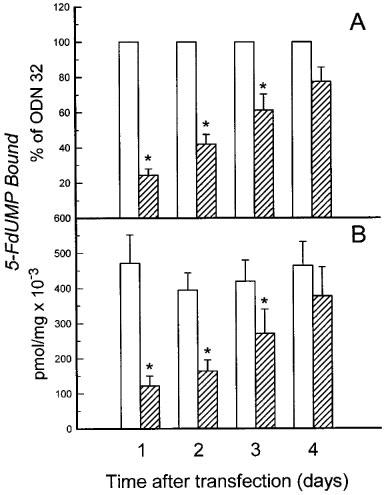
TS protein levels (inferred by measurements of 5-FdUMP binding) are diminished by antisense TS ODN 83 but not scrambled control ODN 32. 5-FdUMP binding was measured in cells transfected with ODN 83 (hatched bars) or ODN 32 (open bars) at different times following transfection. (A) Results are plotted as a per cent of 5-FdUMP binding in cells transfected with control ODN 32±s.e. (n=5). The values for ODN 32 (n=5) were normalized to 100% and are shown without error bars. (B) Results are plotted as pmol 5-FdUMP bound per mg total protein (×10−3) to reveal that transfection with control ODN 32 had no significant effect on TS protein levels. Error bars indicate s.e.mean calculated according to a Student t-test, and indicate error due to differences in experimental conditions in four measurements taken on different days, as well as differences due to transfection with different ODNs. The asterisks indicate significant differences (P⩽0.02) according to a paired Student t-test, which controls for differences in experimental conditions.
Effect of ODN 83 on cytotoxicity of chemotherapy drugs
Tomudex is a potent inhibitor of TS activity and cell proliferation and is active in the nanomolar range (Keyomarsi et al., 1993). It is transported into cells by the reduced folate transporter and polyglutamated, resulting in extended retention by cells (McGuire et al., 1997). Treatment of HeLa cells with antisense TS ODN 83 sensitized the cells to the cytotoxic effect of Tomudex, added to cells 24 h post-transfection, as demonstrated in a representative dose-response assay (Figure 5). The proliferation rates shown reflect the effect of the drug alone, since cells treated with ODN 83 or ODN 32 were assigned a proliferation value of 100% and all data points showing proliferation in the presence of drug after ODN treatment are relative to that 100% value. Therefore, the data presented in Figure 5 and Table 1 indicate ODN enhancement of drug toxicity, and do not include the growth inhibitory effect of ODN 83 shown in Figures 1 and 2. In summarizing the results of multiple experiments, ODN 83 treatment enhanced sensitivity to 5-FU by between 40% (at 8 μM 5-FU) and 67% (at 1–2 μM 5-FU), and sensitivity to 5-FUdR (the active metabolite of 5-FU) by between 10% (at 8 nM 5-FUdR) and 73% (at 4 nM 5-FUdR). In other terms, ODN 83 reduced the IC50 of 5-FUdR by approximately 70% (Table 1 and data not shown), and the IC90 by approximately 66%. Cellular resistance to Tomudex was also reduced by ODN 83, albeit to a lesser degree. Among six separate experiments, the mean reduction in the IC50 of Tomudex was 44±7% (s.e.mean). The enhancement of sensitivity to cytotoxic drugs was separate from, and additional to, the cytostatic effect of ODN 83 alone (all values in Figure 5 and Table 1 are relative to values obtained from cells transfected with ODN alone, without added cytotoxic drug). In contrast, ODN 83 did not sensitize HeLa cells to the toxic effects of cisplatin or chlorambucil, neither of which is known to target the TS complex (Figure 5).
Table 1.
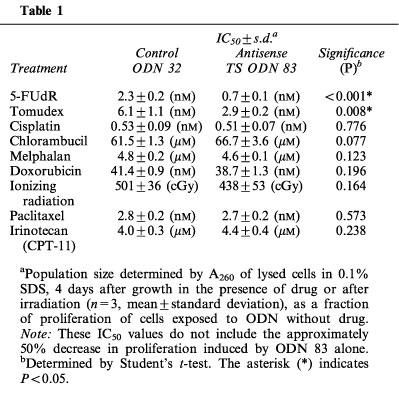
Further experiments to test the capacity of ODN 83 to enhance, in HeLa cells, the toxicity of a variety of agents acting through different mechanisms (Table 1) confirmed and extended the data shown in Figure 5. There was no significant difference in the capacity of CPT-11 (a topoisomerase I inhibitor), doxorubicin (a topoisomerase II inhibitor), melphalan (an alkylating agent), paclitaxel (a tubulin-targeting agent), or ionizing radiation (a generator of reactive oxygen and other radical species) to inhibit the growth of HeLa cells transfected with control ODN 32 or antisense TS ODN 83 (Table 1). In this separate series of experiments, the effect of TS-targeting agents (5-FUdR, Tomudex), but not other drugs, was enhanced by antisense TS ODN 83, similar to the data shown in Figure 5.
Data shown in Figures 1 and 2 revealed the capacity of ODN 83 to exert a cytostatic effect in the absence of cytotoxic drugs, and data in Figure 5 and Table 1 indicate an additional capacity of ODN 83 to enhance the ability of cytotoxic drugs to inhibit cell proliferation. To visualize the overall inhibition of cell proliferation caused by the combination of treatments, proliferation of cells treated with ODN 83 or 32 followed by cytotoxic drug treatment was plotted as a percentage of proliferation of cells not treated with drug or ODN (Figure 6).
Figure 6.
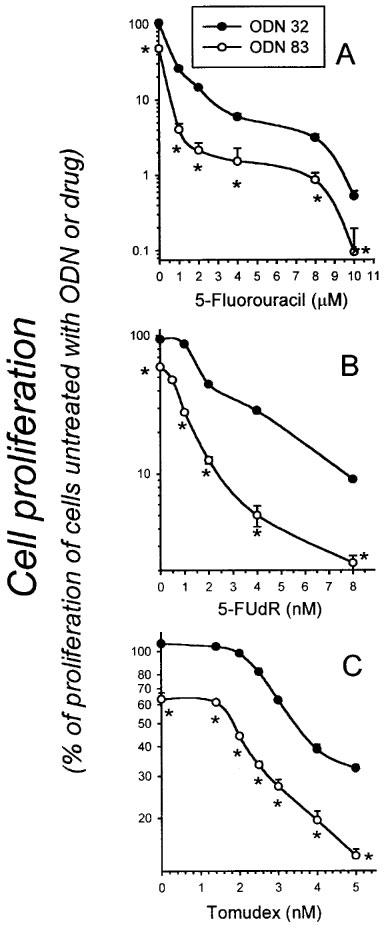
Overall effect of antisense TS ODN 83 on cell proliferation in the absence or presence of 5-FU, 5-FUdR, and Tomudex. As described in the legend to Figure 5, HeLa cells were transfected with ODN 83 or control ODN 32, and then continuously exposed to various concentrations of 5-FU (A), 5-FUdR (B), or Tomudex (C) for 4 days, beginning 24 h after transfection. Data points indicate the number of cells (mean±s.e. of four independent cultures) at the 4 day time point, as a percentage of the number of cells in cultures left untreated with DNA, LFA or toxic drug. Where error bars are not apparent, they are obscured by the symbol. Asterisks (*) indicate significant differences (P⩽0.02, Student t-test).
Discussion
Natural eukaryotic antisense transcripts have been demonstrated to modulate the function of specific mRNAs through interactions that inhibit RNA splicing, transport, and translation, and enhance specific mRNA degradation (Knee & Murphy, 1997). Antisense expression vectors and ODNs introduced experimentally into cells act in a similar fashion to specifically down-regulate a wide variety of gene products (Citro et al., 1998; Dean et al., 1994a; 1996; Denhardt, 1992; Li et al., 1997; Woolf et al., 1992). Although antisense expression vectors can be very specific for the target against which they are directed, and reduce specific protein levels by up to 90%, the use of this technology in vivo may be limited by the low transfection rate usually associated with such treatments. However, specific ODNs, 15–21 bases in length, have been reported to be taken up very efficiently into tissues in mice untreated with transfection-enhancing agents (Dean et al., 1994a; 1996).
An optimal length of 15–21 nucleotides for phosphorothioated ODNs has been demonstrated to balance specificity of targeting and cellular accumulation (Bennett, 1998). Many effective antisense ODNs target regions at or near the TSS, or in the 3′ UTR, while those targeting the coding region tend to be least effective (Dean et al., 1994b). Binding of ODNs to the TSS is believed to block translation (Bennett, 1998; Dean et al., 1994b), while hybridization with the 3′ UTR may initiate degradation of the entire mRNA (Binder et al., 1994). We found that a 20-mer ODN (ODN 83) antisense to the 3′ UTR of TS mRNA specifically down-regulated TS expression, and, when used as a single agent, inhibited HeLa cell proliferation. A scrambled control ODN (ODN 32) had no effect on TS expression or cell proliferation. In addition, ODN 83 enhanced cell sensitivity to Tomudex, 5-FU, and 5-FUdR, compared to cells treated with the control ODN. Sensitivity to drugs that do not target TS (cisplatin, chlorambucil, melphalan, doxorubicin, ionizing radiation, paclitaxel, CPT-11) was unaffected, indicating that toxicity enhancement was a specific response.
Down-regulation of other specific gene products using antisense ODNs targeted to 3′ UTRs of mRNAs has been reported. A phosphorothioated 20-mer antisense to the 3′ UTR of protein kinase C-α mRNA specifically inhibits expression of that isozyme, both in vitro and in vivo (Dean et al., 1994a; 1996). In mice, this antisense ODN inhibited proliferation of human xenografts at doses not toxic to the animal (Dean et al., 1994a; 1996). With respect to altering drug sensitivity, an antisense ODN directed against c-myc was synergistic with cisplatin in treating human melanoma xenografts in nude mice (Citro et al., 1998).
The most important step in natural regulation of TS expression is at the mRNA translational level. In synchronized MCF-7 breast tumour cells, the half-life of TS protein is 5–8 h, fluctuating 4–9 fold throughout the cell cycle, even though TS mRNA levels remain unchanged over the course of three cell cycles (Keyomarsi et al., 1993). TS regulates translation of its own mRNA by binding to the 5′-UTR (Kaneda et al., 1987). Although antisense ODNs targeted to this mRNA region may block translation (Bennett, 1998; Dean et al., 1994b), it is also suspected that such ODNs may bind to TS itself, at the polynucleotide binding site (Ju et al., 1998). This could relieve the repression of translation, as an antisense ODN targeted to the TSS of TS mRNA increased the cellular level of TS protein after 24 h (Ju et al., 1998). A TSS-targeted antisense nucleic acid can also have other effects on TS expression, including induction of TS gene transcription (DeMoor et al., 1998). In light of the potentially antagonistic responses which accompany the intended suppression of TS expression by antisense ODNs targeted to the TSS region, it is more appropriate to target the 3′ region of the mRNA.
Although the effect of ODN 83 treatment in enhancing drug cytotoxicity was assessed relative to the toxicity of the drug in ODN 32-treated cells, it was formally possible that the enhancement of cytotoxicity was a result of a pleiotropic, non-specific effect of ODN 83. In this scenario, the observed down-regulation of TS could be due to a non-specific inhibition of cell proliferation that results only secondarily in down-regulation of TS expression (assuming that TS expression is diminished in cells with lower proliferation levels). However, we observed that ODN 83 transfection inhibited proliferation for the first 48 h following transfection, but proliferation recovered to control levels thereafter (Figure 2). In 3-day post-transfected cells the level of TS was still less than 75% of the controls, indicating that TS was down-regulated in response to ODN 83 even under conditions of high cell proliferation. In addition, TS : GAPDH mRNA ratios in cells treated with LFA alone or LFA plus control ODN 32 were essentially the same, while LFA plus antisense ODN 83 appreciably reduced the TS : GAPDH ratio (Figure 3). These results collectively indicate that: (a) the initial decrease in cell proliferation was due to down-regulation of TS (and not vice versa); and (b) the enhancement of drug cytotoxicity was due to the decreased level of TS mRNA and TS protein. In light of the 5–8-h t1/2 of TS protein in MCF-7 cells (Keyomarsi et al., 1993), a 100% inhibition of new TS synthesis would yield an 88–97% decrease in TS levels 24 h following transfection. Therefore, the 70% decrease in TS protein caused by ODN 83 indicates an effective inhibition of new TS synthesis. The recovery of proliferation could have been due to deterioration of the ODN, although the phosphorothioated ODNs are generally very stable (Dean et al., 1994a; 1994b; 1996; Shaw et al., 1991; Stein & Cheng, 1993; Stein et al., 1988). The intracellular ODN concentration may be diluted by the proliferation of the cells, and there is also the possibility that it slowly diffuses out of the cells into the ODN-free medium. In animals, potential problems with time-dependent loss of ODN activity can be ameliorated by using multiple (2-day) injections.
Since the greatest effect of ODN 83 on TS level was in the first 48 h, subsequent drug treatments were conducted within this time period. The enhanced cytotoxicity of 5-FU, 5-FUdR, or Tomudex, added 24 h following ODN transfection (Figure 5 and Table 1), is in addition to the anti-proliferative activity of ODN 83 on its own (Figures 1 and 2). Therefore, the combination of ODN 83 with a TS-targeted cytotoxic drug has even greater potential as a chemotherapy regimen (Figure 6), particularly if ODN 83 can ultimately be directed preferentially to tumour cells. For example, at a concentration of 5-FUdR (4 nM) that inhibited proliferation by 40–50% in the presence of ODN 32, pretreatment with 50 nM ODN 83 decreased proliferation by a combined total of 95% (Figure 6). Similarly, proliferation of cells treated with 4 nM Tomudex was 39% of control in cells pretreated with scrambled ODN 32, but only 20% of control in cells pretreated with antisense TS ODN 83. These ODN 83-dependent increases in inhibition of cell proliferation by cytotoxic drugs could provide a basis for significant improvement of chemotherapy regimens.
Binding of drug to TS relieves the repression of TS mRNA translation by causing dissociation of TS from the TSS of the mRNA (Chu & Allegra, 1996a; Chu et al., 1991). Human mammary epithelial tumour cells treated with Tomudex or other TS-targeted drugs in vitro exhibit a transient 10–40 fold increase in TS production, half of it in the first 2 h following exposure to the drug (Keyomarsi et al., 1993). This drug-induced increase in TS protein (Chu et al., 1990; 1991; 1993a; Keyomarsi et al., 1993; Van der Wilt et al., 1992) has been observed to enhance resistance to 5-FU therapy in mice (Van der Wilt et al., 1992), and has been reported following treatment with TS-targeted chemotherapeutics in cancer patients (Peters et al., 1994; Swain et al., 1989). It is an important confounding phenomenon to be considered in designing human treatment protocols (Berne et al., 1986; Chu & Allegra, 1996a; Chu et al., 1990; 1991; 1993a; Keyomarsi & Moran, 1988; Peters et al., 1994; Swain et al., 1989; Van der Wilt et al., 1992). An antisense ODN (such as ODN 83) targeted to TS mRNA could potentially play an important role in preventing drug-induced derepression of TS mRNA translation in vivo.
An issue arising from these data is the lack of effect of ODN 83 on the action of S phase-specific agents (for example, CPT-11 and Paclitaxel) which depend upon cellular proliferation rate for toxic effect. It might be predicted that the decreased proliferation following ODN 83 exposure would enhance resistance to these agents, at least during the first 48 h following ODN transfection. We observed a trend toward increased resistance to CPT-11 (Table 1), and experiments are under way to investigate this possibility.
This report demonstrates the ability to use an antisense ODN to down-regulate a specific enzyme resulting in enhancement of cytotoxicity of a drug directed against that enzyme. A TS antisense expression vector was shown by Ju et al. (1998) to down-regulate TS protein and TS activity in KB31 cells, enhancing sensitivity of the cells to 5-FU by 5 fold. In the present report, we demonstrate that down-regulation of TS mRNA and subsequently TS protein using an ODN antisense to a sequence in the 3′ UTR can inhibit cell proliferation when used alone, and can sensitize cells to TS-targeted chemotherapy drugs. The effects of ODN 83 on human tumour growth and drug sensitivity in vivo in immune-deficient mice are currently being investigated.
Acknowledgments
Financial support for this study was provided by Zeneca Pharma Inc., Mississauga, Ontario, Canada.
Abbreviations
- 5-FdUMP
5-fluorodeoxyuridine monophosphate
- 5-FU
5-fluorouracil
- 5-FUdR
5-fluorodeoxyuridine
- D-MEM
Dulbecco's modified Eagle's medium
- GAPDH
glyceraldehyde-3-phosphate-dehydrogenase
- IC50 and IC90 values
concentrations of drug that inhibited proliferation by 50 and 90%, respectively
- LFA
Lipofectamine®
- Me-FH4
5, 10-methylene-tetrahydrofolate
- ODN
oligodeoxynucleotide
- PBS
phosphate-buffered saline (0.15 M NaCl+0.67 mM KH2PO4, pH 7.4)
- RT–PCR
reverse transcriptase–polymerase chain reaction
- Tomudex
N-(5-[N-(3,4-dihydro-2-methyl-4-oxoquinazolin-6-ylmethyl-N-methylamino]-2-thenoyl)-L-glutamic acid, ICI D1694
- TSS
translational start site
- TS
thymidylate synthase (5,10-methylenetetrahydrofolate:dUMP C-methyltransferase; EC 2.1.1.45)
- UTR
untranslated region
References
- BENNETT C.F. Antisense oligonucleotides: is the glass half full or half empty. Biochem. Pharmacol. 1998;55:9–19. doi: 10.1016/s0006-2952(97)00214-1. [DOI] [PubMed] [Google Scholar]
- BERNE M.H.O., GUSTAVSSON B.G., ALMERSJO O., SPEARS P.C., FROSING R. Sequential methotrexate/5-FU: FdUMP formation and TS inhibition in a transplantable rodent colon adenocarcinoma. Cancer Chemother. Pharmacol. 1986;16:237–242. doi: 10.1007/BF00293984. [DOI] [PubMed] [Google Scholar]
- BINDER R., HOROWITZ J.A., BASILION J.P., KOELLER D.M., KLAUSNER R.D., HARFORD J.B. Evidence that the pathway of transferrin receptor mRNA degradation involves an endonucleolytic cleavage within the 3′ UTR and does not involve poly(A) tail shortening. EMBO J. 1994;13:1969–1980. doi: 10.1002/j.1460-2075.1994.tb06466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CHU E., ALLEGRA C.J. The role of thymidylate synthase in cellular regulation. Advan. Enzyme Regul. 1996a;36:143–163. doi: 10.1016/0065-2571(95)00004-6. [DOI] [PubMed] [Google Scholar]
- CHU E., COGLIATI T., COPUR S.M., BORRE A., VOELLER D.M., ALLEGRA C.J., SEGAL S. Identification of in vivo target RNA sequences bound by thymidylate synthase. Nucl. Acids Res. 1996b;24:3222–3228. doi: 10.1093/nar/24.16.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU E., KOELLER D.M., CASEY J.L., DRAKE J.C., CHABNER B.A., ELWOOD P.C., ZINN S., ALLEGRA C.J. Autoregulation of human thymidylate synthase messenger RNA translation by thymidylate synthase. Proc. Natl. Acad. Sci. U.S.A. 1991;88:8977–8981. doi: 10.1073/pnas.88.20.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU E., KOELLER D.M., JOHNSTON P.G., ZINN S., ALLEGRA C.J. Regulation of thymidylate synthase in human colon cancer cells treated with 5-fluorouracil and interferon-γ. Molec. Pharmacol. 1993a;43:527–533. [PubMed] [Google Scholar]
- CHU E., VOELLER D.M., JONES K.L., TAKECHI T., MALEY G.F., MALEY F., SEGAL S., ALLEGRA C.J. Identification of a thymidylate synthase ribonucleoprotein complex in human colon cancer cells. Molec. Cell. Biol. 1994;14:207–213. doi: 10.1128/mcb.14.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU E., VOELLER D., KOELLER D.M., DRAKE J.C., TAKIMOTO C.H., MALEY G.F., MALEY F., ALLEGRA C.J. Identification of an RNA binding site for human thymidylate synthase. Proc. Natl. Acad. Sci. U.S.A. 1993b;90:517–521. doi: 10.1073/pnas.90.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU E., ZINN S., BOARMAN D., ALLEGRA C.J. Interaction of γ interferon and 5-fluorouracil in the H630 human colon carcinoma cell line. Cancer Res. 1990;50:5834–5840. [PubMed] [Google Scholar]
- CHURCH G.M., GILBERT W. Genomic sequencing. Proc. Natl. Acad. Sci. U.S.A. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CITRO G., D'AGNANO I., LEONOTTI C., PERINI R., BUCCI B., ZON G., CALABRETTA B., ZUPI G. c-myc antisense oligodeoxynucleotides enhance the efficacy of cisplatin in melanoma chemotherapy in vitro and in nude mice. Cancer Res. 1998;58:283–289. [PubMed] [Google Scholar]
- DANENBERG P.V. Thymidylate synthase - a target enzyme in cancer chemotherapy. Biochim. Biophys. Acta. 1977;473:73–92. doi: 10.1016/0304-419x(77)90001-4. [DOI] [PubMed] [Google Scholar]
- DEAN N.M., MCKAY R. Inhibition of protein kinase C-α expression in mice after systemic administration of phosphorothioate antisense oligodeoxynucleotides. Proc. Natl. Acad. Sci. U.S.A. 1994a;91:11762–11766. doi: 10.1073/pnas.91.24.11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEAN N.M., MCKAY R., CONDON T.P., BENNET C.F. Inhibition of protein kinase C-α expression in a human A549 cells by antisense oligonucleotides inhibits induction of intercellular adhesion molecule 1 (ICAM-1) mRNA by phorbol esters. J. Biol. Chem. 1994b;269:16416–16424. [PubMed] [Google Scholar]
- DEAN N., MCKAY R., MIRAGLIA L., HOWARD R., COOPER S., GIDDINGS J., NICKLIN P., MEISTER L., ZIEL R., GEIGER T., MULLER M., FABBRO D. Inhibition of growth of human tumor cell lines in nude mice by an antisense oligonucleotide inhibitor of protein kinase C-α expression. Cancer Res. 1996;56:3499–3507. [PubMed] [Google Scholar]
- DEMOOR J.M., VINCENT M.D., COLLINS O.M., KOROPATNICK J.Antisense nucleic acids targeted to the thymidylate synthase (TS) mRNA translation start site stimulate TS gene transcription Exp. Cell Res. 199824311–21.1998 [DOI] [PubMed] [Google Scholar]
- DENHARDT D.T. Mechanism of action of antisense RNA: sometime inhibition of transcription, processing, transport, or translation. Ann. N. Y. Acad. Sci. 1992;660:70–76. doi: 10.1111/j.1749-6632.1992.tb21059.x. [DOI] [PubMed] [Google Scholar]
- JACKMAN A.L., TAYLOR G.A., GIBSON W., KIMBELL R., BROWN M., CALVERT A.H., JUDSON I.R., HUGHES L.R. ICI D1694, a quinazoline antifolate thymidylate synthase inhibitor that is a potent inhibitor of L1210 tumor cell growth in vitro and in vivo: a new agent for clinical study. Cancer Res. 1991;51:5579–5586. [PubMed] [Google Scholar]
- JOHNSON L.F. Posttranscriptional regulation of thymidylate synthase gene expression. J. Cell. Biochem. 1994;54:387–392. doi: 10.1002/jcb.240540405. [DOI] [PubMed] [Google Scholar]
- JU J., KANE S.E., LENZ H.-J., DANENBERG K.D., CHU E., DANENBERG P.V. Desensitization and sensitization of cells to fluoropyrimidines with different antisenses directed against thymidylate synthase messenger RNA. Clin. Cancer Res. 1998;4:2229–2236. [PubMed] [Google Scholar]
- KANEDA S., TAKEISHI K., AYUSAWA D., SHIMIZU K., SENO T., ALTMAN S. Role in translation of a triple tandemly repeated sequence in the 5′-untranslated region of human thymidylate synthase mRNA. Nucl. Acids Res. 1987;15:1259–1270. doi: 10.1093/nar/15.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYOMARSI K., MORAN R.G. Mechanism of the cytotoxic synergism of fluoropyrimidines and folinic acid in mouse leukemic cells. J. Biol. Chem. 1988;263:14402–14409. [PubMed] [Google Scholar]
- KEYOMARSI K., SAMET J., MOLNAR G., PARDEE A.B. The thymidylate synthase inhibitor, ICI D1694, overcomes translational detainment of the enzyme. J. Biol. Chem. 1993;268:15142–15149. [PubMed] [Google Scholar]
- KNEE R., MURPHY P.R. Regulation of gene expression by natural antisense RNA transcripts. Neurochem. Int. 1997;31:379–392. doi: 10.1016/s0197-0186(96)00108-8. [DOI] [PubMed] [Google Scholar]
- LI B., HUGHES J.A., PHILLIPS M.I. Uptake and efflux of intact antisense phosphorothioate deoxyoligonucleotide directed against angiotensin receptors in bovine adrenal cells. Neurochem. Int. 1997;31:393–403. doi: 10.1016/s0197-0186(96)00109-x. [DOI] [PubMed] [Google Scholar]
- MCGUIRE J.J., MAGEE K.J., RUSSELL C.A., CANESTRARI J.M. Thymidylate synthase as a target for growth inhibition in methotrexate-sensitive and -resistant human head and neck cancer and leukemia cell lines. Oncology Res. 1997;9:139–147. [PubMed] [Google Scholar]
- PETERS G.J., VAN DER WILT C.L., VAN GROENINGEN C.J., SMID K., MEIJER S., PINEDO H.M. Thymidylate synthase inhibition after administration of fluorouracil with or without leucovorin in colon cancer patients: implications for treatment with fluorouracil. J. Clin. Oncol. 1994;12:2035–2042. doi: 10.1200/JCO.1994.12.10.2035. [DOI] [PubMed] [Google Scholar]
- SAMBROOK J., FRITSCH E.F., MANIATIS T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY; 1989. [Google Scholar]
- SHAW J.-P., KENT K., BIRD J., FISHBACK J., FROEHLER B. Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucl. Acids Res. 1991;19:747–750. doi: 10.1093/nar/19.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEARS C.P., GUSTAVSSON B.G. Methods for thymidylate synthase pharmacodynamics: serial biopsy, free and total TS, FdUMP and dUMP, and H4PTEGLU and CH2-H4PTEGLU assays. Adv. Exp. Med. Biol. 1988;244:97–104. doi: 10.1007/978-1-4684-5607-3_9. [DOI] [PubMed] [Google Scholar]
- STEIN C.A., CHENG Y-C. Antisense oligonucleotides as therapeutic agents – is the bullet really magical. Science. 1993;261:1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- STEIN C.A., SUBASINGHE C., SHINOZUKA K., COHEN J.S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucl. Acids Res. 1988;16:3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWAIN S.M., LIPPMAN M.E., EGAN E.F., DRAKE J.C., STEINBERG S.M., ALLEGRA C.J. Fluorouracil and high-dose leucovorin in previously treated patients with metastatic breast cancer. J. Clin. Oncol. 1989;7:890–899. doi: 10.1200/JCO.1989.7.7.890. [DOI] [PubMed] [Google Scholar]
- TAKEISHI K., KANEDA S., AYUSAWA D., SHIMIZU K., GOTOH O., SENO T. Nucleotide sequence of a functional cDNA for human thymidylate synthase. Nucl. Acid Res. 1985;13:2035–2043. doi: 10.1093/nar/13.6.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER WILT C., PINEDO H.M., SMID K., PETERS G.J. Elevation of thymidylate synthase following 5-fluorouracil treatment is prevented by the addition of leucovorin in murine colon tumors. Cancer Res. 1992;52:4922–4928. [PubMed] [Google Scholar]
- WOOLF T.M., MELTON D.A., JENNINGS C.G.B. Specificity of antisense oligonucleotides. in vivo. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7305–7309. doi: 10.1073/pnas.89.16.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Z-G., HARSTRICK A., RUSTUM Y.M. Mechanisms of resistance to fluoropyrimidines. Sem. Oncol. 1992;19:4–9. [PubMed] [Google Scholar]


