Abstract
The effects of 5-HT and 5-HT agonists to induce contraction and the 5-HT receptors mediating these effects were investigated in the proximal, central and terminal intestinal segments of Suncus murinus.
The contraction curves to 5-HT (3 nM–30 μM) were shifted to the right by methysergide (1 μM) and ritanserin (0.1 μM), without affecting the maximum response.
In the central and terminal segments (but not the proximal segments) ondansetron (1 μM) and atropine (1 μM) significantly attenuated the contractions to higher concentrations of 5-HT. The selective 5-HT4 receptor antagonist SB204070 (1 nM), failed to modify 5-HT induced contractions in any segment examined.
5-carboxamidotryptamine, α-methyl-5-HT and 5-methoxytryptamine (0.003–3.0 μM) induced contractions but unlike 5-HT, higher concentrations of these three agents failed to increase the response or were associated with a decrease in response. 2-methyl-5-HT (0.03–1.0 μM) was ten times less potent than 5-HT to induce contraction but achieved the same maximum response.
The contractions induced by the lower concentrations of 2-methyl-5-HT (0.03–1.0 μM) in all segments were markedly reduced or abolished by methysergide (1.0 μM); the response to the higher concentrations of 2-methyl-5-HT (3–30.0 μM) were markedly reduced by atropine (1.0 μM) and ondansetron (1.0 μM).
In all segments examined, tetrodotoxin (1 μM) significantly reduced the 5-HT-induced contraction.
It is concluded that the 5-HT-induced contraction was mediated via 5-HT2 (ritanserin sensitive) receptors in all regions of the intestine, with 5-HT3 (ondansetron sensitive) receptors mediating an additional major component in the central and terminal regions.
Keywords: 5-Hydroxytryptamine, contraction response, 5-HT agonists, 5-HT2 and 5-HT3 receptors, Suncus murinus intestine
Introduction
Large amounts of 5-HT are present in the gastro-intestinal tract of many species such as the guinea-pig, mouse, rat and dog (Thompson, 1971; Gabella, 1971; Juorio & Gabella, 1974; Robinson & Gershon, 1971; Gershon et al., 1965; Thompson, 1966; Feldberg & Toh, 1953; Dalgliesh et al., 1953), most of which is present in the enterochromaffin cells of the mucosa (Erspamer, 1954) with smaller amounts being found in the myenteric plexus (Branchek & Gershon, 1987).
5-HT can induce muscle contraction or relaxation responses and enhance secretion in the intestinal tract through a multiplicity of 5-HT receptor subtypes (Buchheit et al., 1985b; Chahl, 1983; Fozard, 1985; 1990; Sanger, 1987; Butler et al., 1990; Bradley et al., 1986; Costall & Naylor, 1990; Dhasmana et al., 1993). For example, 5-HT is known to mediate contraction, relaxation or secretory responses in the guinea-pig ileum, rat oesophagus and intestine and human jejunal mucosa via 5-HT2, 5-HT3, 5-HT4 and 5-ht7 receptors (Craig & Clarke, 1990; Baxter et al., 1991; Reeves et al., 1991; Kellum et al., 1994; Beubler & Horina, 1990; Carter et al., 1995). Stimulation of 5-HT3 receptors located on the vagus afferent nerve terminals of the ferret and dog is also considered to trigger the emetic reflex, evoking a reverse peristalsis in response to challenge with chemotherapeutic agents and radiation treatment (Costall et al., 1986; Miner & Sanger, 1986; Haga et al., 1993; Kamato et al., 1991; King & Sanger, 1989; Leo et al., 1992). In such studies the cat, dog, pigeon, ferret and primate have been the species most frequently used to establish the site(s) and mechanisms of action of emetogenic and antiemetic drugs (Milano et al., 1991; Lucot, 1989; Smith et al., 1986; Navarra et al., 1992; Sancilio et al., 1990; Costall et al., 1986; Wilpizeski et al., 1987), which inevitably effect gastro-intestinal function.
Recent experiments have also described the value of Suncus murinus as a species useful for the study of the actions of many types of emetogenic challenge and including those procedures or drug treatments which effect the 5-HT systems (Ueno et al., 1987; Ito, 1995; Okada, 1994). The role of the 5-HT system in moderating gastro-intestinal function in Suncus murinus has received little investigation (Muraki et al., 1988). The aim of this study was to investigate the potential of 5-HT to induce changes in muscle tension in Suncus murinus intestine and to characterize the receptors that mediate the response.
Methods
Animals and housing conditions
The experiments were carried out using adult Japanese House Musk shrew, Suncus murinus (Bradford University strain) (38–88 g) of either sex. Animals were housed in groups of not more than six in each cage and were allowed food (AQUATIC 3, trout pellets) and water ‘ad libitum'. Animals were also fed with cat food three times per week. The cages were floored with sawdust and cleaned twice a week; water and food were checked daily. The animal room was maintained at a humidity between 45–50% at 24°C and illuminated between 21.00 h to 07.00 h on a reversed light–dark cycle.
Preparation of isolated tissues
Animals were killed by cervical dislocation following a blow to the head. The whole intestine was removed and immediately placed in freshly prepared Krebs' solution (composition mM: NaCl 118, KCl 4.7, KH2PO4 1.2, MgSO4 1.2, CaCl2 2.5, NaHCO3 25 and glucose 10) and gassed with 95% O2 and 5% CO2 at room temperature. The mesentery and fatty tissue were removed and the intestine was emptied of its contents by flushing Krebs' solution gently through the intestine using a narrow tipped pipette. The length of the intestine was approximately 25–35 cm in its relaxed form and 10–15 cm in its contracted form. The intestine of the Suncus murinus lacks a caecum and it is difficult to distinguish between a duodenum, jejunum, ileum and colon. In order to create a reproducible dissection of specified segments, the intestine was cut into eight segments defined as S1 to S8 respectively of approximately 1 cm length (in contracted form) taken 1–8 cm distal to the pyloric sphincter (S1 to S7) and also 2 cm proximal to the anal region (S8). Segments S1, 2, 3 and 4 (taken 1, 2–3, 3–4 and 4–5 cm from the pyloric sphincter, respectively) are referred to as ‘proximal', segments S5, 6 and 7 (taken 5–6, 6–7 and 7–8 cm from the pyloric sphincter) as ‘central' and segment S8 as the ‘terminal' region of the intestine. In this study, since the profiles of response to 5-HT in the absence and presence of different antagonists were revealed to be similar for segments taken from the four proximal regions of the intestine, only representative data taken from segment S1 are shown. For similar reasons, representative data taken from segment S6 are shown for the three central regions of the intestine.
Tissues were bathed in 10 ml water-jacketed organ baths and placed under 0.5 g tension. The Krebs' solution was maintained at a temperature of 37±0.5°C and gassed continuously with a mixture of 95% O2 and 5% CO2. Each tissue was left to equilibrate in the presence or absence of antagonist for 1 h, and washed every 20 min. The resting tension was re-adjusted to 0.5 g when required throughout the experiment. Responses were recorded using isometric Grass transducers which were connected to an Apple Macintosh Computer Performa 630 using MacLab Software 3.5v.
Application of drugs
In preliminary experiments two non-cumulative concentration-response curves to 5-HT were constructed from the same tissue using a 22 min cycle, a 1 min contact time with a 1 h interval between the construction of the response curves and with washing every 15 min. In such studies the magnitude of response to 5-HT changed in the second curve as compared to the first curve. A consistent reduction in the sensitivity of the segments to 5-HT was observed which achieved significance in some tissues and concentrations, with the exception of the most terminal segment (S8). Indeed in segment S8 there was a trend for the contractile response to 5-HT in the second curve to be greater than that in the first curve although it was not statistically significant (Figure 1). These results negated the use of tissues to construct more than a single concentration response curve. Therefore in the subsequent experiments only one concentration-response curve to 5-HT was established in each tissue.
Figure 1.

Representative data showing contraction responses to the non-cumulative addition (1 min contact time 22 min cycle) of 5-HT (30 nM–10 μM) in the proximal S1 and terminal S8 segments of Suncus murinus intestine. The second curve was constructed in the tissues 60 min after the first. Each point represents the mean±s.e.mean, n=3; *P<0.05 taken as significant difference between the two curves.
Non-cumulative dose-response curves to 5-HT (1 nM–30 μM) in the absence (control) or presence of antagonists were constructed. The contact time between tissue and agonist was 1 min which was followed by two washings with 1 min between each wash. 5-HT was added at 22 min intervals between doses. Preliminary experiments showed that the interval was sufficient to avoid either tachyphylaxis or enhancement of the response to a subsequent challenge. The addition of agonist did not exceed 0.3% of bath volume. Tissues were left to equilibrate with the antagonists methysergide (1 μM), ritanserin (0.1 μM), ondansetron (1 μM), atropine (1 μM) or SB204070 (1 nM) for 1 h before the application of agonist. The antagonists were constantly present in the organ bath during the construction of the dose-response curves.
A control contraction to KCl (0.12 M) was also established in each tissue. The effect of agonists in the absence or presence of antagonists were also compared as a percentage of the maximum contractions obtained with KCl (0.12 M). Initial analysis using an internal standard (KCl) revealed that expression of the contractions as a percentage of the KCl control made no discernible difference to the results obtained and comparisons made to the absolute values. Hence most data are expressed in absolute values. None of the 5-HT receptor antagonists or atropine showed any effect on the spontaneous activity of the tissues or the contractions induced by KCl in any segment examined.
The number of observations is shown by ‘n', which represents the number of animals used.
Experimental design
Four different segments (i.e. S1 to S4) of the intestine were taken from any one animal and subjected to one of the following treatments: 5-HT alone and in the presence of ritanserin, methysergide, atropine, ondansetron, a combination of methysergide and ondansetron, SB204070 and a combination of methysergide and SB204070. Also, the response to 2-methyl-5-HT was determined in the absence and presence of methysergide, atropine or ondansetron. The effects of different 5-HT agonists 5-carboxamidotryptamine, 5-methoxytryptamine and α-Methyl-5-HT were also studied. A Latin Square design was used to randomize administrations of the treatments to any one segment.
Analysis of results
Changes in g tension were expressed as either a percentage of the maximal response to KCl (0.12 M) or the mean of the absolute values. The potencies of the 5-HT receptor agonists were expressed as a pEC value and the Emax as the percentage response compared to the KCl induced contraction response. The apparent pA2 values for antagonists were calculated from the Furchgott (1972) equation: pA2=log (CR-1)−log [B], where CR is the concentration ratio of agonist used in the presence and absence of antagonist (B) and is expressed as pA2±s.e.mean. The significance of differences between the control and the test responses was determined using analysis of variance (ANOVA) which was followed by Bonfferroni-Dunnett's t-test, where P values less than 0.05 were taken as significant.
Drugs
5-Hydroxytryptamine maleate (Sigma), 5-carboxamidotryptamine maleate (Research Biochemicals Inc), 5-methoxytryptamine HCl (Sigma), α-methyl-5-hydroxytryptamine (Research Biochemicals Inc), 2-methyl-5-hydroxytryptamine HCl (Tocris), atropine sulphate (Sigma), ondansetron dihydrochloride (Glaxo-Welcome), methysergide maleate (Sandoz), tetrodotoxin (Sigma) and SB204070 hydrochloride (8-amino-7-chloro(N-butyl-4-piperidyl) methylbenzo-1,4-dioxan-5-carboxylate hydrochloride) (Smith Kline Beecham) were dissolved in distilled water; ritanserin (Research Biochemicals Inc) was dissolved in methanol with distilled water being used for further dilutions. Preliminary experiments established that the vehicles used did not show any effect on the tissues.
Results
General observations
The isolated intestine of Suncus murinus showed spontaneous activity under a basal 0.5 g resting tension. The magnitude of the contractions was generally between 0.1–0.8 g and the rate of the spontaneous activity was approximately 25 to 30 cycles per min (Figure 2).
Figure 2.

Representative tracing showing the contractile response induced by increasing concentrations of 5-HT (0.3 nM–30 μM) in the isolated proximal segments of Suncus murinus intestine.
5-HT induced contraction in the intestinal tissues
The non-cumulative addition of 5-HT (10 nM–30 μM) produced concentration-dependent contraction-response curves in all segments. The contractile responses to 5-HT at concentrations below 0.3 μM developed slowly, higher concentrations produced a rapid contraction which was followed by a contraction maintained at a slightly lower level (Figure 2).
The maximum tension changes produced by 5-HT were in the range of 1.5–2.5 g in tissues taken from the proximal and central segments of the intestine; a 5.4±0.7 g maximum tension change was obtained in the terminal segments (Figures 3, 4, 5).
Figure 3.

The contractile response to 5-HT (10 nM–30 μM) in the absence and presence of (a) atropine (atr, 1 μM), ritanserin (rit, 0.1 μM), and (b) methysergide (methy, 1 μM), ondansetron (ond, 1 μM) and a combination of methysergide (1 μM) plus ondansetron (1 μM) in proximal segments taken 1 cm distal to the pyloric sphincter of the Suncus murinus intestine. Each point represents the mean±s.e.mean, n=8; *P<0.05 and **P<0.01 compared to the 5-HT control values. °P<0.05, °°P<0.01 compared to the ondansetron treatment values.
Figure 4.

The contractile response to 5-HT (10 nM–30 μM) in the absence and presence of (a) atropine (atro, 1 μM), ritanserin (rit, 0.1 μM), and (b) methysergide (methy, 1 μM), ondansetron (ond, 1 μM) and a combination of methysergide (1 μM) plus ondansetron (1 μM) in the central segments taken 6–7 cm distal to the pyloric sphincter of the Suncus murinus intestine. Each point represents the mean±s.e.mean, n=8; *P<0.05, **P<0.01 and ***P<0.001 compared to the 5-HT control values. °°P<0.01; °°°P<0.001 compared to the ondansetron treatment values.
Figure 5.

The contractile response to 5-HT (10 nM–30 μM) in the absence and presence of (a) atropine (atro, 1 μM), ritanserin (rit, 0.1 μM) and (b) methysergide (methy, 1 μM), ondansetron (ond, 1 μM) and a combination of methysergide (1 μM) plus ondansetron (1 μM) in the terminal segments taken 2 cm proximal to the anal region of the Suncus murinus intestine. Each point represents the mean±s.e.mean, n=8; *P<0.05, ***P<0.001 compared to the 5-HT control values. °P<0.05 compared to the ondansetron treatment values.
In experiments designed to determine whether tachyphylaxis developed over the time period required to construct the non-cumulative concentration-response curve to 5-HT, the repeated application of 5-HT (1 μM) at 22 min intervals produced reproducible responses over a 4 h period. 5-HT did not produce relaxation in any of the tissues examined.
The ability of the 5-HT receptor antagonists and atropine to modify 5-HT induced contractions
In tissues taken from all three regions of the intestinal tract, methysergide (1 μM) and ritanserin (0.1 μM) abolished or significantly (P<0.05) reduced the contractions induced by concentrations of 5-HT lower than 1 μM (F(3,28) ratios were in the range of 3.9–16.2 with P values of 0.0331–0.0017 for methysergide; F(2,21) ratios were in the range of 5.7–11.3 with P values of 0.0003–0.0048 for ritanserin). In the presence of methysergide or ritanserin higher concentrations of 5-HT induced contractions where the maximum response was comparable to that obtained in control tissues (Figures 345). Estimated pA2 values for methysergide and ritanserin to antagonise the effects of 5-HT in tissues taken from the three regions of the intestine were in the range of 7.78+0.03–8.3+0.1 and 8.8+0.2–9.3+0.2, respectively.
In tissues taken from the proximal region of the intestine, (i.e. S1 segments) atropine (1 μM) and ondansetron (1 μM) alone had no consistent actions to modify the 5-HT-induced contractions (Figure 3). However, in tissues taken from the central and terminal regions, both atropine and ondansetron caused a significant reduction in the contractions induced by concentrations of 5-HT greater than 1 μM (F(3,32) ratios were in the range of 10.5–23.4 with P values of 0.0001 for ondansetron; F(2,24) ratios were in the range of 4.9–26.1 with P values of 0.0001–0.0166 for atropine) (Figures 4 and 5). This reduction was significantly enhanced by the combined administration of methysergide with ondansetron in the central and terminal regions (F(3,28) ratios were in the range of 10.5–21.2 with P values of 0.0001 for the central segments; F(3,28) ratios were in the range of 12.0–23.4 with P values of 0.0001–0.03 for the terminal segments) (Figures 4 and 5). Furthermore, there was also a trend for the combined methysergide-ondansetron treatment to reduce the contractile response to the higher concentrations of 5-HT in the proximal segment (Figure 3).
SB204070 administered alone failed to modify 5-HT induced contractions in any tissue (F(3,28) ratios were in the range of 1.2–16.7 with P values of 0.10–0.97). When SB204070 was administered with methysergide, the antagonism afforded was indistinguishable from that obtained to methysergide alone. Representative data obtained from the proximal segments are shown in Figure 6.
Figure 6.

The contractile response to 5-HT (1 nM–30 μM) in the absence and presence of SB204070 (SB, 1 nM), methysergide (methy, 1 μM) and a combination of methysergide (1 μM) plus SB204070 (1 nM) in the proximal segments taken 1 cm distal to the pyloric sphincter of the Suncus murinus intestine. Each point represents the mean±s.e.mean, n=8; *P<0.05 and **P<0.01 compared to the 5-HT control values.
The effect of 5-HT receptor agonists to modify intestinal muscle tension
The 5-HT agonists 2-methyl-5-HT, 5-carboxamidotryptamine, 5-methoxytryptamine and α-methyl-5-HT (3 nM–30 μM) induced concentration-dependent contractions in all tissues examined. Representative data obtained in the central segments are shown in Figure 7. There was no evidence of a relaxant response with any of the agonists tested. In all the segments examined, 5-HT produced its contractile response with a mean pEC50 of 6.2±0.1 and Emax of 81.02±2.5% of the KCl-induced contraction. 5-methoxytryptamine (pEC50= 7.3±0.1, Emax=35.6±4.2%) and α-methyl-5-HT (pEC50= 7.4±0.1, Emax=21.2±3.4%) over a range of 3 nM–3.0 μM produced similar contraction responses, although the higher concentrations failed to achieve the maximum response attained by 5-HT. 5-carboxamidotryptamine (pEC50= 6.2±0.1, Emax=51.4±3.6%) and 2-methyl-5-HT (pEC50 =5.8±0.1, Emax=82.5±1.8%) (0.03–1.0 μM) were approximately ten times less potent but whereas 5-carboxamidotryptamine failed to produce the same maximum response as 5-HT, this was achieved using 2-methyl-5-HT in all the segments examined.
Figure 7.
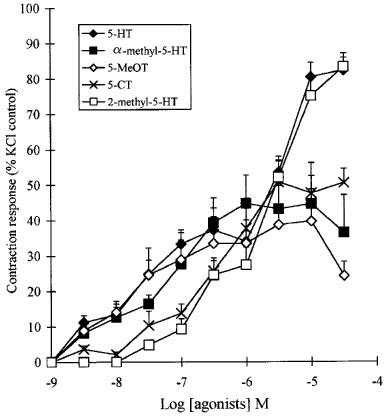
The contractile effect of 5-HT, α-methyl-5-HT, 5-methoxytryptamine (5-MeOT), 2-methyl-5-HT and 5-carboxamidotryptamine (5-CT) (30 nM–30 uM) on central segments taken 6–7 cm distal to the pyloric sphincter from the Suncus murinus intestine. Responses are expressed as a percentage of the KCl (0.12 M) induced contraction. Each point represents the mean±s.e.mean, n=6.
The effect of 5-HT receptor antagonists to inhibit the contractions induced by 2-methyl-5-HT
Neither atropine (1 μM) nor ondansetron (0.01, 0.1, and 1 μM) reduced the contractions induced by the 1 μM and lower concentrations of 2-methyl-5-HT. Both atropine and ondansetron caused a non-surmountable reduction in the contractions induced by concentrations of 2-methyl-5-HT higher than 1 μM (F(3, 28) ratios were in the range of 2.6–65.6 with P values of 0.0001–0.05 for ondansetron; F(2, 21) ratios were in the range of 3.4–18.7 with P values of 0.0016–0.05 for atropine). In contrast, methysergide (1 μM) significantly (P<0.05) antagonized the contractions induced by concentrations of 2-methyl-5-HT lower but not higher than 1.0 μM (F(2, 21) ratios were in the range of 7.1–18.7 with P values of 0.0001–0.0044 for methysergide). These profiles of action were recorded in proximal, central and terminal segments; representative data from the proximal segments are shown in Figure 8.
Figure 8.

The contractile response to 2-methyl-5-HT (30 nM–30 μM) in the absence and presence of (a) ondansetron (ond, 0.01, 0.1 and 1 μM), and (b) atropine (atr, 1 μM) and methysergide (methy, 1 μM) in the central segments taken 6–7 cm distal to the pyloric sphincter of the Suncus murinus intestine; Each point represents the mean±s.e.mean, n=8; *P<0.05, **P<0.01 and ***P<0.001 compared to the 2-methyl-5-HT control values.
The effect of tetrodotoxin (TTX) to modify the contractile response to 5-HT
TTX (1 μM) significantly reduced by approximately 40% the contractions induced by lower concentrations of 5-HT (0.03–3.0 μM) in the proximal, central and terminal regions of the intestine (F(1, 12) ratios were in the range of 8.5–300.9 with P values of 0.0001–0.01) (Figure 9). However, the contractions to higher concentrations of 5-HT (10 and 30 μM) were maximally reduced by approximately 45, 80 and 85 in the proximal, central and terminal regions of the intestine respectively.
Figure 9.

The contractile effect of 5-HT (0.3 μM–30 μM) in the absence and presence of TTX (1 μM) on the (a) proximal segments (1 cm distal to the pyloric sphincter), (b) central segments (6–7 cm distal to the pyloric sphincter) and (c) terminal segments (2 cm proximal to the anal region) of the Suncus murinus intestine. Each point represents the mean±s.e.mean, n=7; *P<0.05, **P<0.01 and ***P<0.001 compared to the 5-HT control values.
Discussion
This study has shown that the non-cumulative addition of 5-HT induced concentration related contraction responses in all segments of the Suncus murinus intestine examined, relaxation was never observed. A single response curve was constructed in each tissue since attempts to use the tissues to construct a second curve were compromised by an increase in response to 5-HT. The mechanisms mediating the contraction response to 5-HT were shown to be complex, involving both tetrodotoxin sensitive and insensitive components, and different 5-HT receptors.
The antagonism afforded by methysergide and ritanserin of the contractions induced by concentrations of 5-HT lower than one micromolar in all the segments suggests that the effects of 5-HT are mediated via 5-HT2 receptors (Bradley et al., 1986; Zifa & Fillion, 1992). 5-HT2 receptors have previously been shown to mediate 5-HT induced contractions in the guinea-pig ileum and colon and the rat stomach fundus (Adam-Vizi & Vizi, 1978; Costa & Furness, 1979; Engel et al., 1984; Eglen et al., 1992). However, the concentrations of 5-HT required to induce contractions in the intestine of Suncus murimus were lower than those required to induce 5-HT2 mediated contractions in the guinea-pig ileum (greater than three micromolar) (Buchheit et al., 1985b). Also, they were lower than required in binding and functional assays, where 5-HT has micromolar affinity for the 5-HT2 antagonist recognition sites (Zifa & Fillion, 1992; Muramatsu et al., 1988; Chahl, 1983). Whether or not the 5-HT2 receptors in Suncus murinus intestine are linked to a more effective transduction mechanism is not known, and elucidation of the 5-HT2 receptor subtype mediating the response will require further experiments using more selective 5-HT2A, 5-HT2B and 5-HT2C receptor antagonists (Baxter et al., 1995).
Ondansetron when administered alone in a concentration known to cause a highly specific and selective blockade of 5-HT3 receptors (Butler et al., 1990) failed to modify the contractions induced by 5-HT in the proximal segments (one to four centimeters distal to the pyloric sphincter) of the Suncus murinus intestine. It could therefore be concluded that 5-HT3 receptors have no involvement in mediating the contractile actions of either high or low concentrations of 5-HT in the proximal segments of the Suncus murinus intestine. However, this conclusion may be premature, since there was a trend for a combined treatment with methysergide and ondansetron to cause a greater antagonism of the contractile actions of micromolar concentrations of 5-HT than observed to methysergide alone. In addition, the contractile effect obtained by concentrations of 2-methyl-5-HT higher than one micromolar and shown to stimulate 5-HT3 receptors (Buchheit et al., 1985b; Clarke et al., 1989) was antagonized by ondansetron, suggesting the presence of 5-HT3 receptors in the proximal segments to mediate a contraction response (Ismaiel et al., 1990; Wilson et al., 1990). Atropine also reduced the contraction induced by concentrations of 2-methyl-5-HT higher than one micromolar, providing further support for the involvement of 5-HT3 receptors mediating the release of acetylcholine as has been reported from other species (Bianchi et al., 1970; Buchheit et al., 1985a).
It remains more likely that in the proximal tissues of the Suncus murinus intestine the 5-HT3 receptors play a minor role in mediating the contractile effects of 5-HT. This contrasts with data obtained in the guinea-pig where 5-HT3 receptors in the intestine mediate a major contraction response that is sensitive to 5-HT3 receptor blockade (Costa & Furness, 1979; Buchheit et al., 1985b). In the guinea-pig ileum, a 5-HT3 receptor-mediated depolarization of cholinergic neuronal terminals causes a release of acetylcholine to induce a contraction response which is antagonized by atropine (Bianchi et al., 1970; Buchheit et al., 1985a; Cohen et al., 1985; Fozard, 1990; Fox & Morton, 1989; 1990). It is relevant that in the proximal segments of the Suncus murinus intestine, atropine failed to antagonize 5-HT induced contractions, providing further support for a minor involvement of 5-HT3 receptors to mediate a cholinergic response. The presence of a limited neuronal involvement in the proximal segment also came from the modest actions of TTX to attenuate the contraction induced by concentrations of 5-HT higher than one micromolar.
The necessity for the use of a combined 5-HT2 and 5-HT3 receptor blockade to indicate a 5-HT3 receptor involvement in 5-HT induced contraction responses in the proximal segments, contrasts with the data obtained in the central and terminal segments. When using micromolar concentrations of 5-HT appropriate for 5-HT3 receptor stimulation (Buchheit et al., 1985b; Butler et al., 1988), both ondansetron and atropine when administered alone antagonized the 5-HT induced contractions in the central and terminal segments of Suncus murinus intestine. In both the central and terminal segments examined, the antagonism afforded by ondansetron appeared non-competitive, the maximum response being reduced to approximately 50% of the control values. The apparent non-competitive antagonism afforded by 5-HT3 receptor antagonists is well established (Woollard et al., 1994; Sanger, 1987), although ondansetron normally exerts a competitive antagonism (Butler et al., 1988; 1990). The data remains strongly supportive that a significant component of the 5-HT induced contraction in the central and terminal segments of Suncus murinus is induced via a 5-HT3 receptor stimulation involving an enhanced cholinergic function (Fox & Morton, 1989). A significant component of the residual contraction reflected activation of the 5-HT2 receptor, since a combined methysergide plus ondansetron treatment further reduced the contraction as compared to the ondansetron treatment alone. Further evidence for the involvement of 5-HT3 receptors in mediating a contractile response came from the antagonism by ondansetron and atropine of the responses induced by 2-methyl-5-HT in the central and terminal regions, similar to the antagonism observed in the proximal region of the intestine.
2-methyl-5-HT was used as a ligand with a modest selectivity and affinity for 5-HT3 receptors and a lower affinity for 5-HT2, 5-HT1A, 5-HT1B, 5-HT1D and 5-HT1E receptor sites (Ismaiel et al., 1990; Wilson et al., 1990; Zifa & Fillion, 1992). In the present study, methysergide was able to antagonize the contractions induced by concentrations of 2-methyl-5-HT lower than one micromolar. The results may reflect the known affinity of 2-methyl-5-HT for 5-HT2 receptors (Zifa & Fillion, 1992). The possibility that in Suncus murinus 2-methyl-5-HT has an unusual high affinity for 5-HT receptors other than 5-HT3 receptors remains to be investigated.
The 5-HT4 receptor has an established role to mediate contraction responses via a cholinergic mechanism in the guinea-pig intestine and colon (Craig & Clarke, 1990; Elswood et al., 1991). SB204070 is a highly selective and specific 5-HT4 receptor antagonist (Wardle et al., 1994) which failed when administered alone to attenuate the contractile effects of any concentration of 5-HT in any of the Suncus murinus tissues examined. Also, when SB204070 was administered with methysergide, the antagonism afforded was indistinguishable from that afforded by methysergide alone in any segment. Therefore it is unlikely that 5-HT4 receptors are involved in mediating the contraction response to 5-HT. Further evidence for the unlikely involvement of 5-HT4 receptors in mediating a contraction response came from the failure of GR125487D, another very potent 5-HT4 receptor antagonist (Gale et al., 1993), to antagonize the response to 5-HT in any of segments taken from Suncus murinus intestine (unpublished observations).
The contractile effect of 5-HT was mimicked by a number of indole 5-HT agonists. The agonist potency values in the present study are in general agreement with those recorded by McLean & Coupar (1996) studying a contraction response in the rat jejunum; 5-HT (6.94±0.19), 5-methoxytryptamine (6.95±0.21), α-methyl-5-HT (6.84±0.44) and 5-carboxamidotryptamine (7.56±0.47). 5-carboxamidotryptamine appeared to be less potent in Suncus murinus intestine. The potency of 5-methoxytryptamine and 2-methyl-5-HT in the present study is in good agreement with that in guinea-pig ileum (5-methoxytryptamine (7.4, 6.7), 2-methyl-5-HT (5.9) Buchheit et al., 1991; 1985b; Fozard, 1990). Further experiments are required to investigate the affinity of these agonists for a particular 5-HT receptor type or subtype in Suncus murinus intestine.
The attenuation of the contractile response to 5-HT by TTX suggests the existence of a neuronal mechanism to mediate 5-HT induced contractions. The involvement of a non-neuronal mechanism in response to 5-HT is also apparent since TTX failed to abolish the response completely. The residual response is likely to be a direct effect of 5-HT on the muscle in the isolated segments. Furthermore, the greater attenuation afforded by TTX of the response in the central and terminal segments to 5-HT at concentrations higher than three micromolar may suggest the greater contribution of neuronal over non-neuronal mechanism/s in mediating 5-HT induced contractions than in the proximal region of the intestine. However, the profile of attenuation of response afforded by TTX to 5-HT at concentrations lower than three micromolar appeared to be similar in all the regions of the intestine.
In the present study the contractions induced by 5-HT appeared to be sensitive to the antagonism afforded by methysergide and ritanserin suggesting a dominant role for 5-HT2 in mediating 5-HT induced contraction in all sections of the intestine of Suncus murinus. However, both methysergide and ritanserin have recently been shown to have moderate affinity (7.1–7.9, 7.1–7.7, respectively; Eglen et al., 1997) for 5-HT7 receptors which relates closely to the apparent affinity values of methysergide (7.78±0.03 to 8.3±0.1) and ritanserin (8.8±0.2 to 9.3±0.1) shown in the present study. The development of more selective 5-HT7 receptor antagonists will be required to investigate the role of 5-HT7 receptors in mediating contraction responses in the Suncus murinus intestine.
It is concluded that the contractile response to 5-HT involves 5-HT2 receptors which play a dominant non-neuronal role in mediating the contractile actions in the proximal, central and terminal regions of the intestine of Suncus murinus. 5-HT3 receptors play a relatively minor role in the proximal region and, with 5-HT2 receptors, an important neuronal role in the central and distal regions.
Abbreviations
- 5-CT
5-carboxamidotryptamine
- 5-HT
5-hydroxytryptamine
- α-methyl-5-HT
α-methyl-5-hydroxytryptamine
- 5-MeOT
5-methoxytryptamine
- TTX
tetrodotoxin
References
- ADAM-VIZI V., VIZI E.S. Direct evidence of acetylcholine releasing effect of serotonin in the Auerbach plexus. J. Neural. Trans. 1978;42:127–138. doi: 10.1007/BF01675351. [DOI] [PubMed] [Google Scholar]
- BAXTER G.S., CRAIG D.A., CLARKE D.E. 5-HT4 receptors mediate relaxation of the rat oesophageal tunica muscularis mucosae. Naunyn Schem. Arch. Pharmacol. 1991;343:439–446. doi: 10.1007/BF00169544. [DOI] [PubMed] [Google Scholar]
- BAXTER G.S., KENNETT G., BLANEY F., BLACKBURN T. 5-HT2 receptor subtypes: a family re-united. TiPS. 1995;16:105–110. doi: 10.1016/s0165-6147(00)88991-9. [DOI] [PubMed] [Google Scholar]
- BEUBLER E., HORINA G. 5-HT2 and 5-HT3 receptor subtypes mediate cholera toxin-induced intestinal secretion in the rat. Gastroenterology. 1990;99:83. doi: 10.1016/0016-5085(90)91233-v. [DOI] [PubMed] [Google Scholar]
- BIANCHI C., BEANI L., CREMA C. Effects of metoclopramide on isolated guinea-pig colon. Eur. J. Pharmac. 1970;12:332–341. doi: 10.1016/0014-2999(70)90085-3. [DOI] [PubMed] [Google Scholar]
- BRADLEY P.B., ENGEL G., FENIUK W., FOZARD J.R., HUMPHREY P.P.A., MIDDLEMISS D.N., MYLECHARANE E.J., RICHARDSON B.P., SAXENA P.R. Proposal for the classification and nomenclature of functional receptors for 5-HT. Neuropharmacology. 1986;25:563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- BRANCHEK T.A., GERSHON M.D. Development of neural receptors for serotonin in the murine bowel. J. Comp. Neurology. 1987;258:597–610. doi: 10.1002/cne.902580409. [DOI] [PubMed] [Google Scholar]
- BUCHHEIT K.H., COSTAL B., ENGLE G., GUNNING S.J., NAYLOR R.J., RICHARDSON B.P. 5-HT receptor antagonism by metoclopramide and ICS 205–930 in the guinea-pig leads to enhancement of contractions of stomach muscle strips induced by electrical field stimulation and facilitation of gastric emptying in vivo. J. Pharm. Pharmac. 1985a;37:664–667. doi: 10.1111/j.2042-7158.1985.tb05109.x. [DOI] [PubMed] [Google Scholar]
- BUCHHEIT K.H., ENGLE G., MUTSCHLER E., RICHARDSON B. Study of the contractile effect of 5-HT in the isolated longitudinal muscle strip from guinea-pig ileum. Evidence for two distinct release mechanisms. Nauny Schmied. Arch. Pharmacol. 1985b;329:36–41. doi: 10.1007/BF00695189. [DOI] [PubMed] [Google Scholar]
- BUCHHEIT K.H., GAMSE R., PFANNKUCHE H.J. SDZ 205557, a selective, surmountable antagonist for 5-HT4 receptors in the isolated guinea-pig ileum. Eur. J. Pharmacol. 1991;200:373–374. doi: 10.1016/0014-2999(91)90601-l. [DOI] [PubMed] [Google Scholar]
- BUTLER A., ELSWOOD C.J., BURRIDGE J., IRELAND S.J., BUNCE K.T., KILPATRIC G.J., TYERS M.B. The pharmacological characterization of 5-HT3 receptors in three isolated preparations derived from guinea-pig tissues. Br. J. Pharmacol. 1990;101:591–598. doi: 10.1111/j.1476-5381.1990.tb14126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTLER A., HILL J.M., IRELAND S.J., JORDAN C.C., TYERS M.B. Pharmacological properties of GR38032F, a novel antagonist at 5-HT3 receptors. Br. J. Pharmacol. 1988;94:387–412. doi: 10.1111/j.1476-5381.1988.tb11542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARTER D., CHAMPNEY M., HWANG B., EGLEN R.M. Characterisation of a postjunctional 5-HT receptor mediating relaxation of guinea-pig isolated ileum. Eur. J. Pharmacol. 1995;280:243–250. doi: 10.1016/0014-2999(95)00195-q. [DOI] [PubMed] [Google Scholar]
- CHAHL L.A. Substance P mediates atropine-sensitive response of guinea-pig ileum to serotonin. Eur. J. Pharmacol. 1983;87:485–489. doi: 10.1016/0014-2999(83)90090-0. [DOI] [PubMed] [Google Scholar]
- COHEN M.L., SCHENCK K.W., COLBERT W., WITTENAUER L. Role of 5-HT receptors in serotonin-induced contractions of nonvascular smooth muscle. J. Pharmacol. Exp. Ther. 1985;232:770–774. [PubMed] [Google Scholar]
- COSTA M., FURNESS J.B. The sites of action of 5-HT in nerve-muscle preparations from the guinea-pig small intestine and colon. Br. J. Pharmacol. 1979;65:237–248. doi: 10.1111/j.1476-5381.1979.tb07824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTALL B., DOMENEY A.M., NAYLOR R.J., TATTERSALL F.D. 5-Hydroxytryptamine M-receptor antagonism to prevent cis-platin-induced emesis. Neuropharmacology. 1986;25:959–961. doi: 10.1016/0028-3908(86)90030-4. [DOI] [PubMed] [Google Scholar]
- COSTALL B., NAYLOR R.J. 5-Hydroxytryptamine: new receptors and novel drugs for gastrointestinal motor disorders. Scand. J. Gastroenterol. 1990;25:769–787. doi: 10.3109/00365529008999215. [DOI] [PubMed] [Google Scholar]
- CLARKE D.E., CRAIG D.A., FOZARD J.R. The 5-HT4 receptor: Naughty, but nice. Trends Pharmacol. Sci. 1989;10:385–386. doi: 10.1016/0165-6147(89)90177-6. [DOI] [PubMed] [Google Scholar]
- CRAIG D.A., CLARKE D.E. Pharmacological characterization of a neuronal receptor for 5-HT in guinea-pig ileum with properties similar to the 5-HT4 receptor. J. Pharmacol. Exp. Ther. 1990;252:1378–1386. [PubMed] [Google Scholar]
- DALGLIESH C.E., TOH C.C., WORK T.S. Fractionation of the smooth muscle stimulants present in extracts of GIT. Identification of 5-HT and its distribution from Substance P. J. Physiol. 1953;120:298. doi: 10.1113/jphysiol.1953.sp004895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHASMANA K.M., ZHU Y.N., CRUZ S.L., VILLALON C.M. Minireview – gastrointestinal effects of 5-HT and related drugs. Life Sci. 1993;53:1651–1661. doi: 10.1016/0024-3205(93)90202-e. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M., JASPER J.R., CHANG D.J., MARTIN G.R. The 5-HT7 receptor: orphan found. TiPS. 1997;18:104–107. doi: 10.1016/s0165-6147(97)01043-2. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M., PERKINS L.A., WALSH L.K.M., WHITING R.L. Agonist action of indole derivatives at 5-HT1-like, 5-HT2, 5-HT3 and 5-HT4 receptors in vitro. J. Auton. Pharmacol. 1992;12:321–333. doi: 10.1111/j.1474-8673.1992.tb00381.x. [DOI] [PubMed] [Google Scholar]
- ELSWOOD C.J., BUNCE K.T., HUMPHREY P.P.A. Identification of putative 5-HT4 receptors in guinea-pig ascending colon. Eur. J. Pharmacol. 1991;196:149–155. doi: 10.1016/0014-2999(91)90421-l. [DOI] [PubMed] [Google Scholar]
- ENGEL G., HOYER D., KALKMAN H.O., WICK M.B. Identification of 5-HT2 receptors on longitudinal muscle of the guinea-pig ileum. J. Rec. Res. 1984;4:113–126. doi: 10.3109/10799898409042543. [DOI] [PubMed] [Google Scholar]
- ERSPAMER V. Pharmacology of indolealkylamines. Pharmacol. Rev. 1954;6:425–487. [PubMed] [Google Scholar]
- FELDBERG W., TOH C.C. Distribution of 5-HT in the wall of the digestive tract. J. Physiol. 1953;119:352. doi: 10.1113/jphysiol.1953.sp004850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX A.J., MORTON I.K.M. Activities of some 5-HT analogues in 5-HT3 receptor mediated release of [3H]-Ach in guinea-pig ileum. Br. J. Pharmacol. 1989;96:53P. [Google Scholar]
- FOX A.J., MORTON I.K.M. An examination of 5-HT3 receptor mediating contraction and evoked tritiated-acetylcholine release in the guinea-pig ileum. Br. J. Pharmacol. 1990;101:553–556. doi: 10.1111/j.1476-5381.1990.tb14119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOZARD J.R. 5-Methoxytryptamine (5-MeOT) discriminates between excitatory neuronal 5-HT receptors in guinea-pig ileum. J. Pharmacologie. 1985;16:498. [Google Scholar]
- FOZARD J.R.Agonists and antagonists of 5-HT3 receptors Cardiovascular pharmacology of 5-HT 1990Dordrecht: Kluwer Academic; 101–115.(eds) Saxena, P.R., Wallis, D.I., Wouters, W., Bevan, P [Google Scholar]
- FURCHGOTT R.F.The classification of adrenoceptors. An evaluation from the standpoint of receptor theory Catecholamines. Hand. Exp. Pharmacol. 1972Berlin, Heideberg, New York: Springer-Verlag; 283eds H.R. Blaschko & E. Muscholl [Google Scholar]
- GABELLA G. Glial cells in the myenteric plexus. Zeit. Naturforsch. 1971;26B:244–245. doi: 10.1515/znb-1971-0313. [DOI] [PubMed] [Google Scholar]
- GALE J.D., REEVES J.J., BUNCE K.T. Antagonism of gastroprokinetic effect of metoclopramide by GR125487D, a potent and selective 5-HT4 receptor antagonist. J. Gastrointes. Motil. 1993;5:192. [Google Scholar]
- GERSHON M.D., DRAKONTIDES A.B., ROSS L.L. Serotonin: Synthesis and release from myenteric plexus of the mouse intestine. Science. 1965;149:197–199. doi: 10.1126/science.149.3680.197. [DOI] [PubMed] [Google Scholar]
- HAGA K., INABA K., SHOJI H., MORIMOTO Y., FUKUDA T., SETOGUCHI M. The effects of orally administered Y-25130, a selective 5-HT3 receptor antagonist, on chemotherapeutic agent-induced emesis. Jpn. J. Pharmacol. 1993;63:377. doi: 10.1254/jjp.63.377. [DOI] [PubMed] [Google Scholar]
- ISMAIEL M., TITELER M., MILLER K.J., SMITH T.S., GLENNON R.A. 5-HT1 and 5-HT2 binding profile of serotonergic agents α-methyl-5-HT and 2-methyl-5-HT. J. Med. Chem. 1990;33:755–758. doi: 10.1021/jm00164a046. [DOI] [PubMed] [Google Scholar]
- ITO C., ISOBE Y., KIJIMA H., KIUCHI Y., OHTSUKI H., KAWAMURA R., TSUCHIDA K., HIGUCHI S. The antiemetic activity of GK-128 in Suncus murinus. Eur. J. Pharmacol. 1995;285:37–43. doi: 10.1016/0014-2999(95)00372-r. [DOI] [PubMed] [Google Scholar]
- JUORIO A.V., GABELLA G. Noradrenaline in the guinea-pig alimentary canal: regional distribution and sensitivity to denervation and reserpine. J. Neurochem. 1974;221:851–858. doi: 10.1111/j.1471-4159.1974.tb04304.x. [DOI] [PubMed] [Google Scholar]
- KAMATO T., MIYATA K., ITO H., YUKI H., YAMANO M., HONDA K. Anti-emetic effects of YM-060, a potent and selective 5-HT3 receptor antagonist in ferrets and dogs. Jpn. J. Pharmacol. 1991;57:387. doi: 10.1254/jjp.57.387. [DOI] [PubMed] [Google Scholar]
- KELLUM J.M., BUDHOO M.R., SIRIWARDENA A.K., SMITH E.P., JEBRAILI S.A. Serotonin induces chloride ion secretion in human jejunal mucosa in vitro via a nonneural pathway at a 5-HT4 receptor. Am. Physiol., Soc. 1994. pp. G357–G363. [DOI] [PubMed]
- KING F.D., SANGER G.J. 5-HT3 receptor antagonist. Drugs Future. 1989;14:876. [Google Scholar]
- LEO R.F., RENA M.L., CHARLES E.P., GEORGY E.M., JEFFREY S.B., GEORGE W.G., JOHN E.A., RAYMOND D.Y., ROBERT G.P., DENNIS L.D. RG12915: a potent 5-HT3 antagonist that is an orally effective inhibitor of cytotoxic drug-induced emesis in the ferret and dog. J. Pharmacol. Exp. Ther. 1992;254:450. [PubMed] [Google Scholar]
- LUCOT J.B. Blockade of 5-HT3 receptors prevents cisplatin-induced but not motion or xylazine-induced emesis in the cat. Pharmacol. Biochem. Behav. 1989;32:207–210. doi: 10.1016/0091-3057(89)90235-9. [DOI] [PubMed] [Google Scholar]
- MCLEAN P.G., COUPAR I.M. Characterisation of a postjunctional 5-ht7-like and a prejunctional 5-HT3 receptor mediating contraction of rat isolated jejunum. Eur. J. Pharmacol. 1996;312:215–225. doi: 10.1016/0014-2999(96)00456-6. [DOI] [PubMed] [Google Scholar]
- MILANO S., SIMON C., GRELOT L. In vitro release and tissue levels of ileal serotonin after cisplatine-induced emesis in the cat. Clin. Auton. Res. 1991;1:175. doi: 10.1007/BF01819832. [DOI] [PubMed] [Google Scholar]
- MINER W.D., SANGER G.R. Inhibition of cis-platin-induced vomiting by selective 5-HT M-receptor antagonism. Br. J. Pharmacol. 1986;88:497. doi: 10.1111/j.1476-5381.1986.tb10228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAKI T., FUJII E., UCHIDA Y., FURUKAWA K., IRIE K., NOMOTO T. Characterization of drug responses of the intestine of the house musk shrew (Suncus murinus) Comp. Biochem. Physiol. 1988;91C No 2:613–617. [Google Scholar]
- MURAMATSU M., TAMAKI-OHASHI J., USUKI C., ARAKI H., AIHARA H. Serotonin-2 receptor mediated regulation of release of Ach by minaprine in cholinergic nerve terminal of hippocampus of rat. Neuropharm. 1988;27:603–609. doi: 10.1016/0028-3908(88)90181-5. [DOI] [PubMed] [Google Scholar]
- NAVARRA P., MARTIRE M., CARMINE R.D., POZZOLI G., PREZIOSI P. A dual effect of some 5-HT3 receptor antagonists on cisplatin-induced emesis in the pigeon. Toxicology Letters. 1992;64:745–749. doi: 10.1016/0378-4274(92)90256-j. [DOI] [PubMed] [Google Scholar]
- OKADA F., TORII Y., SAITO H., MATSUKI N. Antiemetic effects of serotonergic 5-HT1A receptor agonists in Suncus murinus. 1994. [DOI] [PubMed]
- REEVES J.J., BUNCE K.T., HUMPHREY P.P.A. Investigation into the 5-HT receptor mediating smooth muscle relaxation in the rat oesophagus. Br. J. Pharmacol. 1991;103:1067–1072. doi: 10.1111/j.1476-5381.1991.tb12301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON R., GERSHON M.D. Synthesis and uptake of 5-HT by the myenteric plexus of the small intestine of the guinea-pig. J. Pharmacol. Exp. Ther. 1971;179:29–41. [PubMed] [Google Scholar]
- SANCILIO L.F., PINKUS L.M., JACKSON C.B., MUNSON H.R. Emetic activity of zacopride in ferrets and its antagonism by pharmacological agents. Eur. J. Pharmacol. 1990;181:303–306. doi: 10.1016/0014-2999(90)90094-m. [DOI] [PubMed] [Google Scholar]
- SANGER G.J. Increased gut cholinergic activity and antagonism of 5-hydroxytryptamine M-receptor by BRL 24924: potential clinical importance of BRL 24924. Br. J. Pharmacol. 1987;91:77–87. doi: 10.1111/j.1476-5381.1987.tb08985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH W.L., JACKSON C.B., PROAKIS A.G., LEONARD C.A., MUNSON H.R., ALPHIN R.S. Zacopride: a unique and potent inhibitor of cancer chemotherapy induced emesis in dogs. Proc. Am. Soc. Clin. Oncol. 1986;5:260. [Google Scholar]
- THOMPSON J.H. Serotonin and alimentary tract. Res. Commun. Chem. Pathol. Pharmacol. 1971;2:687–781. [PubMed] [Google Scholar]
- THOMPSON J.H.The distribution of serotonin in the gastrointestinal mucosa of the rat Ir. J. Med. Sc. 1966490411–422.Sixth Series No [Google Scholar]
- UENO S., MATSUKI N., SATIO H. Suncus murinus: a new experimental model in emesis research. Life Sci. 1987;41:513–518. doi: 10.1016/0024-3205(87)90229-3. [DOI] [PubMed] [Google Scholar]
- WARDLE K.A., ELLIS E.S., BAXTER G.S., KENNETT G.A., GASTER L.M., SANGER G.J. The effect of SB204070 a highly potent and selective 5-HT4 receptor antagonist on guinea-pig distal colon. Br. J. Pharmacol. 1994;112:789–794. doi: 10.1111/j.1476-5381.1994.tb13148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILPIZESKI C.R., LOWRY L.D., MILLER R.A., SMITH B.D., JR, GOLDMAN W. Adaptation and habituation of motion-induced vomiting in squirrel monkeys. Aviat. Space Environ. Med. 1987;58:A22–A28. [PubMed] [Google Scholar]
- WILSON H., COFFMAN W.J., COHEN M.L. 5-HT3 receptors mediate tachycardia in conscious instrumented dogs. J. Pharmacol. Exp. Ther. 1990;252:683–688. [PubMed] [Google Scholar]
- WOOLLARD D.J., BORNSTEIN J.C., FURNESS J.B. Characterization of 5-HT receptors mediating contraction and relaxation of the longitudinal muscle of guinea-pig distal colon in vitro. Naunyn-Schmied. Arch. Pharmacol. 1994;349:455–462. doi: 10.1007/BF00169133. [DOI] [PubMed] [Google Scholar]
- ZIFA M., FILLION G. 5-Hydroxytryptamine receptors. Pharmacol. Exp. Ther. 1992;44:401–458. [PubMed] [Google Scholar]


