Abstract
We have utilized the human monocytic cell line, THP-1, and freshly isolated adherent human monocytes with the compounds pyridoxalphosphate-6-azophenyl-2′,4′-disuphonic acid (PPADS), oxidized ATP, and 1-(N,O-bis{5-isoquinolinesufonyll}-N-methyl-L-tyrosyl)-4-phenylpiperazine (KN-62) to pharmacologically characterize the P2 receptor involved in ATP-induced release of interleukin 1β (IL-1β). We have also investigated the involvement of P2 receptors in lipopolysaccharide (LPS)-induced IL-1β release from both cell types.
ATP caused release of IL-1β from LPS primed THP-1 cells in both a time- and concentration-dependent manner, with a minimal effective ATP concentration of 1 mM. Stimulation of cells with 5 mM ATP resulted in detectable concentrations of IL-1β in cell supernatants within 30 min.
The ATP analogue benzoylbenzoyl ATP (DBATP), a P2X7 receptor agonist, was approximately 10 fold more potent than ATP at eliciting IL-1β release.
KN-62 (1 μM), PPADS (100 μM) or oxidized ATP (100 uM) significantly inhibited 5 mM ATP-induced IL-1β release by 81, 90 and 66% respectively, but failed to significantly inhibit LPS-induced IL-1β release in both THP-1 cells and in freshly isolated human monocytes.
In both THP-1 cells and freshly isolated human monocytes, addition of the ATP degrading enzyme apyrase (0.4 U ml−1) to cell supernatants prior to LPS activation failed to significantly inhibit the LPS-induced IL-1β release. In addition there was no correlation between extracellular ATP concentrations and IL-1β release in THP-1 cells when studied over a 6 h time period.
In conclusion our data confirm the involvement of P2X7 receptors in ATP-induced IL-1β release in human monocytes. However no evidence was obtained which would support the involvement of either endogenous ATP release or P2X7 receptor activation as the mechanism by which LPS-induces IL-1β release in either the THP-1 cell line or in freshly isolated human monocytes.
Keywords: P2X7, IL-1β, human monocytes, P2X7 receptor, ATP, LPS
Introduction
Interleukin-1β (IL-1β) is a potent mediator of inflammation and the immune response, produced primarily by activated monocytes (Dinarello, 1994). Stimulation of monocytes with bacterial endotoxin, lipopolysaccharide (LPS) induces IL-1β production via activation of the pro-IL-1 gene (March et al., 1985; Clark et al., 1986), expression of pro-IL-1β mRNA, and subsequent translation of the pro-IL-1b message. Pro-IL-1β is processed to mature, biologically active IL-1β by the cysteine protease interleukin-1 converting enzyme (ICE; Kostura et al., 1989; Black et al., 1989).
In contrast to the understanding of IL-1β production, the mechanism of release of this protein, which lacks a classical signal sequence (Rubartelli et al., 1990), remains elusive. At present there is considerable debate about whether the protein is simply released as a consequence of cellular damage, be it necrosis or apoptosis (Hogquist et al., 1991a,1991b) or whether a novel release mechanism exists, which occurs in the absence of cellular damage (Rubartelli et al., 1993; Perregaux & Gabel, 1994; Ferrari et al., 1997a). Many studies have utilized the ability of bacterial endotoxin to release mature IL-1β, although this is often not an efficient stimulant (Hogquist et al., 1991a; Laliberte et al., 1994; Perregaux & Gabel, 1994). A variety of structurally unrelated compounds, all sharing the ability to decrease intracellular K+, have been demonstrated to be more efficient stimulants of IL-1β release (Perregaux & Gabel, 1994). Of these compounds, only ATP is naturally occurring, and thus may play an important role in modulating release of this cytokine in vivo.
Extracellular ATP mediates a wide range of effects by acting on P2 receptors expressed on a variety of tissues throughout the body. P2 receptors have been classified into P2X (ligand-gated ion channels) and P2Y (G protein-coupled receptors; Fredholm et al., 1994; Burnstock et al., 1996). A further receptor, the P2Z receptor, is found mainly on cells of immune and haemopoietic origin and was originally thought to be distinct from these other classes of P2 receptor due to its unique ability to induce membrane permeabilization by formation of large pores, with consequent cell lysis (Pizzo et al., 1992; Chiozzi et al., 1996). Recently, however a P2Z receptor has been cloned from rat brain, and designated P2X7 on the basis of its homology with other P2X receptors (Surprenant et al., 1996). The human homologue of this receptor has also been identified (Rassendren et al., 1997).
Exogenous application of high concentrations of ATP has been reported to cause release of IL-1β from a variety of activated immunocytes (Rubartelli et al., 1993; Perragaux & Gabel, 1994). The involvement of a P2 receptor in this response was assumed, as ATP was more effective than ADP or AMP, and GTP and UTP were inactive (Perregaux & Gabel, 1994). More recently Ferrari and co-workers (1997a) demonstrated that oxidized ATP (OxATP), an irreversible and relatively weak antagonist at the P2Z receptor (Murgia et al., 1993), abolished ATP-induced IL-1β release in human macrophages, thus suggesting the involvement of P2Z receptors in ATP-induced IL-1β release. Importantly, OxATP has also been shown to block other P2X receptors (Evans et al., 1995), and thus this evidence alone does not unequivocally confirm the involvement of P2X7 in this response. In addition LPS has been shown to release ATP from both mouse microglial cells and culture derived human macrophages (Ferrari et al., 1996; 1997b). Since preincubation with OxATP or the ATP degrading enzymes, apyrase and hexokinase, were shown to modulate LPS-induced IL-1β release it was proposed that LPS-induced IL-1β release occurred indirectly through release of ATP and subsequent activation of P2X7 receptors.
In the present study we have further characterized P2X-mediated IL-1β release from human monocytes, by utilizing two compounds, pyridoxalphosphate-6-azophenyl-2′-4′-disulphonic acid (PPADS), and 1-(N,O,-bis[5-isoquinolinesulphonyl]-N-methyl-L-tyrosyl)-4-phenylpiperazine (KN-62), both of which have been shown to be potent antagonists at the recombinant human P2X7 receptor (Michel et al., 1998; Chessell et al., 1998; Blanchard et al., 1995). The present study also sought to investigate whether the proposed ATP-dependent mechanism of LPS-induced IL-1β release from murine microglial cells (Ferrari et al., 1997b) also occurs in human monocytes. A preliminary account of these studies has been presented to the British Pharmacological Society (Grahames et al., 1998).
Methods
Materials
1 -(N,O,-bis[5 -isoquinolinesulphonyl]- N-methyl -L-tyrosyl) -4-phenylpiperazine (KN-62), oxidized ATP, lipopolysaccharide, benzoylbenzoylATP (DBATP), and phorbol-12-myristate-13-acetate were obtained from Sigma. Pyridoxalphosphate-6-azophenyl-2′-4′-disulphonic acid (PPADS), was obtained from Tocris Cookson. RPMI and foetal calf serum were obtained from Gibco.
Cell isolation and culture
Isolation of human monocytes
Blood was collected in 1/7 volume of citrate buffer (0.56% trisodium citrate, 2.5% glucose) to prevent clotting. Blood was centrifuged and the plasma removed, leaving the buffy coat intact. Erythrocytes were lysed. Blood was centrifuged and supernatants, containing lysed erythrocytes, were discarded. Pellets were resuspended in PBS containing 0.4% human serum albumin and 0.38% trisodium citrate. The cell suspension was then layered onto a Ficoll (Pharmacia) gradient and centrifuged at 2600 r.p.m. for 30 min. Mononuclear cells were then removed from the interface. Cells were plated onto 24 well plates at a density of 3×106 cells per well. After 3 h monocytes were selected by adherence to tissue culture plastic by washing wells thoroughly with media.
THP-1 cells
THP-1 cells were maintained in suspension culture in RPMI+10% heat inactivated foetal calf serum (complete media). They were incubated in a water saturated atmosphere of 95%: 5% CO2, and passaged 1 : 10 every 5 days. Two days prior to experimentation, cells were differentiated with 0.5 μM phorbol-12-myristate-13-acetate (PMA) for 3 h. Cells were washed three times in complete media, and plated onto 24-well plates at a density of 4×105 cells per well and left to adhere overnight. The next day cells were treated with 10 ug ml−1 LPS, for 24 h. All experiments were performed in complete media on PMA differentiated, LPS primed THP-1 cells, unless otherwise stated.
Agonist studies
THP-1 cells or freshly isolated adherent monocytes were washed three times with complete media prior to agonist application. Preliminary experiments showed that removal of the IL-1β produced from the overnight LPS stimulation by washing, resulted in an increase in the ATP-induced IL-1β release (data not shown). ATP and DBATP were applied to cells at varying concentrations, usually for 30 min at 37°C and supernatants removed and assayed for IL-1β as described below.
Antagonist studies
Antagonists vs ATP-induced IL-1β release
THP-1 cells, or freshly isolated adherent human monocytes, were washed three times with complete media and incubated either for 2 h at 37°C in the presence of OxATP (THP-1 cells only) or for 30 min at 37°C in the presence of either PPADS or KN-62, prior to addition of 5 mM ATP for 30 min at 37°C. After agonist stimulation supernatants were removed and both IL-1β and lactate dehydrogenase (LDH) concentrations were determined.
Antagonists vs LPS-induced release of IL-1β
PMA differentiated THP-1 cells or freshly isolated adherent human monocytes were plated onto 24-well plates as described above. Prior to addition of 10 μg ml−1 LPS, cells were preincubated with OxATP, PPADS or KN-62 as described above. After a 24 h incubation in the presence of LPS, supernatants were removed and IL-1β concentrations were determined.
Extracellular ATP measurements
PMA differentiated THP-1 cells were plated onto 24-well plates and left to adhere overnight as described above. Cells were then divided into three test groups, control cells, cells treated with LPS only and cells treated with 0.4 U ml−1 apyrase 1 min prior to LPS treatment. LPS (10 μg ml−1) was added to the appropriate wells and supernatants were removed at various time intervals over a 6 h period. At each time point, supernatants were removed and extracellular ATP concentrations were determined using the firefly luciferase assay (Deluca, 1976). IL-1β concentrations in these same supernatants were also determined.
Western analysis
PMA-treated THP-1 cells were plated onto 6-well plates at a density of 1×106 cells per well. THP-1 cells were primed with 10 μg ml−1 LPS overnight as previously described. The next day cells were washed three times with complete media prior to 5 mM ATP application. Supernatants were removed. Adherent cells were washed in water and lysed by freeze thawing (×3). High molecular weight solutes >50,000 kDa were removed from both supernatants and cell lysates by filtration (Amicon). Filtrates were then collected and concentrated 40 times, using an Amicon concentrator.
Proteins were electrophoretically resolved on 15% polyacrylamide gels. Following transfer onto nitrocellulose, membranes were washed briefly in Tris buffered saline (TBS: Tris (pH 7.5) 50 mM, NaCl 250 mM) and blocked overnight in TBS supplemented with 0.1% Tween-20 and 5% dried milk. The anti-IL-1β antibody (R & D systems) was routinely used at 1 : 500 dilution in TBST (TBS+0.1% Tween-20) and all primary incubations were for 1 h at 22°C. Membranes were washed five times in TBST for 10 min and incubated for 1 h at 22°C with a 1 : 1000 dilution of a horseradish peroxidase-conjugated secondary. Excess antibody was removed by washing as above and immunocomplexes were visualised using enhanced chemiluminescence (ECL) detection (Amersham) according to the manufacturer's instructions.
IL-1β and LDH determination
Mature IL-1β concentrations in cell supernatants were measured with the Medgenix IL-1β EASIA, according to the manufacturer's instructions. The monoclonal capture antibody supplied with this kit does not cross react with the pro form of the IL-1β protein (up to 20 ng). LDH concentrations in cell supernatants were measured using the Promega, Cytotox 96 kit.
Data analysis
Experiments shown are representative of at least one other performed on a separate occasion statistical analyses were carried out using GraphPad Prism. In the antagonists studies data were normalized to the appropriate control response, either 5 mM ATP-, or 10 μg ml−1 LPS-induced IL-1β release, and data are the mean of three or four separate experiments with error bars showing 95% confidence intervals. Data for LDH release are shown as percentages of total cell LDH measured in parallel samples by lysis of cells with 0.1% Triton-X. All other data points are mean±s.e.mean.
Results
THP-1 cells were routinely primed with 10 μg ml−1 LPS for 24 h. Cells exposed to 10 μg ml−1 LPS for 24 h released 1429±355 pg ml−1 IL-1β compared to control levels of 41±25 pg ml−1 (n=9). Cells pretreated with LPS (24 h), washed, and then challenged with 5 mM ATP, released mature IL-1β in a time-dependent manner (Figure 1). ATP did not affect cell integrity since levels of LDH, even after a 4 h exposure to the agonist, remained below 10% of the total LDH content determined by lysis of cells with 0.1% Triton-X (Figure 1B). Exposure of cells to 5 mM ATP for 30 min released 1268±470 pg ml−1 (n=9) IL-1β compared to levels found in washed PMA differentiated LPS primed, non ATP-treated cells of 34±14 pg ml−1 (n=9). Exposure of cells to 5 mM ATP for 30 min at 37°C was subsequently used to stimulate IL-1β release. Using Western analysis it was confirmed that the IL-1β released by ATP application was the mature 17 kDa form of IL-1β, with no detectable 35 kDa pro-IL-1β found in cell supernatants, although this protein was clearly detectable in cell lysates (Figure 2).
Figure 1.
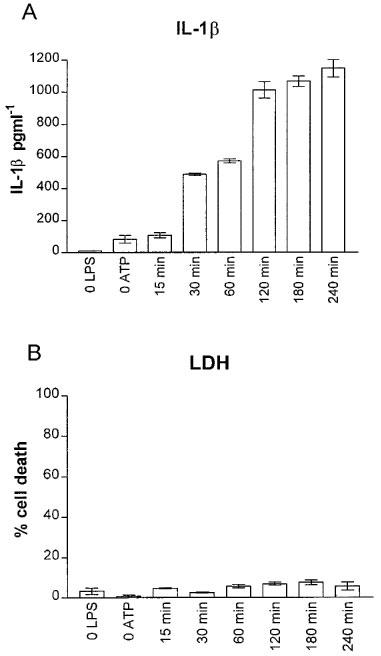
Time course of ATP-induced IL-1β (A), and lactate dehydrogenase (LDH; B) release in PMA differentiated and LPS-primed (24 h) THP-1 cells. Cells were treated with 5 mM ATP, supernatants were removed at the indicated times and assayed for both IL-1β and LDH. Control points (0 LPS, 0 ATP) are from cells treated as above, except for the omission of LPS or omission of exogenous ATP, respectively. For these points, IL-1β concentrations were measured at 240 min. Data are the mean±s.d. of triplicate determinations from one representative experiment; similar results were obtained on two other separate occasions.
Figure 2.
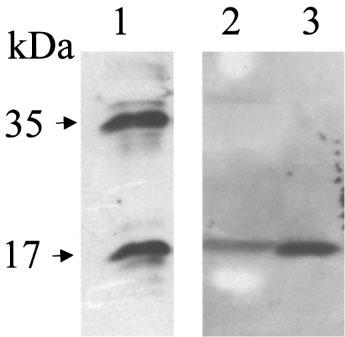
Absence of 35 kDa pro-IL-1β in LPS primed 5 mM ATP-treated THP-1 cell supernatants. Samples were prepared as described in the Methods section. Proteins were resolved on 15% polyacrylamide gels and electrophoretically transferred onto nitrocellulose. Lane 1: THP cell lysates; Lane 2: Cell supernatants. The immunoreactivity detected had the electrophoretic mobility expected for recombinant human IL-1β (lane 3).
Both ATP (100 μM–5 mM) and DBATP (30 μM–1 mM) released IL-1β from THP-1 cells in a concentration-dependent manner (data not shown). The threshold DBATP concentration of 100 μM was at least an order of magnitude lower than that required for ATP to induce release of IL-1β from the THP-1 cells.
Effect of antagonists on IL-1β release
OxATP (100 μM), PPADS (30 and 100 μM) or KN-62 (0.3 and 1 μM) inhibited the ATP-induced IL-1β release from THP-1 cells in a concentration-dependent manner (Figure 3). It was also confirmed that both PPADS and KN-62 markedly antagonized ATP-induced IL-1β release in freshly isolated adherent human monocytes (Figure 4). Preincubation of cells with OxATP, PPADS or KN-62, however caused no significant reduction in LPS-induced IL-1β release from either PMA differentiated THP-1 cells or freshly isolated adherent human monocytes (Figure 5). Paradoxically, in THP-1 cells, a 37% increase in LPS-induced IL-1β release was seen in cells preincubated with KN-62. In addition, in both cell types, concentrations of IL-1β in supernatants of cells treated with the ATP degrading enzyme, apyrase, prior to LPS treatment were not significantly different from levels of the cytokine detected in control cell supernatants (Figure 5).
Figure 3.
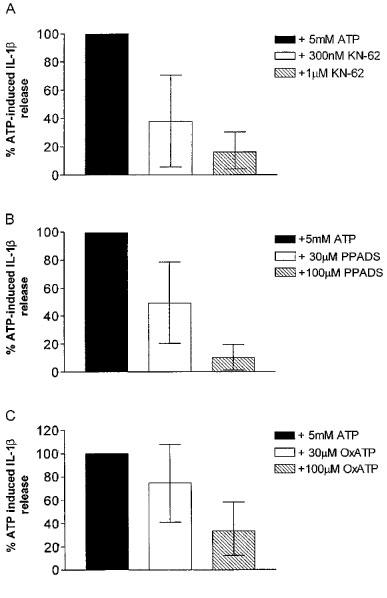
Effects of antagonists on 5 mM ATP-induced IL-1β release in the THP-1 cell line. KN-62 (A), PPADS (B) and oxidized ATP (C) at the indicated concentrations were preincubated for 30 min (2 h for oxidized ATP) with the cells prior to 5 mM ATP stimulation. Data are the mean (with 95% confidence limits) of four determinations.
Figure 4.
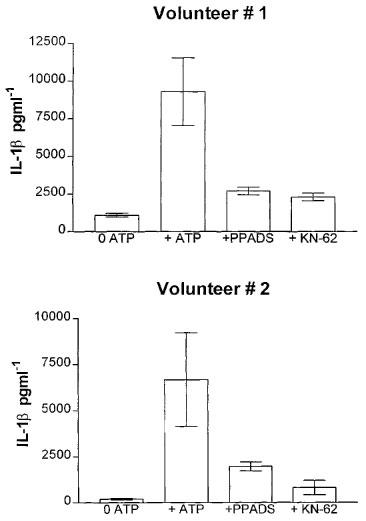
Effect of antagonists on 5 mM ATP-induced IL-1β release in freshly isolated adherent human monocytes. One μM KN-62 or 100 μM PPADS were preincubated for 30 min with the cells prior to 5 mM ATP stimulation. Data are the mean±s.d. of triplicate determinations of experiments carried out on cells isolated from two healthy volunteers.
Figure 5.
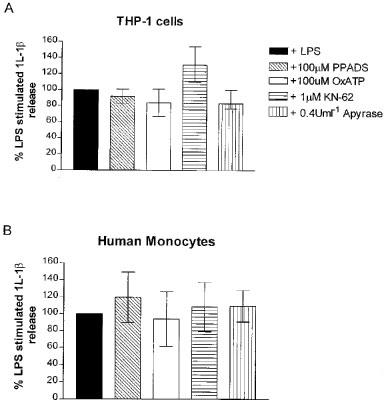
Effect of antagonists on LPS-induced IL-1β release in (A) THP-1 cells and (B) freshly isolated adherent monocytes. KN-62, PPADS and oxidized ATP at the concentrations indicated were preincubated for 30 min (2 h for oxidized ATP) with the cells prior to LPS stimulation. Data are the mean (with 95% confidence limits) of at least three separate determinations.
LPS-induced ATP release
Cells were divided into three groups as described in the Methods section and supernatants were removed at various time intervals. Concentrations of both ATP and IL-1β were determined. ATP concentrations determined in cell supernatants were relatively low (no greater than 10 nM), and no marked differences in extracellular ATP levels were observed in LPS-treated cell supernatants compared to controls (Figure 6). Addition of the ATP degrading enzyme, apyrase, effectively removed any extracellular ATP present in the cell supernatants i.e. readings obtained from cell supernatants treated with apyrase (Figure 6) were similar to reading obtained in complete media in the absence of cell (data not shown). Concentrations of IL-1β were also determined in these same supernatants, showing that removal of ATP from the cells extracellular environment had no effect on LPS-induced IL-1β release in the THP-1 cell line (Figure 6).
Figure 6.
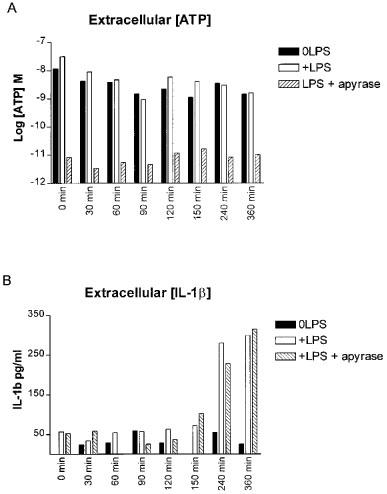
Lack of correlation between LPS-induced IL-1β and ATP release in the THP-1 cell line. Cells were treated as indicated and supernatants were removed at the times indicated. Extracellular ATP concentrations were determined using the firefly luciferase assay (A), and in some samples IL-1β concentrations were determined using an ELISA (B). Data are from one representative experiment, similar results were obtained on two other separate occasions.
Discussion
Exogenous ATP application (>1 mM) to PMA differentiated, LPS primed THP-1 cells, resulted in the externalization of mature IL-1β. This release was rapid, and after a 30 min stimulation of cells with 5 mM ATP, significant levels of mature IL-1β were detected extracellularly. This concentration- and time-dependence observed in the present study was similar to that previously reported for ATP-induced IL-1β release from activated immunocytes of both mouse and human origin (Perregaux & Gabel, 1994; Ferrari et al., 1996; 1997a,1997b).
In the present study ATP induced release of IL-1β in the THP-1 cell line occurred in the absence of significant release of the cytoplasmic enzyme LDH; even after a 4 h incubation with 5 mM ATP, levels of extracellular LDH were not different from control cells. In addition the 35 kDa pro form of IL-1β was undetectable in supernatants of ATP-stimulated cells, although large amounts of this protein were detected in cell lysates. This ability of ATP to release mature IL-1β in the absence of cell lysis is similar to data obtained in freshly isolated human monocytes (Rubartelli et al., 1993), mouse peritoneal macrophages (Perregaux & Gabel, 1994), and human macrophages (Ferrari et al., 1997a) but differs from reports on LPS-induced release of IL-1β in freshly cultured human monocytes, where release of IL-1β correlated well with an increase in LDH (Hogquist et al., 1991; Jessop & Hoffman, 1993).
In the present study the threshold concentration for DBATP-induced IL-1β release was approximately 10 fold lower than that required for ATP to elicit IL-1β release. This agonist profile of DBATP>ATP is similar to that obtained for release of IL-1β in human macrophages (Ferrari et al., 1997a) and given the requirement for high micromolar effective concentrations, is consistent with the release of IL-1β occurring via the activation of the human P2X7 receptor (Rassendren et al., 1997).
All of the three antagonists tested in the present study significantly inhibited 5 mM ATP-induced IL-1β release from PMA differentiated, LPS primed THP-1 cells in a concentration-dependent manner. KN-62 was the most potent of the antagonists tested. Ferrari and co-workers (1997a) previously reported that a 2 h preincubation with 300 μM of the irreversible P2X inhibitor, OxATP, abolished ATP-induced IL-1β release in human macrophages. Similarly in this study we showed that preincubation of 100 μM OxATP for 2 h resulted in 66% reduction in ATP-induced IL-1β release in THP-1 cells.
The non-selective P2 antagonist, PPADS, also significantly inhibited the ATP-induced IL-1β release in a concentration dependent manner, with a 30 min preincubation with 100 μM PPADS causing a 90% reduction in ATP-induced release. This relatively high concentration of PPADS is similar to that reported to block the currents at the recombinant human P2X7 receptor where PPADS antagonized DBATP-induced responses with an IC50 value of 62 μM (Rassendren et al., 1997). However much lower concentrations of PPADS have been shown to block both DBATP-induced currents, and YO-PRO1 influx in HEK-293 cells expressing the recombinant human P2X7 receptor (Michel et al., 1998; Chessell et al., 1998). It is presumed that these differences were due to the presence of 10% FCS in the present study, as we have found that increasing concentrations of serum protein decreases the apparent potency of PPADS (A.D. Michel, unpublished observations).
The Cam kinase II inhibitor, KN-62, was the most potent inhibitor of ATP-induced IL-1β release. A 30 min preincubation of 1 μM KN-62 caused an 81% reduction in ATP-induced release of IL-1β. This high potency of KN-62 is consistent with its effect at blocking ion flux, ethidium entry and phospholipase D activation in human lymphocytes (Gargett & Wiley, 1997), as well as ATP-mediated lysis in human macrophages (Blanchard et al., 1995), events which are all thought to be mediated by P2X7 receptors. This is the first demonstration of the ability of this compound to potently inhibit IL-1β release. A number of other unrelated compounds have been reported to inhibit ATP-induced IL-1β release; tenidap, DIDS, meclofenamate, flufenamic acid (Laliberte et al., 1994), and glyburide (Hamon et al., 1997). Whether or not these compounds are acting directly on the P2X7 receptor or through some other mechanism as suggested (Laliberte et al., 1994; Hamon et al., 1997) requires further investigation.
It was recently suggested by Ferrari and co-workers (1997b) that LPS-induced release of IL-1β in a mouse microglial cell line occurred via LPS-induced release of endogenous ATP, which subsequently activated P2X7 receptors. In order to study the involvement of ATP and P2X7 receptors in the LPS-induced IL-1β release in human monocytes, OxATP, PPADS and KN-62, as well as the ATP degrading enzyme, apyrase, were tested for their ability to inhibit LPS-induced IL-1β release in both the THP-1 cell line and in freshly isolated adherent human monocytes. Although in the present study these compounds antagonized ATP-induced IL-1β release from both PMA differentiated, LPS primed THP-1 cells and freshly isolated adherent human monocytes, none of these compounds significantly reduced LPS-induced IL-1β release in either cell type tested. In addition apyrase did not significantly alter LPS-induced release of IL-1β in either the THP-1 cells or the adherent human monocytes. The significant increase in LPS-induced IL-1β release in THP-1 cells after treatment with KN-62 may reflect one of the other known actions of this compound, such as inhibition of Cam kinase II. This potentiation, although intriguing, was not investigated further in the present study. Notably, the inhibition of P2X7 mediated effects by KN-62 appears to be independent of inhibition of Cam kinase II as KN-04, a related compound lacking the ability to block Cam kinase II, has also been reported to be a potent P2X7 antagonist (Gargett & Wiley, 1997).
Ferrari and co-workers (1997b) reported that mouse microglial cells chronically treated with LPS and then washed, released more ATP into the extracellular medium than non-LPS treated cells. The amount of ATP released was shown to be LPS concentration-dependent, and closely mirrored the concentration dependence of LPS-induced IL-1β release in mouse microglial cells (Ferrari et al., 1997b). We therefore measured the amount of extracellular ATP after LPS treatment in THP-1 cells over time. However we observed no marked differences in extracellular ATP concentrations between LPS treated and non-treated cells over a 6 h time period, although extracellular IL-1β levels rose significantly in a time-dependent manner in LPS treated THP-1 cells. We have also recently repeated this study on thioglycollate-elicited mouse peritoneal macrophages. Once again we saw no increase in extracellular ATP levels in cell supernatants over time following LPS treatment (C.B.A. Grahames, unpublished observations). This observation does not therefore support the idea that the differences observed between the THP-1 cells and mouse microglial cells (Ferrari et al., 1997b) can simply be explained by the differing species of origin of the two cell types. Furthermore, in THP-1 cells treated with apyrase prior to LPS stimulation, we confirmed that apyrase removed any ATP present in the extracellular environment, and that this reduction in extracellular ATP did not affect the ability of LPS to induce IL-1β release in the THP-1 cells.
Both this lack of antagonism (at least by OxATP) and lack of effect of apyrase on LPS-induced IL-1β release in THP-1 cells is in contrast to results obtained on LPS-induced IL-1β release in mouse microglial cells (Ferrari et al., 1997b), but is similar to results obtained in cultured derived human macrophages, where addition of hexokinase (an ATP degrading enzyme) caused no alteration in IL-1β release, and preincubation with OxATP (200–400 uM) caused only a 50% reduction in LPS-induced IL-1β release compared to almost complete inhibition of ATP-induced IL-1β release in the same cells (Laliberte et al., 1997).
Furthermore, in the LPS-induced ATP release studies in microglial cells the actual amounts of ATP measured were relatively low (<3 μM) compared to that required to elicit IL-1β release in the same study (>1 mM; Ferrari et al., 1997b). In addition, the possibility that LPS-treated cells were more susceptible to damage by washing than control cells was also not investigated in that study, and it is possible that the ATP ‘release' was actually a consequence of greater cell death in LPS treated cells. Variations in LPS signalling in different cell types is, however, not without precedence. In astrocytes, elevated intracellular cyclic AMP levels were found to negatively regulate LPS activation of IL-1β via the MAP kinase signalling pathway, whereas in contrast this same pathway was not significantly activated by LPS in monocytic cells (Willis & Nisen, 1996).
We conclude that in human monocytes, exogenous application of ATP results in the release of mature IL-1β, and the pharmacological profile of this release is consistent with activation of a P2X7 receptor. The LPS-induced release of IL-1β in human monocytes, however, appears to be independent of LPS-induced ATP release and subsequent activation of the P2X7 receptor.
Abbreviations
- ATP
Adenosine 5′-triphosphate
- DBATP
benzoylbenzoyl ATP
- ICE
interleukin-1 converting enzyme
- IL-1β
interleukin 1β
- LPS
lipopolysaccharide
- OxATP
oxidized ATP
- PMA
phorbol-12-myristate-13-acetate
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid
References
- BLACK R.A., KRONHEIM S.R., SLEATH P.R. Activation of interleukin-1 beta by a co-induced protease. FEBS Lett. 1989;247:386–390. doi: 10.1016/0014-5793(89)81376-6. [DOI] [PubMed] [Google Scholar]
- BLANCHARD D.K., HOFFMAN S.L., DJEU J.Y. Inhibition of extracellular ATP-mediated lysis of human macrophages by calmodulin antagonists. J. Cell Biochem. 1995;57:452–462. doi: 10.1002/jcb.240570311. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., FISCHER B., HOYLE C.H.V., MAILLARD M., ZIGANSHIN A.U., BRIZZOLARA A.L., ISAKOVICA A., BOYER J.L., HARDEN T.K., JACOBSON K.A. Structure activity relationships for derivatives of adenosine-5′-triphosphate as agonists at P2 purinoceptors: Heterogeneity within P2X and P2Y subtypes. Drug Develop. Res. 1996;31:206–219. doi: 10.1002/ddr.430310308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHESSELL I.P., MICHEL A.D., HUMPHREY P.P.A. Effects of antagonists at the human recombinant P2X7 receptor. Br. J. Pharmacol. 1998;124:1314–1320. doi: 10.1038/sj.bjp.0701958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIOZZI P., MURGIA M., FALZONI S., FERRARI D., DI VIRGILIO F. Role of the purinergic P2Z receptor in spontaneous cell death in J774 macrophage cultures. Biochem. Biophys. Res. Commun. 1996;218:176–181. doi: 10.1006/bbrc.1996.0031. [DOI] [PubMed] [Google Scholar]
- CLARK B.D., COLLINS K.L., GANDY M.S., WEBB A.C., AURON P.E. Genomic sequence from human prointerleukin 1 beta: possible evolution from a reverse transcribed prointerleukin 1 alpha gene. Nucleic Acids Res. 1986;14:7897–7914. doi: 10.1093/nar/14.20.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELUCA M. Firefly luciferase. Adv Enzymol. Relat. Areas Mol. Biol. 1976;44:37–68. doi: 10.1002/9780470122891.ch2. [DOI] [PubMed] [Google Scholar]
- DINARELLO C.A. Interleukin-1 in disease. Keio J.Med. 1994;43:131–136. doi: 10.2302/kjm.43.131. [DOI] [PubMed] [Google Scholar]
- EVANS R.J., LEWIS C., BUELL G., VALERA S., NORTH R.A., SURPRENANT A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors) Mol. Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- FERRARI D., CHIOZZI P., FALZONI S., DALSUSINO M., MELCHIORRI L., BARICORDI O.R., DI VIRGILIO F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J. Immunol. 1997a;159:1451–1458. [PubMed] [Google Scholar]
- FERRARI D., CHIOZZI P., FALZONI S., HANAU S., DI VIRGILIO F. Purinergic modulation of interleukin-1 beta from microglial cells stimulated with bacterial endotoxin. J. Exp. Med. 1997b;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRARI D., VILLALBA M., CHIOZZI P., FALZONI S., RICCIARDI-CASTAGNOLI P., DI VIRGILIO F. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J. Immunol. 1996;156:1531–1539. [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DALY J.W., HARDEN T.K., JACOBSON K.A., LEFF P., WILLIAMS M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- GARGETT C.E., WILEY J.S. The isoquinoline derivative KN-62 a potent antagonist of the P2Z-receptor on human lymphocytes. Br. J. Pharmacol. 1997;120:1483–1490. doi: 10.1038/sj.bjp.0701081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAMES C.B.A., CHESSELL I.P., MICHEL A.D., HUMPHREY P.P.A. Characterisation of ATP- and LPS-induced IL-1 beta release in THP-1 cells. Br. J. Pharmacol. 1998;123:104P. doi: 10.1038/sj.bjp.0702732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMON Y., LUCIANI M.F., BECQ F., VERRIER B., RUBARTELLI A., CHIMINI G. Interleukin-1 beta secretion is impaired by inhibitors of the ATP binding cassette transporter, ABC1. Blood. 1997;90:2911–2915. [PubMed] [Google Scholar]
- HOGQUIST K.A., UNANUE E.R., CHAPLIN D.D. Release of IL-1 from mononuclear phagocytes. J. Immunol. 1991a;147:2181–2186. [PubMed] [Google Scholar]
- HOGQUIST K.A., NETT M.A., UNANEU E.R., CHAPLIN D.D. Interleukin 1 is processed and released during apoptosis. Proc. Natl. Acad. Sci. U.S.A. 1991b;88:8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JESSOP J.J., HOFFMAN T. Production and release of IL-1 beta by human peripheral blood monocytes in response to diverse stimuli: possible role of ‘microdamage' to account for unregulated release. Lymphokine Cytokine Res. 1993;12:51–58. [PubMed] [Google Scholar]
- KOSTURA M.J., TOCCI M.J., LIMJUCO G., CHIN J., CAMERON P., HILLMAN A.G., CHARTRAIN N.A., SCHMIDT J.A. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc. Natl. Acad. Sci. U.S.A. 1989;86:5227–5231. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LALIBERTE R., PERREGAUX D., SVENSSON L., PAZOLES C.J., GABEL C.A. Tenidap modulates cytoplasmic pH and inhibits anion transport in vitro. II. Inhibition of IL-1 beta production from ATP-treated monocytes and macrophages. J. Immunol. 1994;153:2168–2179. [PubMed] [Google Scholar]
- LALIBERTE R., PERREGAUX D., MCNIFF P., GABEL C.A. Human monocyte ATP-induced IL-1 beta posttranslational processing is a dynamic process dependent on in vitro growth conditions. J. Leukocyte Biol. 1997;62:227–239. doi: 10.1002/jlb.62.2.227. [DOI] [PubMed] [Google Scholar]
- MARCH C.J., MOSLEY B., LARSEN A., CERRETTI D.P., BRAEDT G., PRICE V., GILLIS S., HENNEY C.S., KRONHEIM S.R., GRABSTEIN K. Cloning, sequence and expression of two distinct human interlekin-1 complementary DNAs. Nature. 1985;315:641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- MICHEL A.D., CHESSELL I.P., HUMPHREY P.P.A. Inhibition of human P2X7 receptor-mediated YO-PRO-1 influx by PPADS and KN-62. Br. J. Pharmacol. 1998;123:130P. [Google Scholar]
- MURGIA M., HANAU S., PIZZO P., RIPPA M., DI VIRGILIO F. Oxidised ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J. Biol. Chem. 1993;268:8199–8203. [PubMed] [Google Scholar]
- PERREGAUX D., GABEL C.A. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- PIZZO P., MUGIA M., ZAMBON A., ZANOVELLO P., PIETROBON D., DI VIRGILIO F. Role of P2Z purinergic receptors in ATP-mediated killing of tumour necrosis factor (TNF)-sensitive and TNF-resistant L929 fibroblasts. J. Immunol. 1992;149:3372–3378. [PubMed] [Google Scholar]
- RASSENDREN F., BUELL G.N., VIRGINIO C., COLLO G., NORTH R.A., SURPRENANT A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J. Biol. Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- RUBARTELLI A., COZZOLINO F., TALIO M., SITIA R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO. J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBARTELLI A., BAJETTO A., ALLAVENA G., COZZOLINO F., SITIA R. Post translational regulation of interleukin 1 beta secretion. Cytokine. 1993;5:117–124. doi: 10.1016/1043-4666(93)90050-f. [DOI] [PubMed] [Google Scholar]
- SURPRENANT A., RASSENDREN F., KAWASHIMA E., NORTH R.A., BUELL G. The cytolitic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X(7)) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- WILLIS S.A., NISEN P.D. Differential induction of the mitogen-activated protein kinase pathway by bacterial lipopolysaccharide in cultured monocytes and astrocytes. Biochem. J. 1996;313:519–524. doi: 10.1042/bj3130519. [DOI] [PMC free article] [PubMed] [Google Scholar]


