Abstract
The effects of adenosine 5′-triphosphate (ATP), uridine 5′-triphosphate (UTP) and analogues on forskolin-stimulated absorption of Na+ by porcine thyroid epithelial cells were analysed in cultures grown as confluent monolayers on permeable supports in Transwell Ussing chambers.
85% of the forskolin (10 μM)-stimulated short-circuit current was inhibited by phenamil (1 μM), which is a selective antagonist for epithelial type Na+ channels.
Phenamil-sensitive current was inhibited in a dose dependent manner by nucleotides added to the apical compartment of Ussing chambers. In contrast, the phenamil-resistant current, previously shown to represent anion secretion, was unaffected by nucleotides.
The order of potency (with EC50 values given in μM) was UTP (0.08)>>ATP (6.3)=uridine 5′-diphosphate (UDP) (6.6)>2methyl-thio-adenosine-5′-triphosphate (2MeSATP) (84.5)>adenosine 5′-diphosphate (ADP) (147.8)>α,β-methylene ATP (>150)>>adenosine (>1000).
P2 receptors mediating inhibition of sodium absorption were present on the apical membrane of the cells since addition of UTP (1–1000 μM) to the basal compartment of the Ussing chambers had little effect while subsequent addition to the apical compartment produced a normal response.
Cibachron blue (Reactive blue 2) (1–100 μM), an antagonist at some P2 receptor subtypes, inhibited phenamil sensitive current in a dose dependent manner with half maximal inhibition occurring at 14.25 μM.
Suramin (100 μM), pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) (100 μM) and pyridoxal 5′-phosphate (P5P) (100 μM) showed only slight competitive antagonism against the response to UTP.
These results indicate that a UTP-preferring P2 receptor located on the apical membrane of thyroid epithelial cells mediates inhibition of Na+ absorption.
Keywords: Purinergic, P2U, epithelia, thyroid, sodium transport, UTP, Cibachron blue
Introduction
The thyroid gland epithelium possesses a bidirectional ion transport system with absorptive and secretory activities. Absorption is dependent on active transport of Na+ (Bourke et al., 1987) while secretion is driven by secondary active transport of Cl− (Armstrong et al., 1992). This system appears to be capable of controlling the volume of the follicles (Yap et al., 1991; 1993). We have documented the activation of parts of this system by adenosine 3′,5′-cyclic monophosphate (cyclic AMP) (Bourke et al., 1990; Armstrong et al., 1992) and inhibition by Ca2+ (Manley et al., 1988). The bidirectional transport system involves a variety of cation channels and a Cl− channel on the apical membrane of the cells (Bourke et al., 1995; 1996), with an NaK2Cl symporter and Na+/K+ ATPase on the basolateral membrane (Armstrong et al., 1992).
There are many examples of hormones and diffusable mediators regulating Na+ transport in epithelia, including a number of instances of purinergic regulation of epithelial ion transport (Harden et al., 1995; Burnstock 1995). Bennett et al. (1996) reported an effect of the combination of uridine 5′-triphosphate (UTP) and the sodium channel antagonist amiloride on mucociliary clearance from lungs in patients with cystic fibrosis, which was interpreted in terms of changes in secretory and absorptive dynamics in airway epithelium. The airways have a bidirectional ion transport system (Liedtke, 1989) with similarities to that of the thyroid epithelium (Armstrong et al., 1992).
In the thyroid, purinergic mechanisms are known to activate signalling cascades and modulate ionic conductances. Adenosine 5′-triphosphate (ATP) has been shown to activate phospholipase C in a transformed line of Fischer rat thyroid cells (FRTL-5) (Sato et al., 1992), and the Ca2+ phosphatidylinositol cascade in human (Raspe et al., 1991a) and dog thyroid (Raspe et al., 1991b). Efflux of Ca2+ was recorded in response to ATP in cultured human thyrocytes (Raspe et al., 1989). In FRTL-5 cells, ATP promoted efflux of iodide (Okajima et al., 1988) and Cl− (Martin, 1992). In the intact follicles of guinea-pig thyroid slices in vitro, thyroid stimulating hormone (TSH) was found to enhance efflux of iodide (Manley et al., 1972).
However, regulation by purinergic mechanisms of the transepithelial active transport of salt by the thyroid epithelium appears not to have been described. In this paper we report that a UTP-preferring P2 receptor inhibits Na+ absorption by the thyroid epithelium, and speculate that this may play a role in co-ordination of opposing absorptive and secretory activities.
Methods
Culture preparation
Porcine thyroid cultures were established as previously described (Bourke et al., 1981; Armstrong et al., 1992). Briefly, about 30 porcine thyroid glands were collected at a local abattoir, cleared of fat and connective tissue, sliced, washed and subjected to enzymatic digestion for three 30-min periods at 37°C in Ca2+- and Mg2+-free Spinner's salt solution (Eagle, 1959; 100 ml per 100 g tissue) which contained 1 g neutral protease 1−1 with 0.1 g collagenase 1−1.
Cells were separated by filtration through stainless steel mesh (0.5 mm) and centrifugation (200×g for 10 min), and then washed three times by resuspension and centrifugation in incubation medium consisting of Minimal Essential Medium supplemented with (in mM): N-[2-Hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid] (HEPES) 20, L-glutamine 1, NaHCO3 10, nystatin (50,000 units 1−1), gentamicin (50 mg 1−1), porcine insulin (68 μM), 10% (v v−1) heat-inactivated newborn calf serum and NaOH to adjust pH to 7.4.
The cells were cultured on Costar Transwells with collagen-coated transparent membrane supports 24.5 mm in diameter at 1×106 cells/well in 1.8 ml incubation medium at 37°C in a humidified atmosphere of 2.5% CO2 in air. The medium was changed every 2 days. Cultures reached microscopically visible confluence within 4 days, and the transepithelial resistance increased to ∼8,000Ω• cm2 over the next 3–7 days (studies reported were carried out between days 9 and 14).
Electrical measurements on Ussing chambers
The cells were changed into a simplified serum-free medium, composed of (in mM): NaCl 116, NaHCO3 10, KCl 4.4, KH2PO 1, CaCl2 1.77, MgS04.7H20 0.81, glucose 5.55 and HEPES 20, requiring exactly 7.63 ml of 1 M NaOH/1 of medium to adjust the pH to 7.34 in the presence of CO2 (2.5% in air). The Transwells were maintained under a humidified atmosphere of 2.5% CO2 in air, at 37°C in a thermostatically controlled heated box.
Short-circuit current (ISC), transepithelial potential difference (TEP), and resistance were recorded by a computer-controlled apparatus taking measurements every 10 s (Armstrong et al., 1992). Transwells were maintained with identical media in upper (4 ml) and lower (8 ml) chambers, at a controlled temperature of 37°C in a humidified atmosphere of 2.5% CO2 in air. Electrodes were isolated from the Transwell by agar bridges (2% agar). Electrode chambers contained electrode buffer composed of (in mM): NaCI 150, KCl 5, and NaH2PO4 1, adjusted to pH 7.4 with NaOH. Agar bridges were equilibrated in electrode buffer. Transepithelial potential was recorded from Ag-AgCl half-cells connected to the upper and lower chambers by agar bridges 0.5 mm in diameter. Current was passed through platinum electrodes in 20-ml baths coupled to the upper and lower chambers by large (3-mm)-diameter agar bridges to isolate the culture from electrolytic products forming at the current electrodes.
Transwells were allowed to settle down for 15–20 min after installation in the apparatus and then forskolin (10 μM) was added to the basal compartment. After a spike of CI− current lasting 7 min (Armstrong et al., 1992), the short-circuit current rose over 20–30 min to a plateau, predominantly of Na+ current. After the current had stabilized, doses of test substances or control medium were added to develop cumulative dose-response curves. Nucleotides typically evoked a response with a damped train of oscillation: usually 12–15 min was needed for a steady state to be approached, and values recorded, before the next dose was added. At the completion of the series of test or control additions, phenamil (1 μM; a selective antagonist of epithelial type Na+ channels) was added. Total Na+ current was taken as the plateau values of short-circuit current minus the current remaining after phenamil. Na+ current remaining in the presence of a test dose was taken as the total current in the presence of that dose minus the current remaining after phenamil. Data were expressed as the percentage inhibition of Na+ current by test substance, and means±s.e.mean of values from replicated experiments are reported.
The concentration of agonist producing a response which was 50% of maximal (EC50) was determined by fitting dose-response data to a Michaelis-Menten type equation:
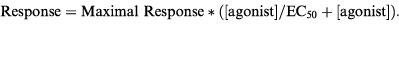 |
The fitting was performed by the Marquard-Levenberg algorithm in the program SigmaPlot (version 1.02; Jandel Corporation, PO Box 7005, San Rafael, CA, U.S.A. 94912-7005). The coefficient of variation of the estimate of EC50 was always less than 2%, and the maximal response was in the range of 71–88% for purine and pyrimidine derivatives, except for α, β-methylene ATP and adenosine whose potency was too low for a full dose-response curve to be obtained. For these latter compounds, the data were interpreted by eye, and EC50 stated as greater than certain values.
Drugs and reagents
Neutral protease (dispase, grade II, 0.5 units mg−1) was purchased from Boehringer Mannheim, Sydney, Australia, and collagenase (Worthington Type 1, 200 units mg−1), Minimal Essential Medium, glutamine and nystatin from Flow Laboratories, Sydney, Australia. Newborn calf serum was purchased from ICN Biomedicals, Sydney, Australia. Costar Transwells with collagen-coated transparent membrane supports 24.5 mm in diameter (Transwell-COL 3425) were obtained from Costar, Cambridge, MA, U.S.A.
Phenamil, pyridoxalphosphate-6-azophenyl-2′, 4′-disulphonic acid (PPADS), pyridoxal 5′-phosphate (P5P) and suramin were purchased from Research Biochemicals International, (Natick, MA, U.S.A.). Phenamil was dissolved in dimethyl sulphoxide (DMSO; Sigma Australia, Castle Hill, NSW, Australia) as 10 mM stock solution. The maximal concentration of DMSO in the final incubation media was 0.1% vol vol−1, appropriate control experiments showed this solvent to be without effect at this concentration. PPADS and P5P were dissolved in water. ATP, UTP and other mononucleotides were purchased from Sigma. All other reagents were analytical grade.
Results
Cultured porcine thyroid epithelial cells grown as a monolayer in Transwell Ussing chambers exhibited a basal positive TEP and ISC which increased in a characteristic pattern on stimulation with cyclic AMP secretagogues, such as forskolin (Figure 1 and Armstrong et al., 1992). After a biphasic rising zone, the plateau current remained constant or declined only slightly over 3 h (Figure 1). The majority of the current (85±1.2%, mean±S.E.M, n=13) was inhibited by the antagonist phenamil, which has a high affinity and specificity for epithelial Na+ channels (Figure 1).
Figure 1.
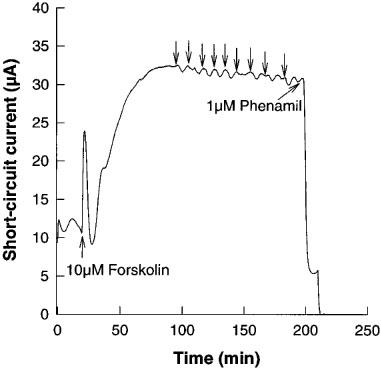
Response of the short-circuit current in a porcine thyroid epithelial cell monolayer cultured on a permeable support in a Transwell Ussing chamber to addition of forskolin (10 μM), multiple additions of control medium (arrows), followed by the sodium channel antagonist, phenamil (1 μM). Data shown are a representative experiment from six replications.
Addition of purinergic agonists resulted in inhibition of the majority of the phenamil-sensitive ISC. The responses had a characteristic damped oscillation, however it was practicable to obtain cumulative dose-response curves within 3 h (Figure 2 shows a typical response to ATP). Means of repeated experiments with purinergic agonists gave the following order of potency (with EC50 values given in μM) UTP (0.08)>>ATP (6.3)=uridine 5′ diphosphate (UDP) (6.6)>2methyl-thio-adenosine-5′ triphosphate (2MeSATP) (84.5)>adenosine 5′ diphosphate (ADP) (147.8)>α, β-methylene ATP (>150)>>adenosine (>1000) (Figure 3).
Figure 2.
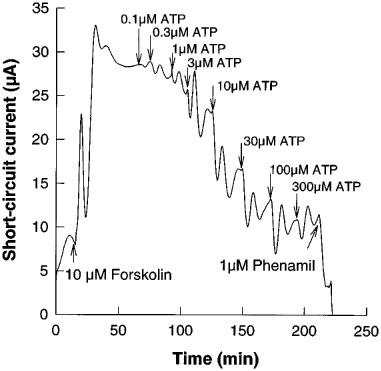
Response of the short-circuit current in a porcine thyroid epithelial cell monolayer cultured on a permeable support in a Transwell Ussing chamber to addition of forskolin (10 μM), followed after settling time by cumulative additions of adenosine triphosphate (ATP), and finally the sodium channel antagonist, phenamil (1 μM). Data shown are a representative experiment from seven replications.
Figure 3.
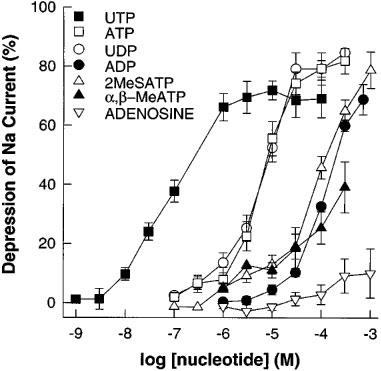
Inhibition of forskolin stimulated (10 μM) short-circuit current in porcine thyroid epithelial cell monolayers cultured on permeable supports in Transwell Ussing chambers by purines and pyrimidines. Data shown are cumulative dose response curves of the depression of short-circuit current expressed as a per cent of the total phenamil sensitive current (1 μM) (see Figure 2); means±s.e.mean (n=5–10). Compounds used were uridine 5′-triphosphate (UTP); uridine 5′-diphosphate (UDP); adenosine 5′-triphosphate (ATP); 2-methyl-thio-adenosine-5′-triphosphate (2MeSATP); adenosine 5′-diphosphate (ADP); α,β-methylene adenosine triphosphate (α,β-MeATP).
Purinergic agonists were conspicuously more effective on addition to the apical than the basal compartment of the Transwells (Figure 4). Indeed, it was possible to add up to 1000 μM UTP to the basal compartment with only a modest effect, then obtain an essentially normal dose-response curve to UTP added to the apical compartment. It appears therefore, that the receptor mediating inhibition of Na+ transport is located on the apical membrane of these polarized epithelial cells.
Figure 4.
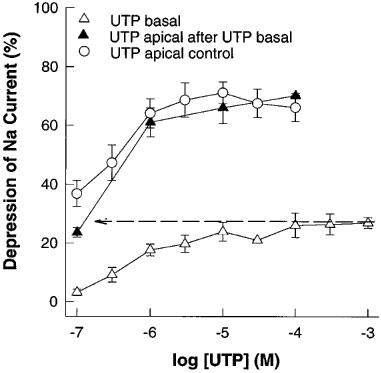
Inhibition of forskolin stimulated (10 μM) short-circuit current in porcine thyroid epithelial cell monolayers by uridine 5′-triphosphate (UTP) added to the basal, and then apical, compartments of Transwell Ussing chambers. Data shown are cumulative dose response curves of the depression of short-circuit current expressed as a per cent of the total phenamil sensitive current (1 μM) (see Figure 2). After completion of the cumulative dose response curve for UTP added to the basal compartment 1000 μM UTP remained in the basal compartment while doses of UTP commencing at 0.1 μM were added to the apical compartment. Data are means±s.e.mean (n=6).
Cibachron blue (Reactive blue 2) which is an antagonist at a number of typical P2 purinergic receptor subtypes (Burnstock, 1995), inhibited phenamil sensitive ISC with an EC50 of 14.3 μM (Figure 5). Suramin, PPADS and P5P were essentially without effect alone (Figure 5) or as antagonists of the UTP response (Figure 6).
Figure 5.
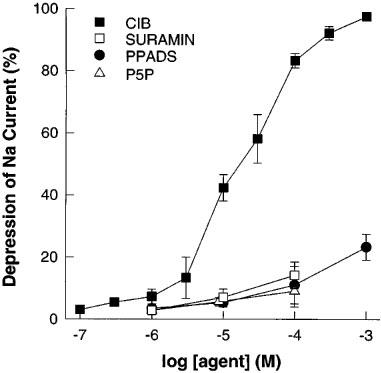
Effect on forskolin stimulated (10 μM) short-circuit current in porcine thyroid epithelial cell monolayers cultured on permeable supports in Transwell Ussing chambers of putative antagonists of purinergic receptors. Data shown are cumulative dose response curves of the depression of short-circuit current expressed as a per cent of the total phenamil sensitive current (1 μM) (see Figure 2). Compounds used were Suramin, Cibachron blue (Reactive Blue 2) (CIB), pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS); pyridoxal 5′-phosphate (P5P). Data are means±s.e.mean (n=4–6).
Figure 6.
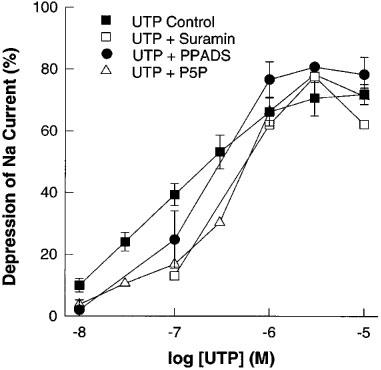
Effect of the purinergic receptor antagonists suramin (100 μM), pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) (100 μM) and pyridoxal 5′-phosphate (P5P) (100 μM) on the response to UTP of the forskolin stimulated (10 μM) short-circuit current in porcine thyroid epithelial cell monolayers cultured on permeable supports in Transwell Ussing chambers. Data shown are cumulative dose response curves of the depression of short-circuit current expressed as a per cent of the total phenamil sensitive current (1 μM) (see Figure 2). Data are means±s.e.mean (n=4–5 for UTP+antagonists, or n=10 for UTP control).
Discussion
The thyroid epithelium possesses opposing ion transport systems, which are able to alter follicular volume by secretion or absorption of ions and osmotically obliged water (Yap et al., 1991; Armstrong et al., 1992). Absorption of Na+ through phenamil-sensitive epithelial Na+ channels on the apical membrane of the cells (Bourke et al., 1996), followed by extrusion of Na+ through the Na+/K+ ATPase on the basolateral membrane, drives fluid absorption, which reduces follicle volume (Yap et al., 1991). Conversely, when Na+ absorption is blocked, secondary active transport of Cl− drives fluid secretion (Armstrong et al., 1992), which increases follicle volume, and is essential for the formation of the lumen in reassembled thyroid follicles in culture (Yap et al., 1994). We have shown that altering follicle volume by osmotic challenge leads to a corrective response mediated by changes in ion transport (Yap et al., 1993), suggesting that this system plays a role in physiological regulation of follicle fluid dynamics.
Understanding of the regulation of such a complex system remains elusive, however. Activation of the cyclic AMP-dependent protein kinase A pathway results in immediate (seconds) activation of Cl− secretion (Armstrong et al., 1992), through 6 pS Cl− channels on the apical membrane (Bourke et al., 1995). Agents promoting cyclic AMP accumulation cause a slower (minutes) increase in short-circuit current due to Na+ absorption (Armstrong et al., 1992). In studies of transepithelial movement of fluid as assessed by changes in the height of domes (detachments of the epithelial monolayer from the culture dish substrate; Bourke et al., 1987), we found that increasing intracellular Ca2+ with the Ca2+ ionophore A23187, inhibited Na+ absorption (Manley et al., 1988). In addition to regulation of ion channels on the apical membrane, processes on the basolateral membrane may be important in activation of ion transport in response to cyclic AMP (Bourke et al., 1990) and follicle stretch induced by hypotonic media (Yap et al., 1993).
The present studies suggest a possible mechanism for co-ordination of opposing secretory and absorptive activities. It has been proposed that a mechanism involving (or linked to) the CFTR Cl− channel releases ATP (al-Awqati, 1995; Cantiello et al., 1998). The Cl− channels on the apical membrane of thyroid epithelial cells have properties consistent with CFTR (Bourke et al., 1995). The P2 receptor mechanism we describe here could mediate reciprocal regulation of Cl− and Na+ transport since the activation of secretion through Cl− channels would lead to P2 receptor-mediated inhibition of absorption through Na+ channels. The P2 receptor in these cells is on the apical membrane, where it will potentially be exposed to nucleotides released on activation of apical membrane Cl− channels.
The classification of receptors responding to nucleotides is complex and evolving rapidly. Cloning of a number of receptors has placed on a firm footing the classification of purinergic receptors into G protein-coupled P2Y and intrinsic ion channel P2X families (Fredholm et al., 1997).
There is evidence for a class of P2Y receptors, which, although not constituting a separate molecular family, behave functionally as pyrimidinergic receptors, preferring UTP to ATP (Communi & Boeynaems, 1997). Communi et al. (1995) cloned a G protein-coupled receptor with this property, exhibiting 51% sequence identity with the human P2Y2 receptor. A functional pyrimidinergic receptor (Communi et al., 1996), initially classified as P2Y4 was, like the system described in the present studies, insensitive to suramin, and preferred UTP. However, it differed in that PPADS strongly inhibited the UTP response.
Cibachron blue (Reactive blue 2) is an antagonist at a number of typical P2 purinergic receptor subtypes (Burnstock, 1995). However, in the present studies it inhibited phenamil sensitive ISC. This effect may have been non-specific, or, if mediated via the receptor, would have involved stimulation instead of inhibition.
Although the present studies provide no evidence about the signal transduction cascade activated by the P2 receptor, it has been shown in a number of cell types that UTP mobilizes intracellular Ca2+ through a G protein coupled receptor mechanism activating phospholipase C to produce inositol triphosphate (IP3) (Communi & Boeynaems, 1997). In the present data, the response to nucleotides showed marked oscillations, a phenomenon which has been widely observed in Ca2+ mediated responses to purinergic agents (Morley et al., 1994) and has been subjected to theoretical analysis (Sneyd et al., 1995). Since we have shown that increased intracellular Ca2+ inhibits Na+ absorption (Manley et al., 1988), such a mechanism would be the leading hypothesis for testing in future work on the response of thyroid epithelial cells to nucleotides.
In conclusion, these studies demonstrate a UTP-preferring P2 receptor, located on the apical membrane of thyroid epithelial cells. Activation of this receptor leads to inhibition of Na+ absorption. If release of ATP were coupled to cyclic AMP dependent activation of secretion, this purinergic receptor mechanism could play a role in co-ordination of the opposing absorptive and secretory activities of the thyroid epithelium.
Acknowledgments
This research was supported by the National Health and Medical Research Council of Australia.
Abbreviations
- A23187
calcium ionophore A23187
- ADP
adenosine 5′-diphosphate
- α,βMeATP
α,β-methylene ATP
- 2MeSATP
2methyl-thio-adenosine-5′-triphosphate
- ATP
adenosine 5′-triphosphate
- ATPase
adenosine 5′-triphosphatase
- CFTR
cystic fibrosis transmembrane conductance regulator
- CIB
Cibachron blue=Reactive blue 2
- cyclic AMP
adenosine 3′,5′-cyclic monophosphate
- EC50
concentration exerting a half-maximal effect
- DMSO
dimethylsulphoxide
- FRTL5 cells
transformed line of Fischer rat thyroid cells
- G protein
guanyl nucleotide binding protein
- HEPES
(N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid])
- IP3
inositol 1,4,5-trisphosphate
- ISC
short-circuit current
- P2
class of purinergic receptors
- P5P
pyridoxal 5′-phosphate
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid
- TEP
transepithelial potential difference
- TSH
thyroid stimulating hormone
- UDP
uridine 5′-diphosphate
- UTP
uridine 5′-triphosphate
References
- AL-AWQATI Q. Regulation of ion channels by ABC transporters that secrete ATP. Science. 1995;269:805–806. doi: 10.1126/science.7543697. [DOI] [PubMed] [Google Scholar]
- ARMSTRONG J., MATAINAHO T., CRAGOE E.J., JR, HUXHAM G.J., BOURKE J.R., MANLEY S.W. Bidirectional ion transport in thyroid: secretion of anions by cultured porcine thyroid cell monolayers which absorb sodium. Am. J. Physiol. 1992;262:E40–E45. doi: 10.1152/ajpendo.1992.262.1.E40. [DOI] [PubMed] [Google Scholar]
- BENNETT W.D., OLIVIER K.N., ZEMAN K.L., HOHNEKER K.W., BOUCHER R.C., KNOWLES M.R. Effect of uridine 5′-triphosphate plus amiloride on mucociliary clearance in adult cystic fibrosis. Am. J. Respir. Crit. Care Med. 1996;153:1796–1801. doi: 10.1164/ajrccm.153.6.8665037. [DOI] [PubMed] [Google Scholar]
- BOURKE J.R., ABEL K.C., HUXHAM G.J., SAND O., MANLEY S.W. Sodium channel heterogeneity in the apical membrane of thyroid epithelial cells. J. Endocrinol. 1996;149:101–108. doi: 10.1677/joe.0.1490101. [DOI] [PubMed] [Google Scholar]
- BOURKE J.R., CARSELDINE K.L., FERRIS S.H., HUXHAM G.J., MANLEY S.W. Changes in membrane potential of cultured porcine and human thyroid cells in response to thyrotrophin and other agents. J. Endocrinol. 1981;88:187–196. doi: 10.1677/joe.0.0880187. [DOI] [PubMed] [Google Scholar]
- BOURKE J.R., CRAGOE E.J., JR, HUXHAM G.J., PEARSON J.V., MANLEY S.W. Control of ion transport in the thyroid: Prostaglandin E2 activates cation transport on the basal membrane of cultured porcine thyroid cell monolayers. J. Endocrinol. 1990;127:197–202. doi: 10.1677/joe.0.1270197. [DOI] [PubMed] [Google Scholar]
- BOURKE J.R., MATAINAHO T., HUXHAM G.J., MANLEY S.W. Cyclic AMP-stimulated fluid transport in the thyroid: influence of thyroid stimulators, amiloride and acetazolamide on dynamics of domes in monolayer cultures of porcine thyroid cells. J. Endocrinol. 1987;115:19–26. doi: 10.1677/joe.0.1150019. [DOI] [PubMed] [Google Scholar]
- BOURKE J.R., SAND O., ABEL K.C., HUXHAM G.J., MANLEY S.W. Chloride channels in the apical membrane of thyroid epithelial cells are regulated by cyclic AMP. J. Endocrinol. 1995;147:441–448. doi: 10.1677/joe.0.1470441. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Current state of purinoceptor research. Pharm. Acta Helv. 1995;69:231–242. doi: 10.1016/0031-6865(94)00043-u. [DOI] [PubMed] [Google Scholar]
- CANTIELLO H.F., JACKSON G.R., JR, GROSMAN C.F., PRAT A.G., BORKAN S.C., WANG Y., REISIN I.L., O'RIORDAN C.R., AUSIELLO D.A. Electrodiffusional ATP movement through the cystic fibrosis transmembrane conductance regulator. Am. J. Physiol. 1998;274:C799–C809. doi: 10.1152/ajpcell.1998.274.3.C799. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., BOEYNAEMS J.M. Receptors responsive to extracellular pyrimidine nucleotides. Trends Pharmacol. Sci. 1997;18:83–86. doi: 10.1016/s0165-6147(96)01035-8. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., MOTTE S., BOEYNAEMS J.M., PIROTTON S. Pharmacological characterization of the human P2Y4 receptor. Eur. J. Pharmacol. 1996;317:383–389. doi: 10.1016/s0014-2999(96)00740-6. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., PIROTTON S., PARMENTIER M., BOEYNAEMS J.M. Cloning and functional expression of a human uridine nucleotide receptor. J. Biol. Chem. 1995;270:30849–30852. doi: 10.1074/jbc.270.52.30849. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DUBYAK G.R., HARDEN T.K., JACOBSON K.A., SCHWABE U., WILLIAMS M. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol. Sci. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDEN T.K., BOYER J.L., NICHOLAS R.A. P2-purinergic receptors: subtype-associated signaling responses and structure. Annu. Rev. Pharmacol. Toxicol. 1995;35:541–579. doi: 10.1146/annurev.pa.35.040195.002545. [DOI] [PubMed] [Google Scholar]
- LIEDTKE C.M. Regulation of chloride transport in epithelia. Annu. Rev. Physiol. 1989;51:143–160. doi: 10.1146/annurev.ph.51.030189.001043. [DOI] [PubMed] [Google Scholar]
- MANLEY S.W., BOURKE J.R., HAWKER R.W. Kinetic aspects of the depression of 131-iodide concentration by thyrotrophin in thyroid tissue in vitro. J. Endocrinol. 1972;54:387–398. doi: 10.1677/joe.0.0540387. [DOI] [PubMed] [Google Scholar]
- MANLEY S.W., ROSE D.S., HUXHAM G.J., BOURKE J.R. Role of calcium ion in the secretomotor response of the thyroid: effects of A23187 and medium calcium concentration on radioiodine turnover in cultured porcine thyroid cells. J Endocrinol. 1988;116:373–380. doi: 10.1677/joe.0.1160373. [DOI] [PubMed] [Google Scholar]
- MARTIN S.C. ATP activates a Ca2+-dependent Cl− current in the rat thyroid cell line, FRTL-5. J. Membr Biol. 1992;125:243–253. doi: 10.1007/BF00236437. [DOI] [PubMed] [Google Scholar]
- MORLEY P., VANDERHYDEN B.C., TREMBLAY R., MEALING G.A., DURKIN J.P., WHITFIELD J.F. Purinergic receptor-mediated intracellular Ca2+ oscillations in chicken granulosa cells. Endocrinology. 1994;134:1269–1276. doi: 10.1210/endo.134.3.8119167. [DOI] [PubMed] [Google Scholar]
- OKAJIMA F., SHO K., KONDO Y. Inhibition by islet-activating protein, pertussis toxin, of P2-purinergic receptor-mediated iodide efflux and phosphoinositide turnover in FRTL-5 cells. Endocrinology. 1988;123:1035–1043. doi: 10.1210/endo-123-2-1035. [DOI] [PubMed] [Google Scholar]
- RASPE E., ANDRY G., DUMONT J.E. Adenosine triphosphate, bradykinin, and thyrotropin-releasing hormone regulate the intracellular Ca2+ concentration and the 45 Ca2+ efflux of human thyrocytes in primary culture. J. Cell Physiol. 1989;140:608–614. doi: 10.1002/jcp.1041400328. [DOI] [PubMed] [Google Scholar]
- RASPE E., LAURENT E., ANDRY G., DUMONT J.E. ATP, bradykinin, TRH and TSH activate the Ca2+-phosphatidylinositol cascade of human thyrocytes in primary culture. Mol. Cell. Endocrinol. 1991a;81:175–183. doi: 10.1016/0303-7207(91)90216-f. [DOI] [PubMed] [Google Scholar]
- RASPE E., LAURENT E., CORVILAIN B., VERJANS B., ERNEUX C., DUMONT J.E. Control of the intracellular Ca(2+)-concentration and the inositol phosphate accumulation in dog thyrocyte primary culture: evidence for different kinetics of Ca2+-phosphatidylinositol cascade activation and for involvement in the regulation of H2O2 production. J. Cell Physiol. 1991b;146:242–250. doi: 10.1002/jcp.1041460208. [DOI] [PubMed] [Google Scholar]
- SATO K., OKAJIMA F., KONDO Y. Extracellular ATP stimulates three different receptor-signal transduction systems in FRTL-5 thyroid cells. Activation of phospholipase C, and inhibition and activation of adenylate cyclase. J. Biochem. 1992;283:281–287. doi: 10.1042/bj2830281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNEYD J., KEIZER J., SANDERSON M.J. Mechanisms of calcium oscillations and waves: a quantitative analysis. FASEB J. 1995;9:1463–1472. doi: 10.1096/fasebj.9.14.7589988. [DOI] [PubMed] [Google Scholar]
- YAP A.S., ARMSTRONG J.W., CRAGOE E.J., JR, BOURKE J.R., HUXHAM G.J., MANLEY S.W. Regulation of thyroid follicular volume by bidirectional transepithelial ion transport. Mol. Cell. Endocrinol. 1991;82:R1–R5. doi: 10.1016/0303-7207(91)90017-m. [DOI] [PubMed] [Google Scholar]
- YAP A.S., ARMSTRONG J.W., CRAGOE E.J., JR, BOURKE J.R., HUXHAM G.J., MANLEY S.W. Activation of sodium transport mediates regulation of thyroid follicle volume in response to hypotonic media. Am. J. Physiol. 1993;264:E644–E649. doi: 10.1152/ajpendo.1993.264.4.E644. [DOI] [PubMed] [Google Scholar]
- YAP A.S., STEVENSON B.R., ARMSTRONG J.W., KEAST J.R., MANLEY S.W. Thyroid epithelial morphogeneis in vitro: a role for bumetanide-sensitive Cl− secretion during follicular lumen development. Experimental Cell Research. 1994;213:319–326. doi: 10.1006/excr.1994.1205. [DOI] [PubMed] [Google Scholar]


