Abstract
The actions of the α1-adrenoceptor antagonist indoramin have been examined against the contractions induced by noradrenaline in the rat vas deferens and aorta taking into account a putative neuronal uptake blocking activity of this antagonist which could result in self-cancelling actions.
Indoramin behaved as a simple competitive antagonist of the contractions induced by noradrenaline in the vas deferens and aorta yielding pA2 values of 7.38±0.05 (slope=0.98±0.03) and 6.78±0.14 (slope=1.08±0.06), respectively.
When the experiments were repeated in the presence of cocaine (6 μM) the potency (pA2) of indoramin in antagonizing the contractions of the vas deferens to noradrenaline was increased to 8.72±0.07 (slope=1.10±0.05) while its potency remained unchanged in the aorta (pA2=6.69±0.12; slope=1.04±0.05).
In denervated vas deferens, indoramin antagonized the contractions to noradrenaline with a potency similar to that found in the presence of cocaine (8.79±0.07; slope=1.09±0.06).
It is suggested that indoramin blocks α1-adrenoceptors and neuronal uptake in rat vas deferens resulting in Schild plots with slopes not different from unity even in the absence of selective inhibition of neuronal uptake. As a major consequence of this double mechanism of action, the pA2 values for this antagonist are underestimated when calculated in situations where the neuronal uptake is active, yielding spurious pKB values.
Keywords: Indoramin, α1-adrenoceptors, vas deferens, aorta
Introduction
Indoramin is an antihypertensive drug which act as a competitive antagonist at α1-adrenoceptors (Cubeddu, 1988). Recent studies with both native and cloned α1-adrenoceptors showed that indoramin has higher affinity for α1A-adrenoceptors than α1B or α1D subtypes (Forray et al., 1994; Eltze, 1996; Ford et al., 1996; 1997) suggesting that it could be used as a useful tool for the characterization of α1-adrenoceptors.
In addition to its ability to block α1-adrenoceptors, it was shown that indoramin blocks H1-histaminergic and serotonergic receptors (Alps et al., 1972; Black & Mylecharane, 1984; Nedergaard, 1986) and also noradrenaline neuronal uptake (Sugden, 1974; Nedergaard, 1986). This latter activity, inhibition of neuronal uptake, could lead to a self-cancelling mechanism of action because the increases in the concentrations of noradrenaline in the receptor compartment could counteract the antagonism of α1-adrenoceptors induced by indoramin.
Therefore, the potency of indoramin in antagonizing an α1-adrenoceptor subtype in a tissue with intense neuronal uptake activity could be lower than its potency against this same subtype in a tissue without, or at least, with weak neuronal uptake activity because the counteraction or self-cancellation will be effective in the former but not in the latter tissue. Additionally, it could be expected that the antagonism showed by a drug which blocks simultaneously the receptor and an agonist removal process yield a regression line in the Schild plot with slope not different from the theoretical unity even in the absence of selective inhibition of the agonist removal process, but resulting in spurious pKB values (Kenakin & Beek, 1985; Kenakin, 1997).
To test these hypotheses we compared the actions of indoramin against noradrenaline contractions in the rat vas deferens, a tissue with intense neuronal uptake activity, with that obtained in a surgically-denervated vas deferens and rat aorta, tissues where neuronal uptake is absent or at least not so effective in reducing the concentrations of noradrenaline in the receptor compartment. The effects of neuronal uptake blockade with cocaine were also studied on the antagonism showed by indoramin in the vas deferens and aorta.
Methods
Isolated vas deferens and aorta
Male Wistar rats weighing between 280–360 g (16–20 weeks old) were killed by ether inhalation. The thoracic aorta and both vasa deferentia were removed. For the recording of isometric contractions, the whole vasa deferentia were mounted under 9.80 mN (N, Newton) of tension in 10 ml organ baths containing a nutrient solution of the following composition (mM): NaCl 138; KCl 5.7, CaCl2 1.8, NaH2PO4 0.36, NaHCO3 15, dextrose 5.5, prepared in glass-distilled, de-ionized water and maintained at 30°C, pH 7.4. Denervated vas deferens was obtained as described by Kasuya et al. (1969). The rats were anaesthetized by ether inhalation, after a 2 cm suprapubic incision the deferential artery and vein were exposed and gently separated from the prostatic portion of the vas deferens. After this procedure the incision was sutured and the rat was killed 7–10 days later. The effectiveness of the denervation was checked by the absence of contractile response to the indirect sympathomimetic agent tyramine (100 μM).
Rings of thoracic aorta were denuded of endothelium by gentle rubbing and mounted under 9.80 mN of tension for isometric registration of the contractions in 10 ml organ baths containing a nutrient solution of the following composition (mM): NaCl 119, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, dextrose 5.5, prepared in glass-distilled, de-ionized water and maintained at 37°C, pH 7.4.
Experimental protocols
Vasa deferentia from control or denervated rats were equilibrated for 30 min before the start of the experiments. After this period, two or three cumulative concentration-response curves for noradrenaline were obtained, and then corticosterone (10 μM) and propranolol (0.1 μM) were incubated in order to block extraneuronal uptake and β-adrenoceptors, respectively (Pupo, 1998). The interval between each concentration-response curve was 45 min. When specified, cocaine (6 μM) was added in order to block neuronal uptake.
Aorta were equilibrated for 45 min before the start of the experiments. After this period, the organs were challenged with noradrenaline (1 μM) and at peak response, carbachol (10 μM) was added to check for the absence of endothelium-dependent relaxations. Corticosterone (10 μM) and propranolol (0.1 μM) were included to block extraneuronal uptake and β-adrenoceptors, respectively. When specified, cocaine (6 μM) was added to block neuronal uptake.
Indoramin was incubated for 45 min before and during the contractile responses of the vas deferens and aorta to noradrenaline.
Calculation of pA2 values
The pA2 values (the negative logarithim to base 10 of the antagonist concentration that makes it necessary to double the agonist concentration needed to elicit half-maximal response) for indoramin were calculated by Schild regression analysis (Arunlakshana & Schild, 1959). The ratios between the half-maximal concentrations of noradrenaline (concentration-ratios, r) were calculated only when the maximal amplitude of the concentration-response curve in the presence of indoramin was similar to that obtained in its absence. Data were plotted as log antagonist concentrations (M) vs log (r−1). For calculation purposes the slope parameter was constrained to 1.0 when statistically not different from unity.
Statistical analysis
All values are shown as means±standard error of mean (s.e.mean) of n experiments. Differences between mean values were tested for statistical significance (P<0.05) using Student's paired or unpaired t-tests.
Drugs
Drugs were obtained from the following sources: cocaine (Cocainum Hydrochloricum puriss., C.H. Boehringer, Germany); corticosterone, noradrenaline [(±)-arterenol HCl]; from Sigma Chemical Co, U.S.A. Indoramin hydrochloride was a gift from Wyeth-Fontoura (Brazil). Drugs were dissolved in distilled water or dimethyl sulphoxide (1 mM), kept frozen and discarded after 20 days. Noradrenaline solutions were dissolved in 0.01 N HCl each day shortly before the experiments.
Results
Indoramin antagonized the contractions induced by noradrenaline in the rat vas deferens showing simple competitive antagonism characterized by the slope of the line in the Schild plot not different from the theoretical unity and by the absence of changes in the maximal response to noradrenaline (Figure 1a and d, Table 1). Indoramin also behaved as a competitive antagonist against noradrenaline in the rat aorta resulting in a pA2 value similar to that found in the vas deferens (Figure 2a and c, Table 1). The incubation of cocaine (6 μM) in the rat vas deferens induced a leftward shift in the concentration-response curve to noradrenaline (Table 2). In the presence of cocaine, the potency of indoramin in antagonizing the contractions of the vas deferens to noradrenaline was higher than its potency in the absence of cocaine as showed by the pA2 values found (Figure 1b and d, Table 1). On the other hand, the incubation of cocaine (6 μM) had not shifted the noradrenaline concentration-response curve in the aorta (Table 2) nor changed the potency of indoramin in inhibiting these contractions, as expressed by its pA2 value (Figure 2b, Table 1).
Figure 1.
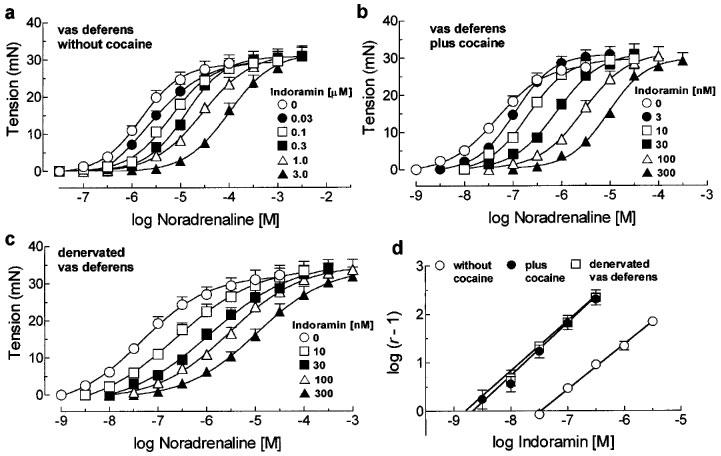
Concentration-response curves for noradrenaline in the absence and presence of increasing concentrations of indoramin in vas deferens without cocaine (a), plus cocaine (b) and in denervated vas deferens (c). In (d) are shown the respective Schild plots obtained for indoramin. Each symbol represents the mean and the vertical line, when larger than the symbol, the s.e.mean of 5–8 experiments.
Table 1.
pA2 and slope values* obtained for indoramin against noradrenaline in vas deferens and aorta

Figure 2.
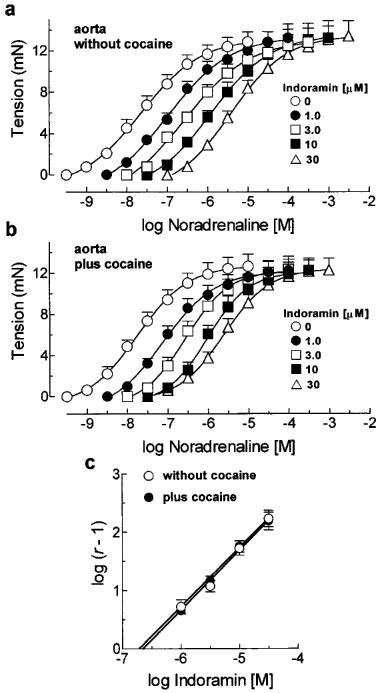
Concentration-response curves for noradrenaline in the absence and presence of increasing concentrations of indoramin in aorta without cocaine (a) and aorta plus cocaine (b). In (c) are shown the respective Schild plots for indoramin. Each symbol represents the mean and the vertical line, when larger than the symbol, the s.e.mean of six experiments.
Table 2.
pD2 values* for noradrenaline in rat vas deferens and aorta

In a denervated vas deferens, indoramin antagonized noradrenaline contractions with a potency similar to that found in a control vas deferens in the presence of cocaine (Figure 1c and d, Table 1). Note that the extent of the change of the potency of indoramin in the absence of cocaine and presence of cocaine or in a denervated vas deferens (≈1.4 log units) is similar to the extent of the leftward shift that cocaine or denervation induced in the noradrenaline concentration-response curve (≈1.5 log units).
Discussion
In the present study the effects of indoramin against the contractions induced by noradrenaline in the rat vas deferens and aorta were investigated taking into account a putative neuronal uptake blocking activity showed by indoramin. Our results suggest that indoramin, besides its competitive antagonism at α1-adrenoceptors, is also an effective neuronal uptake blocker in the rat vas deferens. Two main results indicate that indoramin blocks neuronal uptake of noradrenaline in the rat vas deferens: (1) the slope in the Schild plot is not different from 1.0 in the absence of cocaine; this is in contrast to what is observed with other antagonists in the rat vas deferens such as phentolamine or piperoxan where it is necessary to use a neuronal uptake blocker to obtain a slope not different from unity (Jurkiewicz & Jurkiewicz, 1976); and (2) the very similar pA2 values found in presence of cocaine and in denervated vas deferens.
In the absence of cocaine, the pA2 values found for indoramin against noradrenaline in the rat aorta and vas deferens were similar and derived from Schild plots with slopes not different from unity. Apparently, these results fulfil the criteria for simple competitive antagonism and allow the assumption that these pA2 values are good estimates of the antagonist dissociation constant (pKB). Furthermore, these results could suggest that indoramin does not differentiate between the α1-adrenoceptors in the vas deferens and aorta. This is in disagreement with the proposals that indoramin has substantially higher affinity at α1A-adrenoceptors than α1B and α1D subtypes (Forray et al., 1994; Eltze, 1996; Ford et al., 1996; 1997) or, moreover, that the rat vas deferens is a suitable preparation for the study of α1A-adrenoceptor-mediated contractions (Burt et al., 1995; Pupo et al., 1997; Docherty; 1998; Pupo, 1998) and the rat aorta for α1D-adrenoceptors (Kenny et al., 1995; Fagura et al., 1997; Hussain & Marshall, 1997). However, these conclusions are not supported when the results obtained in the presence of cocaine or in the denervated vas deferens are compared. There was a 25 fold increase (≈1.4 log units) in the potency of indoramin against the contractions induced by noradrenaline in the rat vas deferens in the presence of cocaine or in denervated vas while in the aorta the potency of indoramin remained unchanged in presence of cocaine. The pA2 value found for indoramin in vas deferens in presence of cocaine or in denervated organs is in agreement with the affinity of this drug at α1A-adrenoceptors as well as the values found in rat aorta for α1D-adrenoceptors (Forray et al., 1994; Eltze, 1996; Ford et al., 1996; 1997). This increase in the potency of indoramin in vas deferens can be explained by the fact that the control concentration-response curve obtained in the absence of cocaine was done under non-equilibrium conditions caused by noradrenaline neuronal uptake resulting in a gradient between the concentrations of agonist in the bathing solution and that in the receptor compartment (biophase). On the other hand, the control concentration-response curves obtained in presence of cocaine or in denervated vas were determined in a situation in which equilibrium steady state was approximated and the concentration gradient was minimized. As expected according to this interpretation, the increase in the potency of indoramin (≈1.4 log units) is similar to the leftward displacement that cocaine or denervation (≈1.5 log units) induced in the noradrenaline concentration-response curve. There was no difference between the potencies of indoramin in the rat aorta in absence or presence of cocaine because in this organ neuronal uptake is not so active in removing noradrenaline from the receptor compartment as indicated by the absence of significant potentiation of noradrenaline contractions in the presence of cocaine.
The Schild analysis is a valuable tool for the investigation of drug properties because besides it furnishes estimates of the antagonist pKB, the slope parameter is indicative of non-equilibrium steady states. Non-compliance of the Schild equation results in slope values different from the theoretical unity precluding valid determinations of pKB. Slope values higher than unity are indicative of insufficient time of incubation of the antagonist, chemical interference or saturation of antagonist removal while slopes lower than unity are indicative of agonist removal saturation, chemical interference or activation of heterogeneous receptor population (for review see Kenakin, 1997). Interestingly, drugs with self-cancelling actions such as indoramin yield slope values not different from unity even in the absence of selective inhibition of neuronal uptake, but resulting in spurious pKB values that will be underestimated depending on the strength of the agonist removal process. This emphasizes the importance of selective inhibition of agonist uptake processes for the correct determination of antagonists pKB as stressed by other authors (Furchgott, 1972; Furchgott et al., 1973; Kenakin & Beek, 1981; Kenakin, 1997).
The behaviour observed for indoramin in the rat vas deferens is very similar to that described by Kenakin & Beek (1985) for the anticholinesterase agent ambenonium which also show anti-muscarinic effects. This double mechanism of action results in self-cancellation of muscarinic receptor antagonism by blockade of acetylcholinesterase. Kenakin & Beek (1985) showed that the potency of ambenonium in antagonizing acetylcholine contractions is inversely proportional to the potency of acetylcholinesterase as a metabolizing enzyme resulting in organ selectivity which could be interesting in some instances.
In conclusion, our results indicate that indoramin blocks α1-adrenoceptors and neuronal uptake in rat vas deferens resulting in Schild plots with slopes not different from unity even in the absence of selective inhibition of neuronal uptake. As a major consequence of this double mechanism of action, the pA2 values for this antagonist are underestimated when calculated in situations where the neuronal uptake is active yielding spurious pKB values.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP - Proc. no. 98/11031-2 to A.S. Pupo). M. Campos and P.L. Morais are MSc students recipient of studentships from FAPESP and CAPES, respectively. We thank Wyeth-Fontoura (Brazil) for gift of indoramin.
Abbreviations
- N
tension in newtons
- r
noradrenaline half-maximal concentration-ratios
- s.e.mean
standard error of the mean
References
- ALPS B.J., HILL M., JOHNSON E.S., WILSON A.B. Quantitative analysis on isolated organs of the autonomic blocking properties of indoramin hydrochloride (Wy 21901) Br. J. Pharmacol. 1972;44:52–62. doi: 10.1111/j.1476-5381.1972.tb07237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–52. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK J.L., MYLECHARANE E.J. Actions of indoramin on rabbit and human vasculature. Clin. Exp. Pharmacol. Physiol. 1984;11:27–35. doi: 10.1111/j.1440-1681.1984.tb00236.x. [DOI] [PubMed] [Google Scholar]
- BURT R.P., CHAPPLE C.R., MARSHALL I. Evidence for a functional α1A-(-α1C-) adrenoceptor mediating contraction of the rat vas deferens and an α1B-adrenoceptor mediating contraction of the rat spleen. Br. J. Pharmacol. 1995;115:467–475. doi: 10.1111/j.1476-5381.1995.tb16356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUBEDDU L.X. New alpha1-adrenergic receptor antagonists for the treatment of hypertension: Role of vascular alpha receptors in control of peripheral resistance. Am. Heart. J. 1988;116:133–162. doi: 10.1016/0002-8703(88)90261-x. [DOI] [PubMed] [Google Scholar]
- DOCHERTY J.R. Subtypes of functional α1- and α2- adrenoceptors. Eur. J. Pharmacol. 1998;361:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- ELTZE M. In functional experiments, risperidone is selective, not for the B, but for the A subtype of α1-adrenoceptors. Eur. J. Pharmacol. 1996;295:69–73. doi: 10.1016/0014-2999(95)00685-0. [DOI] [PubMed] [Google Scholar]
- FAGURA M.S., LYDFORD S.J., DOUGALL I.G. Pharmacological classification of α1-adrenoceptors mediating contractions of rabbit isolated ear artery: comparison with rat isolated thoracic aorta. Br. J. Pharmacol. 1997;120:247–258. doi: 10.1038/sj.bjp.0700917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORD A.P.D.W., ARREDONDO N.F., BLUE D.R., JR, BONHAUS D.W., JASPER J., KAVA M.S., LESNICK J., PFISTER J.R., SHIEH I.A., VIMONT R.L., WILLIAMS T.J., MCNEAL J.E., STAMEY T.A., CLARKE D.E. RS-17053 (N-[2-(2-Cyclopropylmethoxyphenoxy) ethyl]-5-chloro-α, α-dimethyl-1H-indole-3-ethanamine hydrochloride), a selective α1A-adrenoceptor antagonist, displays low affinity for functional α1-adrenoceptors in human prostate: implications for adrenoceptor classification. Mol. Pharmacol. 1996;49:209–215. [PubMed] [Google Scholar]
- FORD A.P.D.W., DANIELS D.V., CHANG D.J., GEVER J.R., JASPER J.R., LESNICK J.D., CLARKE D.E. Pharmacological pleiotropism of the human recombinant α1A-adrenoceptor: implications for α1-adrenoceptor classification. Br. J. Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORRAY C., BARD J.A., WETZEL J.M., CHIU G., SHAPIRO E., TANG H., LEPOR E., HARTIG P.R., WEINSHANK R.L., BRANCHEK T.A., GLUCHOWSKI C. The α1-adrenergic receptor that mediates smooth muscle contraction in human prostate has the pharmacological properties of the cloned α1c subtype. Mol. Pharmacol. 1994;45:703–708. [PubMed] [Google Scholar]
- FURCHGOTT R.F.The classification of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory Handbook of Experimental Pharmacology 197233Springer Verlag: New York; pp, 283–335.In: Blaschko, H., Muscholl, E. (eds) [Google Scholar]
- FURCHGOTT R.F., JURKIEWICZ N.H., JURKIEWICZ A.Antagonism of propranolol to isoproterenol in guinea-pig trachea: some cautionary findings Frontiers in Catecholamine Research 1973Pergamon Press: New York; 295–300.Usdin, E., Snyder, S.H. (eds) [Google Scholar]
- HUSSAIN M.B., MARSHALL I. Characterization of α1-adrenoceptor subtypes mediating contractions to phenylephrine in rat thoracic aorta, mesenteric artery and pulmonary artery. Br. J. Pharmacol. 1997;122:849–858. doi: 10.1038/sj.bjp.0701461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JURKIEWICZ N.H., JURKIEWICZ A. Dual effects of α-adrenoceptors antagonists in isolated rat vas deferens. Br. J. Pharmacol. 1976;56:169–178. doi: 10.1111/j.1476-5381.1976.tb07439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASUYA Y., GOTO K., HASHIMOTO H., WATANABE H., MUNUKATA H., WATANABE M. Nonspecific denervation supersensitivity in the rat vas deferens in vitro. Eur. J. Pharmacol. 1969;8:177–184. doi: 10.1016/0014-2999(69)90074-0. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.Competitive antagonism Pharmacologic Analysis of Drug-Receptor Interaction 1997Lippincott-Raven: Philadelphia; 331–373.In: Kenakin, T. (ed)3rd edn [Google Scholar]
- KENAKIN T.P., BEEK D. The measurement of antagonist potency and the importance of selective inhibition of agonist uptake processes. J. Pharm. Exp. Ther. 1981;219:112–120. [PubMed] [Google Scholar]
- KENAKIN T.P., BEEK D. Self-cancellation of drug properties as a mode of organ selectivity: The antimuscarinic effects of ambenonium. J. Pharm. Exp. Ther. 1985;232:732–740. [PubMed] [Google Scholar]
- KENNY B.A., CHALMERS D.H., PHILPOTT P.C., NAYLOR A.M. Characterization of an α1D-adrenoceptor mediating the contractile response of rat aorta to noradrenaline. Br. J. Pharmacol. 1995;115:981–986. doi: 10.1111/j.1476-5381.1995.tb15907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEDERGAARD O.A. Pre- and Postsynaptic effects of indoramin on sympathetic neuroeffector transmission in rabbit aorta and pulmonary artery. J. Cardiovasc. Pharmacol. 1986;8:1020–1027. doi: 10.1097/00005344-198609000-00021. [DOI] [PubMed] [Google Scholar]
- PUPO A.S. Functional effects of castration on α1-adrenoceptors in the rat vas deferens. Eur. J. Pharmacol. 1998;351:221–227. doi: 10.1016/s0014-2999(98)00315-x. [DOI] [PubMed] [Google Scholar]
- PUPO A.S., JURKIEWICZ N.H., JURKIEWICZ A. Functional change of the balance between α1A and α1B adrenoceptor populations after transplantation of the vas deferens to the intestine. Ann. N.Y. Acad. Sci. 1997;812:193–195. doi: 10.1111/j.1749-6632.1997.tb48171.x. [DOI] [PubMed] [Google Scholar]
- SUGDEN R.F. The action of indoramin and other compounds on the uptake of neurotransmitters into rat cortical slices. Br. J. Pharmacol. 1974;51:467–469. doi: 10.1111/j.1476-5381.1974.tb10687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


