Abstract
Systemic application of U37883A, a blocker of ATP sensitive potassium (KATP) channels, elicits diuresis and natriuresis without significantly altering urinary potassium excretion.
To elucidate tubular sites of action upstream to the distal nephron, micropuncture experiments were performed in nephrons with superficial glomeruli of anaesthetized Munich-Wistar-Frömter rats during systemic application of U37883A (1, 5 or 15 mg kg−1 i.v.).
The observed eukaliuric diuresis and natriuresis in response to U37883A at 15 mg kg−1 was accompanied by an increase in early distal tubular flow rate (VED) from 10–18 nl min−1 reflecting a reduction in fractional reabsorption of fluid up to this site (FR-fluid) of 13%. The latter proposed an effect on water-permeable segments such as the proximal tubule which could fully account for the observed reduction in fractional reabsorption of Na+ up to the early distal tubule (FR-Na+) of 8% and the increase in early distal tubular Na+ concentration ([Na+]ED) from 35–51 mM whereas [K+]ED was left unaltered.
In comparison, furosemide (3 mg kg−1 i.v.), which acts in the water-impermeable thick ascending limb, elicited diuresis, natriuresis and kaliuresis which were associated with a fall in FR-Na+ of 10% with no change in FR-fluid, and a rise in [Na+]ED from 42–117 mM and [K+]ED from 1.2–5.7 mM with no change in VED.
Direct late proximal tubular fluid collections confirmed a significant inhibition of fluid reabsorption in proximal convoluted tubule in response to systemic application of U37883A.
These findings suggest that the diuretic and natriuretic effect upstream to the distal tubule in response to systemic application of U37883A involves actions on water-permeable segments such as the proximal convoluted tubule.
Keywords: Micropuncture, U37883A, thick ascending limb, reabsorption, Munich-Wistar-Frömter rats, KATP channels, proximal tubule
Introduction
There is evidence that ATP sensitive potassium (KATP) channels play a role in tubular reabsorption in the proximal tubule and thick ascending limb of Henle's loop (TALH) as well as in reabsorption and K+ secretion in both distal tubule and cortical collecting duct (for review see Quast, 1996). Indeed, systemic application of the KATP channel blockers U37883A (4-morpholinecarboximidine-N-1-adamantyl-N′-cyclohexyl-hydrochloride) or glibenclamide has been shown to induce diuresis and natriuresis without eliciting a pronounced increase in urinary potassium ion secretion or a change in glomerular filtration rate (Clark et al., 1993; Ludens et al., 1995a; Wang et al., 1995a,1995b; Vallon et al., 1998a,1998b). Furthermore, systemic application of U37883A has been shown to lower renin secretion or plasma renin activity (Linseman et al., 1995; Vallon et al., 1998a,1998b), a feature which is uncommon to other diuretics and which is in accordance with studies showing that the KATP channel opener cromakalim stimulates whereas the KATP channel blocker glibenclamide inhibits renin release from cultured juxtaglomerular cells (Ferrier et al., 1989; Linseman et al., 1995). In contrast to glibenclamide, U37883A was found not to affect insulin secretion (Guillemare et al., 1994; Meisheri et al., 1993a) and therefore appears to be selective for vascular and tubular over pancreatic KATP channels.
Although availability and concentrations in the tubular lumen of U37883A achieved by systemic application are not known, Wang et al. (1995b) employing intratubular application of U37883A in micropuncture studies proposed that the diuresis and natriuresis in response to U37883A may be the result of inhibiting KATP channels and thus impairing reabsorption in the water-impermeable thick ascending limb of Henle's loop (TALH). In addition, blockade of KATP channels in the distal tubule and cortical collecting duct was proposed to suppress K+ secretion which prevents kaliuresis, but further increases Na+ excretion (Wang et al., 1995b).
The present study aimed to elucidate which tubular segment upstream to the distal tubule contributes to the diuresis and natriuresis in response to systemic application of U37883A. U37883A was applied at doses of 1, 5 and 15 mg kg−1, which represents the dose range previously employed to elucidate the diuresis and natriuresis as well as the inhibition of plasma renin activity in response to U37883A (Ludens et al., 1995a; Wang et al., 1995b; Vallon et al., 1998a,1998b). These doses were also shown previously to inhibit the hypotensive response to application of various KATP channel openers (Ludens et al., 1995b; Meisheri et al., 1993b). In a first series of experiments, late proximal tubular flow rate or early distal tubular flow rate and Na+ and K+ concentration were measured concomitantly with urinary excretion during systemic application of U37883A. For comparison, the response to furosemide which is known to inhibit TALH reabsorption was assessed. Furthermore, these experiments were performed in Munich-Wistar-Frömter (MWF) rats which have multiple nephrons with superficial glomeruli which allow to collect tubular fluid from the early distal tubule relatively close to the macula densa. This can help to minimize the input from drug effects on the distal tubule during early distal tubular fluid collection. The latter could be of importance for being able to ascribe drug actions to the TALH, if the drug tested, like U37883A, also acts on the distal tubule. Because experiments with systemic application of U37883A revealed a predominant effect on the proximal convoluted tubule rather than TALH, additional studies were performed with intratubular application of the drug to test for potential different effects of systemic and intratubular application of U37883A.
Methods
All animal experimentation described here was conducted in accord with the NIH Guide for the Care and Use of Laboratory Animals and the German Law on the Protection of Animals. Experiments were predominantly performed in male Munich-Wistar-Frömter (MWF) rats weighing between 200 and 400 g. In the microperfusion series with U37883A in Henle's loop, both male MWF and Sprague-Dawley (SD) rats (220–300 g) were used to assess potential differences between rats strains.
Surgical preparation
Surgical preparation was performed as described before (Huang et al., 1998). Briefly, rats were anaesthetized with thiobutabarbital (Inactin® 120 mg kg−1 i.p.). Animals were then placed on a temperature-controlled operating table to keep rectal temperature at 37°C. After tracheostomy (PE-200), a catheter (PE-50) was inserted into the right jugular vein for infusion of Ringer saline (mmol l−1: NaCl 111, KCl 4.7, NaHCO3 30) at a rate of 1.3% of body weight h−1. For assessment of whole kidney and single nephron glomerular filtration rate (GFR, SNGFR) [3H]-inulin was added to Ringer saline to deliver 100 μCi h−1. Arterial blood pressure was recorded via a catheter (PE-50) which was inserted in the left femoral artery and connected to a pressure transducer (P23dB, Gould-Statham, Oxnard, CA, U.S.A.). The left kidney was exposed by flank incision, carefully freed of perirenal fat and immobilized in a lucite cup. The kidney was covered with prewarmed paraffin oil. Both the urinary bladder and the left ureter were cannulated (PE-50) to maintain free urine drain. After completion of the surgical preparation, the animals were allowed to stabilize for 90 min before starting functional studies.
Series 1: Effect of systemic application of U37883A on reabsorption (i) of the whole kidney, (ii) up to the early distal tubule, and (iii) up to the late proximal convoluted tubule
After starting a timed urine collection from the left kidney, distal or proximal tubular configuration of nephrons with superficial glomeruli were identified by dye injection into Bowman space using a micropipette (1–3 μm tip) filled with coloured artificial tubular fluid (ATF; see Series 3). In one series, a collecting pipette (7–9 μm tip) was inserted into the first accessible distal tubular loop to perform a timed collection of tubular fluid (at least 2½ min in duration) under free-flow conditions, utilizing a short mineral oil block, to obtain ‘Basal' measurements (see Figure 1a). In another series, timed tubular fluid collections were performed in the last accessible loop of the proximal convoluted tubule (10–12 μm tip) (see Figure 2a). Following these collections, tubular fluid was allowed to escape from the collection hole or, after pushing away the short mineral oil block, escaped downstream. Thereafter, the timed urine collection from the left kidney was stopped. Subsequently, U37883A (1, 5 or 15 mg kg−1) or Vehicle (Ringer saline) were applied i.v. as a slow bolus injection. In the series with early distal tubular collection, additional studies were performed with bolus injection of furosemide (3 mg kg−1 i.v.). Ten minutes after completing the bolus injection, a second urine collection from the left kidney was started and at the same time the early distal or late proximal tubular sites were punctured for paired recollection (experimental, ‘EXP' in Figure 1). GFR, urinary flow rate and whole kidney sodium and potassium ion excretion as well as SNGFR, early distal flow rate and sodium and potassium ion concentration, and late proximal tubular flow rate were determined as described below.
Figure 1.
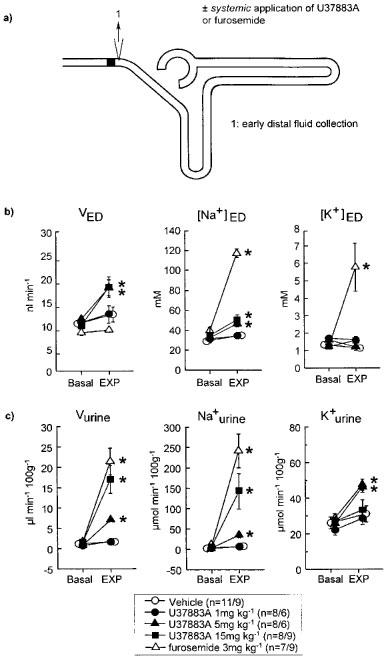
Effect of systemic application of U37883A or furosemide on (i) early distal tubular flow rate and sodium and potassium ion concentration (VED, [Na+]ED, [K+]ED) as well as (ii) urinary fluid, sodium ion and potassium ion excretion (Vurine, Na+urine, K+urine). (a) Schematic illustration of the nephron (with glomerulus, proximal tubule, loop of Henle and distal tubule) and of micropuncture manoeuvres. (b and c) Depicted is the effect of the experimental manoeuvre (EXP: i.v. administration of Vehicle, U37883A, or furosemide on the respective parameters as compared to basal measurement (Basal). Mean±s.e.mean n is given as number of nephrons/number of rats; *P<0.05 vs basal. Notice that the diuresis and natriuresis in response to U37883A at 15 mg kg−1 was associated with an increase in [Na+]ED and VED. In comparison, the diuresis, natriuresis and kaliuresis in response to furosemide, which acts in the water-impermeable TALH, was associated with a substantial rise in [Na+]ED and [K+]ED without altering VED. U37883A at 5 mg kg−1 elicited a similar response up to early distal tubule as 15 mg kg−1 (as indicated by similar rises in VED and [Na+]ED), which, however, was associated with an attenuated diuresis and natriuresis and the presence of kaliuresis.
Figure 2.
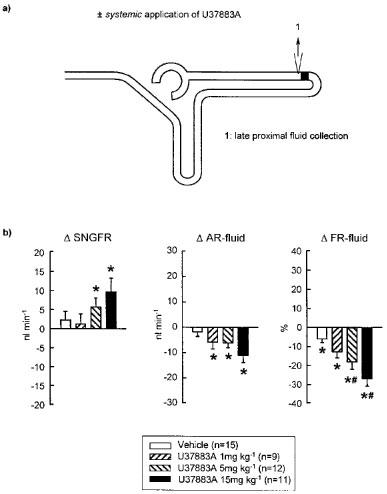
Effect of systemic application of U37883A on single nephron filtration rate (SNGFR) and proximal convoluted tubule fluid reabsorption as determined by free-flow collection from late proximal tubular site. (a) Schematic illustration of micropuncture manoeuvres. (b) Depicted are the changes in SNGFR (Δ SNGFR), and absolute (Δ AR-fluid) and fractional (Δ FR-fluid) reabsorption of fluid in the proximal convoluted tubule in response to systemic application of U37883A or Vehicle as compared to basal measurements. Mean±s.e.mean. *P<0.05 vs basal; Δ FR-fluid: #P<0.05 vs vehicle. Notice that systemic application of U37883A caused a significant inhibition of fluid reabsorption in the proximal convoluted tubule. Whereas during fluid collection from the early distal tubular site, systemic application of U37883A left the SNGFR unaltered (Table 1), an increase in SNGFR was observed during fluid collection from the late proximal tubular site.
Series 2: Effect of systemic application of U37883A on proximal convoluted tubule reabsorption during stopped-flow perfusion
This series aimed to elucidate whether U37883A has to be filtered in the glomerulus in order to affect proximal tubular reabsorption. After identification of proximal nephron configuration, a perfusion pipette directly attached to a calibrated Hampel microperfusion pump and filled with the appropriate type of artificial tubular fluid (ATF, see Series 3) was inserted into the first surface loop of the proximal convoluted tubule (see Figure 3a). A wax block was introduced immediately upstream to the perfusion pipette and venting the tubular system upstream to the wax block allowed the filtrate to escape freely onto the surface. Three to five minutes elapsed at an initial perfusion with ATF (40 nl min−1) before a collecting pipette (9–11 μm tip) was inserted into the last surface loop of the proximal convoluted tubule to perform a timed collection of tubular fluid (at least 2½ min in duration) utilizing a short mineral oil block, and obtain ‘basal' measurements. Subsequently, U37883A (15 mg kg−1) or Vehicle (Ringer saline) were applied i.v. as a slow bolus injection. Ten minutes after completing the bolus injection of U37883A or Vehicle, the late proximal tubular sites were punctured for paired recollection. Late proximal tubular flow rate was determined as described below.
Figure 3.
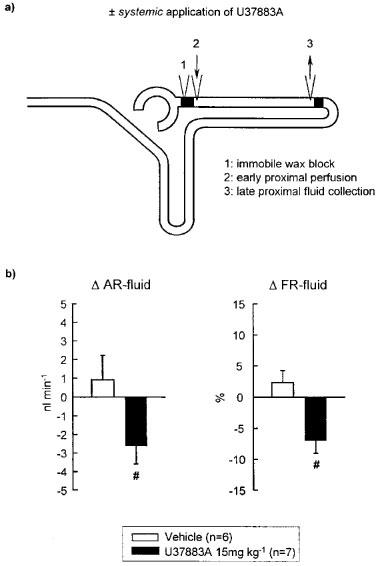
Effect of systemic application of U37883A on proximal convoluted tubule fluid reabsorption as determined by stopped-flow perfusion. (a) Schematic illustration of micropuncture manoeuvres. (b) Depicted are the changes in absolute (Δ AR-fluid) and fractional (Δ FR-fluid) reabsorption of fluid in the proximal convoluted tubule in response to systemic application of U37883A as compared to Vehicle treatment. Mean±s.e.mean. #P<0.05 vs vehicle. Notice that systemic application of U37883A significantly reduced fluid reabsorption in proximal convoluted tubule during stopped-flow perfusion.
Series 3: Effect of intratubular application of U37883A on proximal convoluted tubule or loop of Henle reabsorption during stopped-flow perfusion
Reabsorption in the proximal convoluted tubule and in the loop of Henle were studied during orthograde microperfusion of the respective tubular segment with U37883A added to the perfusate. Different from previous reports (Wang et al., 1995b) intratubular application of U37883A in the present study was found not to alter loop of Henle reabsorption. Therefore, additional experiments were performed to study (i) the effect of U37883A on loop of Henle reabsorption in SD rats in order to exclude potential differences between rats strains, and (ii) the effect on loop of Henle reabsorption of glibenclamide, another KATP channel blocker, as well as its two major metabolites found in urine namely W039580A and W039109B (Heptner et al., 1969). Concentrations of U37883A (10–100 μM) or glibenclamide (250 μM) employed were those used in previous studies (Wang et al., 1995a,1995b). Concentrations for the two metabolites of glibenclamide were set at a somewhat lower concentration than glibenclamide, namely 100 μM. Tubular reabsorption was studied during microperfusion of functionally isolated (i) proximal convoluted tubule (PCT, see Figure 4a) or (ii) loop of Henle (LH, see Figures 5a). After proximal and distal nephron configuration were identified, a perfusion pipette directly attached to a calibrated Hampel microperfusion pump and filled with the appropriate type of ATF (see below) was inserted into (i) the first, or (ii) the last surface loop of the PCT. A wax block was introduced immediately upstream to the perfusion pipette and venting the tubular system upstream to the wax block allowed the filtrate to escape freely onto the surface. Three to five minutes elapsed at an initial perfusion with ATF (40 nl min−1 for PCT, 20 or 25 nl min−1 for LH) before a collecting pipette (7–9 for LH or 9–11 μm tip for PCT) was inserted into (i) the last surface loop of the PCT, or (ii) the first accessible distal tubular loop and a timed collection of tubular fluid was obtained. Subsequently, a second perfusion pipette connected to a second Hampel pump (pump 2) and filled with ATF containing the respective drug or Vehicle was inserted in close proximity to the first pump. Pump 1 was switched off and pump 2 was started. After a time period of 10 min, a second tubular collection was performed.
Figure 4.
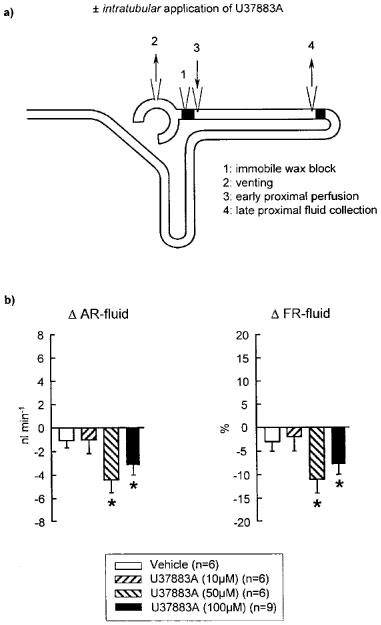
Effect of intratubular application of U37883A on proximal convoluted tubule fluid reabsorption during stopped-flow perfusion. (a) Schematic illustration of micropuncture manoeuvres. (b) Depicted are the changes in absolute (Δ AR-fluid) and fractional (Δ FR-fluid) reabsorption of fluid in the proximal convoluted tubule in response to intratubular application of U37883A or vehicle as compared to basal measurements. Mean±s.e.mean. *P<0.05 vs basal. Notice that intratubular application of U37883A significantly inhibited fluid reabsorption in the proximal convoluted tubule.
Figure 5.
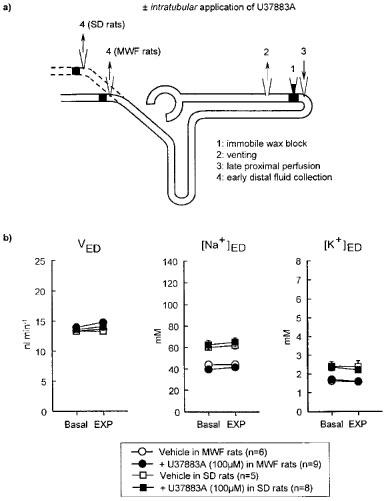
Effect of intratubular application of U37883A on loop of Henle reabsorption during stopped-flow perfusion. (a) Schematic illustration of micropuncture manoeuvres. (b) Depicted is the effect of the experimental manoeuvre (EXP: intratubular application of Vehicle or U37883A) on early distal tubular flow rate (VED) and sodium and potassium ion concentration ([Na+]ED, [K+]ED) as compared to basal measurement (Basal). Experiments were performed in Munich-Wistar-Frömter (MWF) and Sprague-Dawley (SD) rats. In contrast to SD rats, MWF rats have nephrons with superficial glomeruli which allow to puncture the early distal tubule closer to the macula densa. Mean±s.e.mean. *P<0.05 vs basal. Notice that intratubular application of U37883A did not significantly alter VED [Na+]ED, or [K+]ED in MWF or SD rats.
The following types of ATF were employed: For identifier and PCT (in mM): NaCl 113, NaHCO3 25, KCl 4, MgSO4 1, CaCl2 2, Na2HPO4 1, Glucose 5, urea 5, 0.075% FD&C green, pH 7.4. For LH (in mM): NaCl 130, NaHCO3 10, KCl 4, CaCl2 2, urea 7.5, 0.075% FD&C green, pH 7.4.
Materials
The following drugs were used: U37883A was kindly provided by Pharmacia & Upjohn, (Michigan, U.S.A.). Glibenclamide and furosemide were purchased from Sigma (St. Louis, MO, U.S.A.). The metabolites of glibenclamide, W039580A and W039109B, were kindly provided by Dr H. Englert (Hoechst AG, Frankfurt, Germany). Glibenclamide and its two metabolites were firstly dissolved in ethanol/DMSO (1 : 1) to obtain stock solutions and were then further diluted in ATF to achieve final concentrations. For experiments with glibenclamide or its metabolites, ATF plus the respective concentration of ethanol/DMSO was used as ‘Vehicle'. U37883A and furosemide were dissolved in Ringer solution (for systemic application) or ATF (for intratubular application) using an ultrasound bath as performed previously (Vallon et al., 1997a; Huang et al., 1998).
Analytic methods and statistics
Urinary flow rate was determined gravimetrically and plasma and urine were analysed for sodium and potassium ion concentration by flame photometry (ELEX 6361, Eppendorf, Germany). Late proximal and early distal tubular flow rate were measured by transferring the collected fluid sample to a constant bore capillary. Sodium and potassium concentration in the distal tubular fluid were determined as previously described (Vallon et al., 1997b; Huang et al., 1998) with a micro flame photometer which was developed and built by Rolf Englert and Klaus Stieler (Department of Pharmacology, University of Tübingen, Germany) based on the original conception by Wolfgang Hampel (Wissenschaftlicher Gerätebau, Frankfurt/Main, Germany). Concentration of 3H-inulin in plasma, late proximal and early distal tubular fluid, and urine were measured by liquid phase scintillation counting. Paired or unpaired Student's t-test were performed to analyse the data. P<0.05 was considered to be statistically significant.
Results
Series 1: Effect of systemic application of U37883A on reabsorption (i) of the whole kidney, (ii) up to the early distal tubule, and (iii) up to the late proximal convoluted tubule
Systemic application of Vehicle did not significantly alter mean arterial blood pressure (MAP: 110±1 vs 110±1 mmHg, NS), GFR (0.37±0.07 vs 0.34± 0.02 ml min−1 100 g body wt−1 NS), urinary flow rate or the excretion of sodium or potassium ion (see Figure 1c). Likewise, SNGFR, early distal tubular flow rate (VED) and early distal tubular sodium or potassium ion concentration ([Na+]ED, [K+]ED) as well as the fractional reabsorption of fluid or sodium or potassium ion upstream to the early distal tubule (FR-fluid, FR-Na+, FR-K+) were not significantly altered in response to Vehicle application (see Figure 1b and Table 1). Systemic application of U37883A at 1, 5 or 15 mg kg−1 did not significantly alter MAP or GFR as compared to basal measurements (MAP: 114±7 vs 121±4 mmHg, 113±3 vs 124±4 mmHg or 111± 7 vs 111±4 mmHg, NS; GFR: 0.40±0.12 vs 0.46±0.18 ml min−1 100 g body wt−1, 0.28±0.03 vs 0.33±0.03 ml min−1 100 g body wt−1 or 0.39±0.07 vs 0.32±0.04 ml min−1 100 g body wt−1 NS). Application of U37883A at 15 mg kg−1, however, elicited a diuresis and natriuresis without significantly altering urinary potassium excretion (see Figure 1c). The latter response was accompanied by an increase in VED and [Na+]ED (see Figure 1b) and a decrease in FR-fluid, FR-Na+ and FR-K+ without a change in SNGFR (see Table 1). In comparison, systemic application of furosemide elicited a diuresis and natriuresis which was accompanied by a substantial kaliuresis (see Figure 1c). Furthermore, furosemide caused a significant increase in [Na+]ED and [K+]ED without altering VED (see Figure 1b) or SNGFR (see Table 1). The latter changes were associated with a fall in FR-Na+ and FR-K+ but not in FR-fluid. Application of furosemide did not significantly alter MAP (112±3 vs 112±4 mmHg, NS) or GFR (0.25±0.02 vs 0.28± 0.02 ml min−1 100 g body wt−1, NS).
Table 1.
Effect of systemic application of U37883A or furosemide on single nephron glomerular filtration rate (SNGFR) and fractional reabsorption of fluid, and sodium and potassium ion upstream to the early distal tubule (FR-fluid, FR-Na+, FR-K+)

Although systemic application of U37883A at a dose of 5 mg kg−1 elicited almost similar rises in VED and [Na+]ED as well as decreases in FR-fluid and FR-Na+ as 15 mg kg−1 (see Figure 1b and Table 1), which indicated a relatively similar response up to the early distal tubule, that dose caused a significantly lesser diuresis and natriuresis and induced a kaliuresis (see Figure 1c).
It was further observed that systemic application of U37883A at 5 or 15 mg kg−1 reduced absolute and fractional fluid reabsorption in the proximal convoluted tubule during free-flow collection from late proximal tubular site (see Figure 2b). In addition, U37883A increased SNGFR during late proximal tubular collection, a response which had not been observed during early distal tubular collection (see Figure 2b and Table 1).
Series 2: Effect of systemic application of U37883A on proximal convoluted tubule reabsorption during stopped-flow perfusion
As compared to Vehicle, systemic application of U37883A at 15 mg kg−1 significantly inhibited proximal tubular fluid reabsorption during stopped-flow perfusion (see Figure 3b).
Series 3: Effect of intratubular application of U37883A on proximal convoluted tubule or loop of Henle reabsorption during stopped-flow perfusion
Fluid reabsorption in proximal convoluted tubule was significantly reduced during intratubular application of 50 or 100 μM U37883A (see Figure 4b). In comparison, intratubular U37883A (10, 50 or 100 μM) did not significantly alter the reabsorption of fluid, sodium or potassium ion in the loop of Henle (Figure 5b and Table 2). Similarly, loop of Henle reabsorption was unaffected by intratubular application of glibenclamide (250 μM) or its metabolites WO39580A or WO39109B (each 100 μM) (Table 2). All the above experiments have been performed in MWF rats. Assessing loop of Henle reabsorption in SD rats which do not have superficial glomeruli, it was found as expected that early distal tubular concentrations of sodium and potassium ion were significantly higher as compared to MWF rats (Figure 5b). Similar to MWF rats, however, intratubular application of U37883A (100 μM) did not alter the reabsorption of fluid, sodium or potassium ion in the loop of Henle in SD rats.
Table 2.
Effect of intratubular application of U37883A, glibenclamide, or two metabolites of glibenclamide on early distal flow rate, and sodium and potassium ion concentrations (VED, [Na+]ED, [K+]ED) during stopped-flow perfusion of Henle's loop in MWF rats

Discussion
The present study aimed to elucidate which tubular segment upstream to the distal tubule contributes to the diuresis and natriuresis in response to systemic application of the KATP channel blocker U37883A. Confirming the observation that systemic application of U37883A elicits a diuresis and natriuresis, the present study further revealed that effects on water-permeable segments upstream to the distal tubule such as the proximal convoluted tubule contribute to this response.
Performing free-flow collections from the early distal tubular site in nephrons with superficial glomeruli, it was observed that the diuresis and natriuresis in response to systemic application of U37883A at 15 mg kg−1 was associated with an increase in VED from 10–18 nl min−1 and in [Na+]ED from 35–51 mM, reflecting a decrease in fractional reabsorption of fluid and Na+ up to the early distal tubule of 13 and 8%, respectively. These findings indicated an inhibition of reabsorption in water-permeable segments upstream to the early distal tubule such as the proximal tubule. Since the TALH is considered water-impermeable, this also indicated a substantial rise in the fluid and electrolyte load to the TALH in response to U37883A. As previously shown, increasing late proximal perfusion rate downstream from an obstructing wax block from 15–30 nl min−1 causes VED to increase from 10–17 nl min−1 and [Na+]ED from 31–63 mM reflecting the increased load to the water-impermeable TALH (Huang et al., 1998). Thus, the increase in fluid and electrolyte load to the TALH, as proposed from the present study in response to systemic application of U37883A, could fully account for the observed modest rise in [Na+]ED. The latter indicates that U37883A may not have significantly inhibited Na+ reabsorption in TALH, although a minor contribution can not be fully excluded. Further supporting this notion and confirming previous reports (Romano et al., 1995), we found that systemic application of furosemide, the action of which is restricted to inhibiting Na+-2Cl−-K+-cotransport in the water-impermeable TALH, elicited a substantial rise in [Na+]ED (39–117 mM) without altering VED and therefore the fluid load to TALH. Together, these data proposed that, at least in superficial nephrons, effects on water-permeable segments predominantly contributed to the diuretic and natriuretic effect upstream to the early distal tubule in response to systemic application of U37883A.
During systemic application of U37883A and free-flow collection from early distal tubular site it was further observed that a dose of 5 mg kg−1 elicited a similar response up to early distal tubule as 15 mg kg−1 as indicated by almost similar rises in VED and [Na+]ED. However, the observed diuresis and natriuresis were significantly reduced at 5 mg kg−1 of U37883A and a kaliuresis was evident, a response which was not observed with 15 mg kg−1. These findings may indicate that U37883A at 5 mg kg−1 was not as efficient as 15 mg kg−1 to inhibit K+ secretion in the distal nephron. Because a dose of 5 mg kg−1 caused an almost similar increase in fluid and Na+ load to the distal nephron, less efficient inhibition of K+ secretion and thus of Na+ reabsorption in this nephron segment is expected to lower natriuresis and diuresis and elicit kaliuresis.
There is evidence that in the proximal convoluted tubule KATP channels in the basolateral membrane, which are sensitive to glibenclamide, contribute to fluid and Na+ reabsorption (Tsuchiya et al., 1992; Beck et al., 1993; Welling, 1995). Whereas the latter evidence was derived from studies in rabbits, in the present study, the observed significant fall in fractional fluid reabsorption upstream to the early distal tubule in response to systemic application of U37883A could reflect an inhibition of KATP channels in the proximal convoluted tubule in rat kidney. Further supporting such a notion, subsequent experiments with systemic (5 or 15 mg kg−1) or intratubular (50 or 100 μM) application of U37883A revealed a significant inhibition of fluid reabsorption in the proximal convoluted tubule. Because proximal tubule fluid reabsorption takes place by virtually isosmotic Na+ reabsorption, these findings indicated that U37883A inhibited Na+ reabsorption in the proximal convoluted tubule. The finding that the inhibition of fluid reabsorption in proximal tubule by U37883A appeared not quite as high with stopped-flow perfusion downstream from an obstructing wax block as compared to free-flow collection is not unexpected, because in the experiments with stopped-flow perfusion the part of the proximal convoluted tubule studied did not include the segment between Bowman space and the first superficial proximal tubular loop, a segment which is known to have a high reabsorptive capacity. Furthermore, the observation that both systemic as well as intratubular application of U37883A inhibited proximal tubular reabsorption during stopped-flow perfusion indicated that application of the drug to either tubular site, i.e., the luminal or basolateral membrane, can be effective. The latter findings, however, do not necessarily implicate that U37883A exerted actions on both the luminal and basolateral membrane, because for example U37883A after being taken up by the cell across the luminal membrane could affect KATP channels in the basolateral membrane.
It was previously reported that intratubular application of U37883A (50 μM) or glibenclamide (250 μM) inhibited the reabsorption of fluid, Na+, and K+ in the loop of Henle in SD rats (Wang et al., 1995a,1995b). Although the observed significant inhibitory effect of U37883A or glibenclamide on fluid reabsorption in those studies indicated at least an additional action in water-permeable segments, the authors proposed a primary inhibitory effect of U37883A or glibenclamide on reabsorption in the water-impermeable TALH (Wang et al., 1995a,1995b). Because the present experiments with systemic application of U37883A proposed a predominant effect on the proximal convoluted tubule rather than the TALH, additional studies were performed with intratubular application of U37883A. Basically further supporting a predominant effect on the proximal convoluted tubule during systemic application, it was observed that intratubular application of U37883A (10–100 μM) did not significantly affect the reabsorption of fluid, Na+, or K+ in the loop of Henle.
Similar negative findings were observed for glibenclamide (250 μM) as well as for two of its metabolites which in contrast to glibenclamide can be found in the urine (Heptner et al., 1969). In comparison, as shown previously under similar experimental conditions, intratubular application of furosemide substantially reduced Na+ and K+ reabsorption without changing fluid reabsorption in the loop of Henle (Huang et al., 1998), which is in accordance with the effect observed in response to systemic application of furosemide in the present study. The different response in loop of Henle reabsorption in the present and the two previous studies appears not to be related to the rat strain used, since we could confirm our negative results obtained in MWF rats on the effect of U37883A on loop of Henle reabsorption in SD rats. Thus, independent of the interpretation of the results of the two previous studies, the reason(s) for the different response in loop of Henle reabsorption to intratubular application of U37883A or glibenclamide in the present and the two previous studies remains unclear. Two types of KATP channels were identified in the luminal membrane of rat TALH using patch clamp technique (Bleich et al., 1990; Wang, 1994). One of these two channels revealed an intermediate K+ conductance (about 70 pS) and the other a low K+ conductance (about 30 pS). Although both types of KATP channels are present in the apical membrane of TALH, the intermediate conductance K+ channel appears to functionally dominate and represent about 80% of the apical K+ conductance (Wang & Lu, 1995). Interestingly, neither U37883A nor glibenclamide have been reported to inhibit the intermediate conductance KATP channel in TALH. Whereas U37883A and glibenclamide were shown to inhibit the low conductance KATP channel (Wang et al., 1995a,1995b), the intermediate conductance KATP channel in TALH proved insensitive to glibenclamide (Wang, 1994). Whether the functional contribution of small and intermediate conductance KATP channels can be altered in the loop of Henle or TALH under certain conditions, which could account for the different findings with intratubular application of U37883A or glibenclamide in the present and previous studies (Wang et al., 1995a,1995b), remains to be determined.
It was further observed that systemic application of U37883A increased SNGFR during fluid collection from the late proximal but not the early distal tubular site. These findings may indicate that U37883A elicited an upward pressure on SNGFR which might be independent from the tubular effect (e.g., renal vasodilation in response to U37883A could be the result of direct inhibition of renin release (Vallon et al., 1998a,1998b)). The upward pressure on SNGFR of U37883A was balanced out during fluid collection from the early distal tubular site implicating a role of tubuloglomerular feedback which may have been activated as a result of inhibition of proximal tubular reabsorption. The upward pressure became evident and the SNGFR increased, however, when the tubuloglomerular feedback signal was prevented from changing as during fluid collection from the late proximal tubular site.
In summary, the present findings suggest that the diuretic and natriuretic response to systemic application of the KATP channel blocker U37883A involves, in addition to effects on the distal nephron, an inhibition of reabsorption in water-permeable segments upstream to the distal tubule such as the proximal convoluted tubule, where a role in tubular reabsorption for KATP channels has been implicated in the basolateral membrane. Furthermore, evidence is provided that systemic application of U37883A exerted an upward pressure on GFR by an yet unknown mechanism, the influence of which, however, appeared to be balanced by the tubular effects of U37883A, such that GFR remained unchanged.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Os 42/9-1), the Federal Ministry of Education and Research (BMBF 01EC9405) and the Interdisciplinary Clinical Research Center (IKFZ) Tübingen (BMBF 01KS9602). The authors acknowledge the support and helpful discussion by Prof U. Quast.
Abbreviations
- Δ AR-fluid
change in absolute fluid reabsorption up to the late proximal convoluted tubule
- ATF
artificial tubular fluid
- EXP
experimental period
- FR-fluid
FR-Na+, FR-K+, fractional reabsorption of fluid, sodium and potassium ion up to the early distal tubule
- Δ FR-fluid
fractional fluid reabsorption up to the late proximal convoluted tubule
- GFR
glomerular filtration rate
- KATP channels
ATP sensitive potassium channels; early distal tubular K+ concentration
- K+urine
urinary K+ excretion
- LH
loop of Henle
- MAP
mean arterial blood pressure
- MWF
Munich-Wistar-Frömter
- [Na+]ED
early distal tubular Na+ concentration
- Na+urine
urinary Na+ excretion
- PCT
proximal convoluted tubule
- SD
Sprague-Dawley
- SNGFR: single nephron glomerular filtration rate; Δ SNGFR
change in SNGFR
- TALH: thick ascending limb of Henle's loop; VED
early distal tubular flow rate
- Δ VLP
change in late proximal tubular flow rate
- Vurine
urinary flow rate
References
- BECK J.S., HURST A.M., LAPOINTE J.-Y., LAPRADE R. Regulation of basolateral potassium channels in proximal tubule studied during continuous microperfusion. Am. J. Physiol. 1993;264:F496–F501. doi: 10.1152/ajprenal.1993.264.3.F496. [DOI] [PubMed] [Google Scholar]
- BLEICH M., SCHLATTER E., GREGER R. The luminal K+ channel of the thick ascending limb of Henle's loop. Pfluegers. Arch. 1990;415:449–460. doi: 10.1007/BF00373623. [DOI] [PubMed] [Google Scholar]
- CLARK M.A., HUMPHREY S.J., SMITH M.P., LUDENS J.H. Unique natriuretic properties of the ATP-sensitive K+-channel blocker glyburide in conscious rats. J. Pharmacol. Exp. Ther. 1993;265:933–937. [PubMed] [Google Scholar]
- FERRIER C.P., KURTZ A., LEHNER P., SHAW S.G., PUSTERLA C., SAXENHOFER H., WEIDMANN P. Stimulation of renin secretion by potassium-channel activation with cromakalim. Eur. J. Clin. Pharmacol. 1989;36:443–447. doi: 10.1007/BF00558067. [DOI] [PubMed] [Google Scholar]
- GUILLEMARE E., HONORE E., DE WEILLE J., FOSSET M., LAZDUNSKI M., MEISHERI K. Functional receptors in Xenopus oocytes for U-37883A, a novel ATP-sensitive K+ channel blocker: Comparison with rat insulinoma cells. Mol. Pharmacol. 1994;46:139–145. [PubMed] [Google Scholar]
- HEPTNER W., KELLNER H.M., CHRIST O., WEIHRAUCH D. Metabolismus von HB419 am Tier. Arzneim. Forsch./Drug. Res. 1969;19:1400–1404. [PubMed] [Google Scholar]
- HUANG D.Y., OSSWALD H., VALLON V. Intratubular application of sodium azide inhibits loop of Henle reabsorption and tubuloglomerular feedback response in anesthetized rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:367–373. doi: 10.1007/pl00005266. [DOI] [PubMed] [Google Scholar]
- LINSEMAN D.A., LAWSON J.A., JONES D.A., LUDENS J.H. Glyburide attenuates calmodulin antagonist-stimulated renin release from isolated mouse juxtaglomerular cells. Am. J. Physiol. 1995;269:F242–F247. doi: 10.1152/ajprenal.1995.269.2.F242. [DOI] [PubMed] [Google Scholar]
- LUDENS J.H., CLARK M.A., LAWSON J.A. Effects of a K+ channel blocker on glomerular filtration rate and electrolyte excretion in conscious rats. J. Pharmacol. Exp. Ther. 1995a;273:1375–1381. [PubMed] [Google Scholar]
- LUDENS J.H., CLARK M.A., SMITH M.P., HUMPHREY S.J. Renal and vascular effects of chemically distinct ATP-sensitive K+ channel blockers in rats. J. Cardiovasc. Pharmacol. 1995b;25:404–409. doi: 10.1097/00005344-199503000-00009. [DOI] [PubMed] [Google Scholar]
- MEISHERI K.D., HUMPHREY S.J., KHAN S.A., CIPKUS-DUBRAY L.A., SMITH M.P., JONES A.W. 4-Morpholinecarboximidine -N -1-adamantyl-N′-cyclohexylhydrochloride (U-37883A): Pharmacological characterization of a novel antagonist of vascular ATP-sensitive K+ channel openers. J. Pharmacol. Exp. Ther. 1993a;166:655–665. [PubMed] [Google Scholar]
- MEISHERI K.D., KHAN S.A., MARTIN J.L. Vascular pharmacology of ATP-sensitive K+ channels: interactions between glyburide and K+ channel openers. J. Vasc. Res. 1993b;30:2–12. doi: 10.1159/000158969. [DOI] [PubMed] [Google Scholar]
- QUAST U. ATP-sensitive K+ channels in the kidney. Naunyn-Schmiedebergs Arch. Pharmacol. 1996;354:213–215. doi: 10.1007/BF00171051. [DOI] [PubMed] [Google Scholar]
- ROMANO G., FAVRET G., BARTOLI E. Micropuncture study of the effect of furosemide on proximal and distal tubules of rat nephron. Renal Physiol. Biochem. 1995;18:209–218. doi: 10.1159/000173918. [DOI] [PubMed] [Google Scholar]
- TSUCHIYA K., WANG W., GIEBISCH G., WELLING P.A. ATP is a coupling modulator of parallel Na+-K+-ATPase and K+ channel activity in renal proximal tubule. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6418–6422. doi: 10.1073/pnas.89.14.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALLON V., ALBINUS M., BLACH D. Effect of KATP channel blocker U37883A on renal function in experimental diabetes mellitus. J. Pharmacol. Exp. Ther. 1998a;286:1215–1221. [PubMed] [Google Scholar]
- VALLON V., KIRSCHENMANN D., BRENNER I., ALBINUS M., OSSWALD H. Potassium diet as a determinant for the renal response to systemic potassium channel modulation in anesthetized rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998b;358:245–252. doi: 10.1007/pl00005249. [DOI] [PubMed] [Google Scholar]
- VALLON V., OSSWALD H., BLANTZ R.C., THOMSON S.C. Potential role of luminal potassium in tubuloglomerular feedback. J. Am. Soc. Nephrol. 1997a;8:1831–1837. doi: 10.1681/ASN.V8121831. [DOI] [PubMed] [Google Scholar]
- VALLON V., RICHTER K., HEYNE N., OSSWALD H. Effect of intratubular application of angiotensin 1-7 on nephron function. Kidney Blood Press. Res. 1997b;20:233–239. doi: 10.1159/000174151. [DOI] [PubMed] [Google Scholar]
- WANG T., WANG W.H., KLEIN-ROBBENHAAR G., GIEBISCH G. Effects of Glyburide on renal tubule transport and potassium-channel activity. Renal. Physiol. Biochem. 1995a;18:169–182. doi: 10.1159/000173914. [DOI] [PubMed] [Google Scholar]
- WANG T., WANG W., KLEIN-ROBBENHAAR G., GIEBISCH G. Effects of a novel KATP channel blocker on renal tubule function and K+ channel activity. J. Pharmacol. Exp. Ther. 1995b;273:1382–1389. [PubMed] [Google Scholar]
- WANG W. Two types of K+ channels in thick ascending limb of rat kidney. Am. J. Physiol. 1994;267:F599–F605. doi: 10.1152/ajprenal.1994.267.4.F599. [DOI] [PubMed] [Google Scholar]
- WANG W.H., LU M. Effect of arachidonic acid on activity of the apical K channel in the thick ascending limb of the rat kidney. J. Gen. Physiol. 1995;106:727–743. doi: 10.1085/jgp.106.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLING P.A. Cross-talk and the role of KATP channels in the proximal tubule. Kidney Intern. 1995;48:1017–1023. doi: 10.1038/ki.1995.384. [DOI] [PubMed] [Google Scholar]


