Abstract
The effect of spinal administration of the selective cannabinoid CB1 receptor antagonist, SR141716A, and the selective CB2 receptor antagonist, SR144528, on innocuous versus noxious evoked responses of dorsal horn neurones in the spinal cord of the anaesthetized rat was investigated. SR141716A (0.001–1 ng 50 μl−1) dose-relatedly facilitated the non-potentiated component of the electrical C-fibre mediated neuronal response (120±6, 156±13, 192±33 and 192±31% of control respectively; n=6). In contrast, SR144528 (0.001–1 ng 50 μl−1) did not influence the non-potentiated component of the C-fibre evoked neuronal response (n=5). The electrical evoked Aβ-fibre mediated neuronal responses were not influenced by SR141716A or SR144528. The results of this study provide evidence that tonic cannabinoid CB1 receptor activation, but not CB2 receptor activation, attenuates acute nociceptive transmission, at the level of the spinal cord. These results suggest a selective antinociceptive role of the endogenous cannabinoids at spinal CB1 receptors.
Keywords: Nociception, spinal neurones, cannabinoid receptor antagonism
Introduction
The localization of spinal cannabinoid CB1 receptors (Herkenham et al., 1991; Tsou et al., 1997) and an endogenous cannabinoid receptor ligand at this level (see Di Marzo et al., 1998) implicates a functional role of the endogenous spinal cannabinoids at the level of the spinal cord.
SR141716A has been shown to be a potent and selective antagonist of CB1 receptors in rat brain (Rinaldi-Carmona et al., 1994). However, it has also been suggested that SR141716A acts as an inverse agonist at human CB1 and CB2 receptors in transfected Chinese hamster ovary cells (MacLennan et al., 1998).
Spinal administration of SR141716A has been shown to result in thermal hyperalgesia in mice (Richardson et al., 1997, 1998a) and to facilitate formalin evoked pain behaviour in rats (Strangman et al., 1998). These behavioural studies suggest there is tonic control of spinal nociceptive processing by endogenous cannabinoids acting at the CB1 receptor. In contrast, the nociceptive threshold of CB1 receptor knockout mice has been shown to be similar to those of wild-type mice (Ledent et al., 1999). Thus the importance of a tonic control of nociceptive thresholds/responses by the endogenous cannabinoids is unclear.
To our knowledge there have been no reports on the effects of blockade of the endogenous cannabinoids on neuronal measures of nociceptive activity. The effect of spinal administration of SR144528, a selective CB2 receptor antagonist (Rinaldi-Carmona et al., 1998), on nociceptive processing is unknown. The aim of the present study was to ascertain the selectivity of the tonic effects of the endogenous cannabinoids on nociceptive versus non-nociceptive activity. To this end, the effects of spinal administration of SR141716A and SR144528 on Aβ-fibre versus C-fibre evoked responses of dorsal horn neurones have been studied.
Methods
The techniques used have been described previously (Chapman et al., 1994). Extracellular recordings of convergent dorsal horn neurones (depth 500–1000 μm) were made in anaesthetized (1.5% halothane in 66% N2O/33% O2) Sprague-Dawley rats (200–250 g, n=11). Neuronal responses to transcutaneous electrical stimulation (3×C-fibre threshold, trains of 16 stimuli at 0.5 Hz) of the peripheral receptive field were recorded and post-stimulus histograms were constructed. Evoked responses were separated and quantified on the basis of latencies: Aβ-fibre: 0–20 ms post-stimulus; C-fibre: 90–300 ms post-stimulus and post-discharge: 300–800 ms post-stimulus. The non-potentiated C-fibre evoked neuronal response was calculated as the number of action potentials evoked by the first stimulus multiplied by the total number of stimuli (16). The non-potentiated component of the C-fibre evoked response is reflective of the C-fibre input into the dorsal horn prior to the activation of post-synaptic NMDA receptor mediated events and the facilitation of C-fibre evoked responses.
Control responses (less than 10% variance) were established. The effects of spinal administration of SR141716A (0.001–1 ng 50 μl−1 [0.042–42 nM] n=6) and SR144528 (0.001–1 ng 50 μl−1, n=5) on evoked neuronal responses were measured. SR141716A and SR144528 were dissolved in distilled H2O and ethanol [final concentration for highest dose of drugs studied <0.1% ethanol]. Drugs were given cumulatively and effects were measured at 5, 10, 20, 30 and 40 min post-drug administration. Data are presented as percentage of the control response±s.e.mean, statistical analysis was performed with repeated measures (ANOVA) and Dunnett's multiple comparison test.
Results
All of the neurones studied were convergent, located in the dorsal horn of the spinal cord. The mean thresholds for electrical stimulation of C-fibres for the two groups of neurones studied were 1.7±0.2 mA (SR141716A, n=6) and 1.5±0.3 mA (SR144528, n=5).
Spinal administration of SR141716A significantly facilitated the non-potentiated component of the C-fibre evoked neuronal responses in a dose-related manner (Figure 1). Maximal effects of the lower doses of SR141716A (0.001, 0.01 and 0.1 ng 50 μl−1) were observed at 28±5, 27±4 and 30±5 min post-drug administration. The maximal effect of the highest dose of SR141716A (1 ng 50 ml−1) was observed at 18±5 min post-drug administration. SR141716A produced a non-significant facilitation of the post-discharge response, a measure of the level of the hyperexcitability of the neurone (Figure 1).
Figure 1.
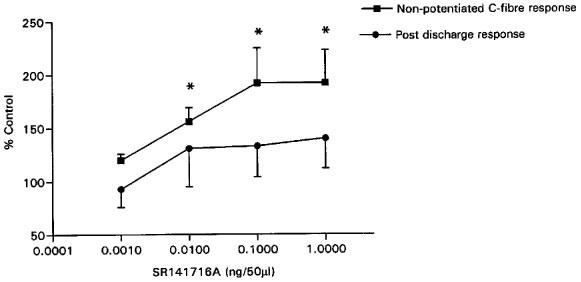
The effect of intrathecal administration of SR141716A on the non-potentiated component of the C-fibre evoked response of dorsal horn neurones (mean control value 320±81 action potentials) and the post-discharge response (mean control value 220±73 action potentials) (n=6 for each dose). Note the significant excitatory effect of SR141716A on the non-potentiated response as compared to control. Data shown are means and s.e.means. Statistical analysis: repeated measures (ANOVA) and Dunnett's multiple comparison test, *P<0.01.
The effects of SR141716A (0.001, 0.01, 0.1 and 1 ng 50 μl−1) on the overall C-fibre evoked neuronal responses (mean control value 370±51 action potentials) were small and non-significant (101±6, 116±5, 120±11 and 126±11% of control respectively). The same concentrations of SR141716A (0.001–1 ng 50 μl−1) did not influence the Aβ-fibre evoked responses (mean control value 90±8 action potentials) of the dorsal horn neurones (80±6, 92±7, 101±13 and 100±15% of control respectively).
Spinal administration of SR144528 (0.001–1 ng 50 μl−1) did not influence the evoked responses of dorsal horn neurones. The mean maximal effect of the highest concentration of SR144528 studied on the non-potentiated component of the C-fibre evoked neuronal response and the post-discharge was 93±18% of control and 103±17% of control respectively. Finally, the mean maximal effect of SR144528 (1 ng 50 μl−1) on the overall C-fibre and Aβ-fibre evoked neuronal response was 82±7 and 85±9% of control respectively.
Discussion
This study has clearly shown for the first time that the cannabinoid CB1 receptor antagonist, SR141716A, facilitates noxious evoked (C-fibre mediated) responses of spinal neurones. The non-potentiated component of the overall C-fibre evoked neuronal response was most strongly, and significantly, facilitated by SR141716A. There was a tendency towards a facilitation of the post-discharge response by SR141716A. Innocuous evoked neuronal responses (Aβ-fibre mediated) were not influenced by SR141716A. Spinal administration of the CB2 receptor antagonist, SR144528, did not influence the C- or Aβ-fibre evoked neuronal responses.
The concentrations of SR141716A used in this study are within the range of concentrations originally shown to be selective for the CB1 receptor and showing no affinity for an array of other receptors and channels studied (Rinaldi-Carmona et al., 1994). Taken with the finding that the CB2 receptor antagonist, SR144528, does not influence spinal nociceptive transmission, the results of this study with SR141716A suggest a tonic control of nociceptive evoked activity of spinal neurones by endogenous inhibitory cannabinoids acting at the CB1 receptor.
The location of the cannabinoid CB1 receptors is an important determinant of the selective effect of this endogenous inhibitory system on nociceptive transmission. A recent autoradiography study has demonstrated that only 16% of spinal CB1 receptors are located on C-fibre afferent endings (Hohmann & Herkenham, 1998). Furthermore, a recent in situ hybridization study has shown that only 10–15% of dorsal root ganglion (DRG) neurones express mRNA for the CB1 receptor and a small percentage of the CB1 receptors in the DRG reside on C-fibres (Hohmann & Herkenham, 1999). Although anatomical data suggests a predominant post-synaptic location of the CB1 receptors, a functional role of CB1 receptors located on C-fibre afferent endings has been demonstrated (Richardson et al., 1998b). Thus there are clear differences in the pre- versus postsynaptic location of the CB1 receptors and opioid receptors which also have anti-nociceptive actions, but are pre-dominantly located presynaptically on C-fibre afferent endings (Besse et al., 1990).
The results of this study are in agreement with previous behavioural studies of the effect of SR141716A and CB1 receptor antisense oligonucleotides, but are not in agreement with a recent study of nociceptive thresholds in CB1 knockout mice (see Introduction). Thus there is some disparity between the effect of acutely blocking spinal cannabinoid CB1 receptors and global CB1 receptor knockout on nociceptive transmission. Compensatory mechanisms in CB1 receptor knockout mice may mask the novel tonic inhibitory control of nociceptive transmission by endogenous cannabinoids acting at the spinal CB1 receptor reported here.
Acknowledgments
SR141716A was provided by Research Biochemicals International as part of the chemical synthesis program of the National Institute of Mental Health, Contract N01MH30003. SR144528 was a gift from Sanofi (Montpellier, France). The Royal Society and the Research Opportunity Fund (Nottingham University) supported this study. Dr D.A. Kendall and Dr M.D. Randall are thanked for their helpful discussion.
Abbreviations
- CB1 receptor
cannabinoid1 receptor
- CB2 receptor
cannabinoid2 receptor
- SR141716A
N-(piperidin-1-yl)-5 - (4-chlorophenyl) - 1-(2,4 - dichlorophenyl) - 4 - methyl-1H-pyrazole - 3 - carboxamide hydrochloride
- SR144528
N-[(1S)-endo-1,3,3-trimethyl bicyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide
- s.e.mean
standard error of mean
References
- BESSE D., LOMBARD M.C., ZAJAC J.M., FOQUES B.P., BESSON J.M. Pre- and postsynaptic distribution of μ, δ and κ opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990;521:15–22. doi: 10.1016/0006-8993(90)91519-m. [DOI] [PubMed] [Google Scholar]
- CHAPMAN V., HALEY J.E., DICKENSON A.H. Electrophysiologic analysis of preemptive effects of spinal opioids on NMDA receptor mediated events. Anesthesiology. 1994;81:1429–1436. doi: 10.1097/00000542-199412000-00018. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., MELCK D., BISOGNO T., DE PETROCELLIS L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. TINS. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- HERKENHAM M., LYNN A.B., JOHNSON M.R., MELVIN L.S., COSTA B.R., RICE K.C. Characterization and localization of cannabinoid receptors in rat brain: quantitative in vitro autoradiographic study. J. Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHMANN A.G., HERKENHAM M. Regulation of cannabinoid and mu opioid receptors in rat lumbar spinal cord following neonatal capsaicin treatment. Neurosci. Letts. 1998;252:13–16. doi: 10.1016/s0304-3940(98)00534-5. [DOI] [PubMed] [Google Scholar]
- HOHMANN A.G., HERKENHAM M. Localisation of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- LEDENT C., VALVERDE O., COSSU G., PETITET F., AUBERT J.-F., BESLOT F., BÖHME G.A., IMPERATO A., PEDRAZZINI T., ROQUES B.P., VASSART G., FRATTA W., PARMENTIER M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- MACLENNAN S.J., REYNEN P.H., KWAN J., BONHAUS D.W. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br. J. Pharmacol. 1998;124:619–622. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON J.D., AANONSEN L., HARGREAVES K.M. SR141716A, a cannabinoid receptor antagonist, produces hyperalgesia in untreated mice. Eur. J. Pharmacol. 1997;319:R3–R4. doi: 10.1016/s0014-2999(96)00952-1. [DOI] [PubMed] [Google Scholar]
- RICHARDSON J.D., AANONSEN L., HARGREAVES K.M. Hypoactivity of the spinal cannabinoid system results in NMDA-dependent hyperalgesia. J. Neurosci. 1998a;18:451–457. doi: 10.1523/JNEUROSCI.18-01-00451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON J.D., AANONSEN L., HARGREAVES K.M. Antihyperalgesic effects of spinal cannabinoids. Eur. J. Pharmacol. 1998b;345:145–153. doi: 10.1016/s0014-2999(97)01621-x. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NELIAT G., CAPUT D., FERRARA P., SOUBRIE P., BRELIERE J.C., LE FUR G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Letts. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J.M., CASELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOUABOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIERE J.C., LEFUR G. SR144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- STRANGMEN N.M., PATRICK S.L., HOHMANN A.G., TSOU K., WALKER J.M. Evidence for a role of endogenous cannabinoids in the modulation of acute and tonic pain sensitivity. Brain Research. 1998;813:323–328. doi: 10.1016/s0006-8993(98)01031-2. [DOI] [PubMed] [Google Scholar]
- TSOU K., BROWN S., SANUDO-PENA M.C., MACKIE K., WALKER J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1997;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]


