Abstract
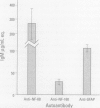
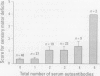
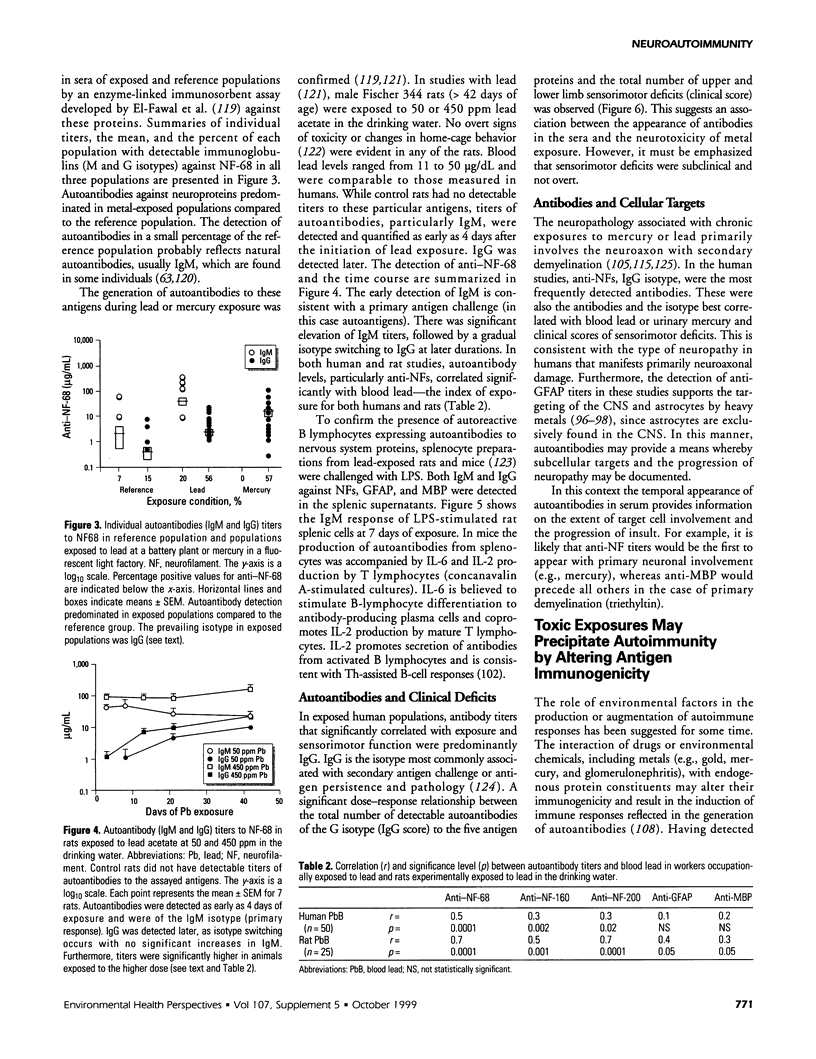
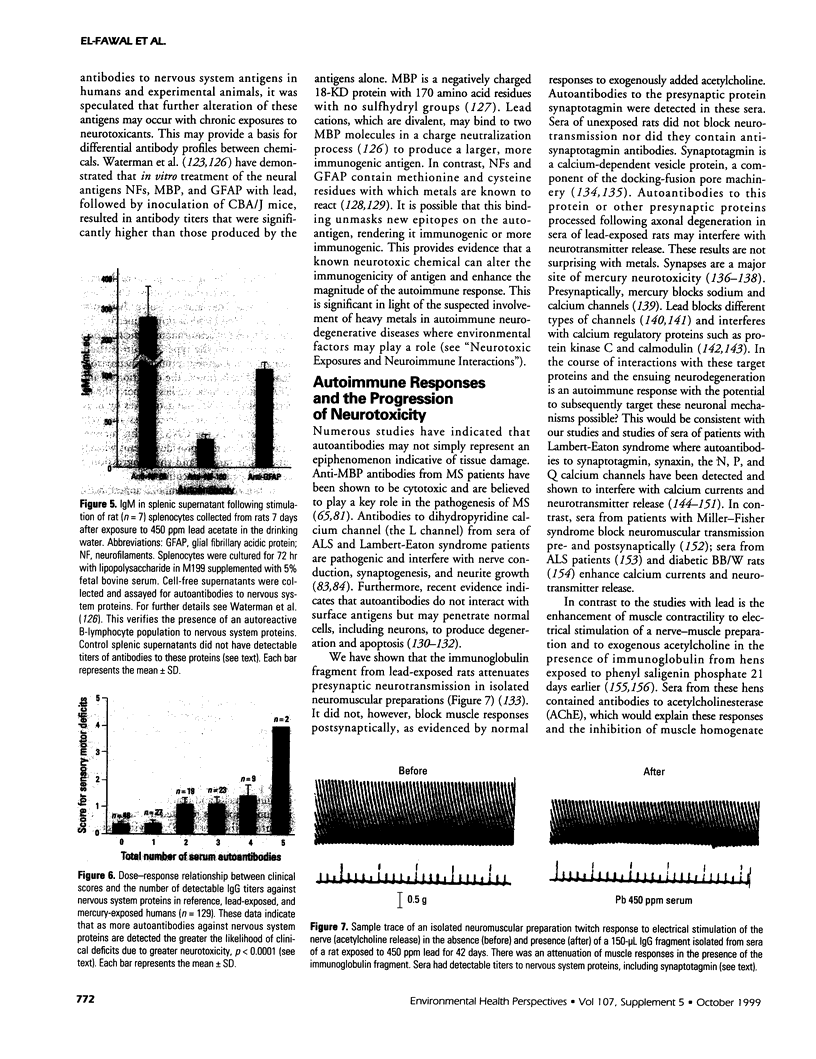
The interactions between the nervous and immune systems have been recognized in the development of neurodegenerative disease. This can be exploited through detection of the immune response to autoantigens in assessing the neurotoxicity of environmental chemicals. To test this hypothesis, the following questions were addressed. a) Are autoantibodies to nervous system (NS) antigens detected in populations exposed to environmental or occupational chemicals? In sera of male workers exposed to lead or mercury, autoantibodies, primarily IgG, to neuronal cytoskeletal proteins, neurofilaments (NFs), and myelin basic protein (MBP) were prevalent. These findings were confirmed in mice and rats exposed to either metal. b) Do autoantibodies to NS antigens relate to indices of exposure? In humans exposed to either metal, and similarly in exposed rats, titers of IgG against NFs and MBP significantly correlated with blood lead or urinary mercury, the typical indices of exposure. c) Do autoantibodies correlate with sensorimotor deficits? In workers exposed to lead or mercury, a significant correlation was observed between IgG titers and subclinical deficits. Doses of metals used in rat exposures were subclinical, suggesting that autoantibodies may be predictive of neurotoxicity. d) Is the detection indicative of nervous system pathology? In rats exposed to metals, histopathology indicated central nervous system (CNS) and peripheral nervous system (PNS) damage. In addition there was evidence of astrogliosis, which is indicative of neuronal damage in the CNS, and the presence of IgG concentrated along the blood-brain barrier, as indicated by immunostaining for antibodies. e) Are immune responses to NS antigens pathogenic? Immunoglobulin fractions from rat and human sera interfered with neuromuscular function. These studies suggest that the detection of autoantibodies to NS-specific antigens may be used to monitor the development of neurotoxicity to environmental chemicals and that immune mechanisms may be involved in the progression of neurodegeneration.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. R., Ziegler D. K., Lin J. T. Mercury intoxication simulating amyotrophic lateral sclerosis. JAMA. 1983 Aug 5;250(5):642–643. [PubMed] [Google Scholar]
- Akiyama H., Itagaki S., McGeer P. L. Major histocompatibility complex antigen expression on rat microglia following epidural kainic acid lesions. J Neurosci Res. 1988;20(2):147–157. doi: 10.1002/jnr.490200202. [DOI] [PubMed] [Google Scholar]
- Alarcon-Segovia D., Ruiz-Argüelles A., Llorente L. Broken dogma: penetration of autoantibodies into living cells. Immunol Today. 1996 Apr;17(4):163–164. doi: 10.1016/s0167-5699(96)90258-3. [DOI] [PubMed] [Google Scholar]
- Armati P. J., Pollard J. D., Gatenby P. Rat and human Schwann cells in vitro can synthesize and express MHC molecules. Muscle Nerve. 1990 Feb;13(2):106–116. doi: 10.1002/mus.880130204. [DOI] [PubMed] [Google Scholar]
- Armon C., Kurland L. T., Daube J. R., O'Brien P. C. Epidemiologic correlates of sporadic amyotrophic lateral sclerosis. Neurology. 1991 Jul;41(7):1077–1084. doi: 10.1212/wnl.41.7.1077. [DOI] [PubMed] [Google Scholar]
- Atchison W. D. Effects of neurotoxicants on synaptic transmission: lessons learned from electrophysiological studies. Neurotoxicol Teratol. 1988 Sep-Oct;10(5):393–416. doi: 10.1016/0892-0362(88)90001-3. [DOI] [PubMed] [Google Scholar]
- Atchison W. D., Narahashi T. Mechanism of action of lead on neuromuscular junctions. Neurotoxicology. 1984 Fall;5(3):267–282. [PubMed] [Google Scholar]
- Barna B. P., Chou S. M., Jacobs B., Yen-Lieberman B., Ransohoff R. M. Interferon-beta impairs induction of HLA-DR antigen expression in cultured adult human astrocytes. J Neuroimmunol. 1989 Jun;23(1):45–53. doi: 10.1016/0165-5728(89)90072-6. [DOI] [PubMed] [Google Scholar]
- Bell C. L., Partington C., Robbins M., Graziano F., Turski P., Kornguth S. Magnetic resonance imaging of central nervous system lesions in patients with lupus erythematosus. Correlation with clinical remission and antineurofilament and anticardiolipin antibody titers. Arthritis Rheum. 1991 Apr;34(4):432–441. doi: 10.1002/art.1780340408. [DOI] [PubMed] [Google Scholar]
- Bergsteinsdóttir K., Kingston A., Jessen K. R. Rat Schwann cells can be induced to express major histocompatibility complex class II molecules in vivo. J Neurocytol. 1992 May;21(5):382–390. doi: 10.1007/BF01191706. [DOI] [PubMed] [Google Scholar]
- Borella P., Giardino A. Lead and cadmium at very low doses affect in vitro immune response of human lymphocytes. Environ Res. 1991 Aug;55(2):165–177. doi: 10.1016/s0013-9351(05)80173-2. [DOI] [PubMed] [Google Scholar]
- Braxton D. B., Williams M., Kamali D., Chin S., Liem R., Latov N. Specificity of human anti-neurofilament autoantibodies. J Neuroimmunol. 1989 Feb;21(2-3):193–203. doi: 10.1016/0165-5728(89)90175-6. [DOI] [PubMed] [Google Scholar]
- Bressler J. P., Goldstein G. W. Mechanisms of lead neurotoxicity. Biochem Pharmacol. 1991 Feb 15;41(4):479–484. doi: 10.1016/0006-2952(91)90617-e. [DOI] [PubMed] [Google Scholar]
- Brose N., Petrenko A. G., Südhof T. C., Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992 May 15;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Buchwald B., Weishaupt A., Toyka K. V., Dudel J. Pre- and postsynaptic blockade of neuromuscular transmission by Miller-Fisher syndrome IgG at mouse motor nerve terminals. Eur J Neurosci. 1998 Jan;10(1):281–290. doi: 10.1046/j.1460-9568.1998.00053.x. [DOI] [PubMed] [Google Scholar]
- Cash E., Weerth S., Voltz R., Kornhuber M. Cells of cerebrospinal fluid of multiple sclerosis patients secrete antibodies to myelin basic protein in vitro. Scand J Immunol. 1992 Jun;35(6):695–701. doi: 10.1111/j.1365-3083.1992.tb02977.x. [DOI] [PubMed] [Google Scholar]
- Chan W. L., Javanovic T., Lukic M. L. Infiltration of immune T cells in the brain of mice with herpes simplex virus-induced encephalitis. J Neuroimmunol. 1989 Aug;23(3):195–201. doi: 10.1016/0165-5728(89)90051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. W. Neurotoxic effects of mercury--a review. Environ Res. 1977 Dec;14(3):329–373. doi: 10.1016/0013-9351(77)90044-5. [DOI] [PubMed] [Google Scholar]
- Chung I. Y., Benveniste E. N. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol. 1990 Apr 15;144(8):2999–3007. [PubMed] [Google Scholar]
- Cohen S. R., Herndon R. M., McKhann G. M. Radioimmunoassay of myelin basic protein in spinal fluid. An index of active demyelination. N Engl J Med. 1976 Dec 23;295(26):1455–1457. doi: 10.1056/NEJM197612232952604. [DOI] [PubMed] [Google Scholar]
- Conradi S., Ronnevi L. O. Further studies on the occurrence of serum autoantibodies against a membrane bound AChE fraction in ALS/MND patients and controls. J Neurol Sci. 1994 Jul;124 (Suppl):67–69. doi: 10.1016/0022-510x(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Conradi S., Ronnevi L. O., Nise G., Vesterberg O. Abnormal distribution of lead in amyotrophic lateral sclerosis--reestimation of lead in the cerebrospinal fluid. J Neurol Sci. 1980 Dec;48(3):413–418. doi: 10.1016/0022-510x(80)90112-4. [DOI] [PubMed] [Google Scholar]
- Conradi S., Ronnevi L. O. Selective vulnerability of alpha motor neurons in ALS: relation to autoantibodies toward acetylcholinesterase (AChE) in ALS patients. Brain Res Bull. 1993;30(3-4):369–371. doi: 10.1016/0361-9230(93)90267-f. [DOI] [PubMed] [Google Scholar]
- Conradi S., Ronnevi L. O., Vesterberg O. Increased plasma levels of lead in patients with amyotrophic lateral sclerosis compared with control subjects as determined by flameless atomic absorption spectrophotometry. J Neurol Neurosurg Psychiatry. 1978 May;41(5):389–393. doi: 10.1136/jnnp.41.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crols R., Saerens J., Noppe M., Lowenthal A. Increased GFAp levels in CSF as a marker of organicity in patients with Alzheimer's disease and other types of irreversible chronic organic brain syndrome. J Neurol. 1986 Jun;233(3):157–160. doi: 10.1007/BF00314423. [DOI] [PubMed] [Google Scholar]
- Dambinova S. A., Granstrem O. K., Tourov A., Salluzzo R., Castello F., Izykenova G. A. Monitoring of brain spiking activity and autoantibodies to N-terminus domain of GluR1 subunit of AMPA receptors in blood serum of rats with cobalt-induced epilepsy. J Neurochem. 1998 Nov;71(5):2088–2093. doi: 10.1046/j.1471-4159.1998.71052088.x. [DOI] [PubMed] [Google Scholar]
- David P., Martin-Moutot N., Leveque C., el Far O., Takahashi M., Seagar M. J. Interaction of synaptotagmin with voltage gated calcium channels: a role in Lambert-Eaton myasthenic syndrome? Neuromuscul Disord. 1993 Sep-Nov;3(5-6):451–454. doi: 10.1016/0960-8966(93)90095-2. [DOI] [PubMed] [Google Scholar]
- Drachman D. B., McIntosh K. R., De Silva S., Kuncl R. W., Kahn C. Strategies for the treatment of myasthenia gravis. Ann N Y Acad Sci. 1988;540:176–186. doi: 10.1111/j.1749-6632.1988.tb27060.x. [DOI] [PubMed] [Google Scholar]
- Ehle A. L. Lead neuropathy and electrophysiological studies in low level lead exposure: a critical review. Neurotoxicology. 1986 Fall;7(3):203–216. [PubMed] [Google Scholar]
- Estes M. L., Iwasaki K., Jacobs B. S., Barna B. P. Interleukin-4 down-regulates adult human astrocyte DNA synthesis and proliferation. Am J Pathol. 1993 Aug;143(2):337–341. [PMC free article] [PubMed] [Google Scholar]
- Evans H. L., Garman R. H., Laties V. G. Neurotoxicity of methylmercury in the pigeon. Neurotoxicology. 1982 Nov;3(3):21–36. [PubMed] [Google Scholar]
- Evans H. L., Little A. R., Gong Z. L., Duffy J. S., Wirgin I., el-Fawal H. A. Glial fibrillary acidic protein (GFAP) indicates in vivo exposure to environmental contaminants: PCBs in the Atlantic tomcod. Ann N Y Acad Sci. 1993 May 28;679:402–406. doi: 10.1111/j.1749-6632.1993.tb18329.x. [DOI] [PubMed] [Google Scholar]
- Fabry Z., Raine C. S., Hart M. N. Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol Today. 1994 May;15(5):218–224. doi: 10.1016/0167-5699(94)90247-X. [DOI] [PubMed] [Google Scholar]
- Fierz W., Endler B., Reske K., Wekerle H., Fontana A. Astrocytes as antigen-presenting cells. I. Induction of Ia antigen expression on astrocytes by T cells via immune interferon and its effect on antigen presentation. J Immunol. 1985 Jun;134(6):3785–3793. [PubMed] [Google Scholar]
- Frei K., Malipiero U. V., Leist T. P., Zinkernagel R. M., Schwab M. E., Fontana A. On the cellular source and function of interleukin 6 produced in the central nervous system in viral diseases. Eur J Immunol. 1989 Apr;19(4):689–694. doi: 10.1002/eji.1830190418. [DOI] [PubMed] [Google Scholar]
- Garman R. H., Weiss B., Evans H. L. Alkylmercurial encephalopathy in the monkey (Saimiri sciureus and Macaca arctoides): a histopathologic and autoradiographic study. Acta Neuropathol. 1975;32(1):61–74. doi: 10.1007/BF00686067. [DOI] [PubMed] [Google Scholar]
- Goin J. C., Leiros C. P., Borda E., Sterin-Borda L. Interaction of human chagasic IgG with the second extracellular loop of the human heart muscarinic acetylcholine receptor: functional and pathological implications. FASEB J. 1997 Jan;11(1):77–83. doi: 10.1096/fasebj.11.1.9034169. [DOI] [PubMed] [Google Scholar]
- Goldstein G. W., Ar D. Lead activates calmodulin sensitive processes. Life Sci. 1983 Sep 5;33(10):1001–1006. doi: 10.1016/0024-3205(83)90757-9. [DOI] [PubMed] [Google Scholar]
- Górny M., Losy J., Wender M. Przeciwciała przeciw kwaśnemu białku włkienkowemu gleju (GFAP) w płynie mózgowo-rdzeniowym chorych na stwardnienie rozsiane i inne choroby neurologiczne. Neurol Neurochir Pol. 1990 Jan-Apr;24(1-2):17–22. [PubMed] [Google Scholar]
- Hajela R. K., Atchison W. D. The proteins synaptotagmin and syntaxin are not general targets of Lambert-Eaton myasthenic syndrome autoantibody. J Neurochem. 1995 Mar;64(3):1245–1251. doi: 10.1046/j.1471-4159.1995.64031245.x. [DOI] [PubMed] [Google Scholar]
- Hansen S. H., Stagaard M., Møllgård K. Neurofilament-like pattern of reactivity in human foetal PNS and spinal cord following immunostaining with polyclonal anti-glial fibrillary acidic protein antibodies. J Neurocytol. 1989 Aug;18(4):427–436. doi: 10.1007/BF01474540. [DOI] [PubMed] [Google Scholar]
- Harrington M. G., Merril C. R. Cerebrospinal fluid protein analysis in diseases of the nervous system. J Chromatogr. 1988 Jul 29;429:345–358. doi: 10.1016/s0378-4347(00)83877-3. [DOI] [PubMed] [Google Scholar]
- Hart M. N., Fabry Z. CNS antigen presentation. Trends Neurosci. 1995 Nov;18(11):475–481. doi: 10.1016/0166-2236(95)92767-k. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Heininger K., Schäfer B., Fierz W., Toyka K. V. Immune mechanisms in inflammatory polyneuropathy. Ann N Y Acad Sci. 1988;540:122–161. doi: 10.1111/j.1749-6632.1988.tb27058.x. [DOI] [PubMed] [Google Scholar]
- Hashim G. A. Myelin basic protein: structure, function and antigenic determinants. Immunol Rev. 1978;39:60–107. doi: 10.1111/j.1600-065x.1978.tb00397.x. [DOI] [PubMed] [Google Scholar]
- Hewett S. J., Atchison W. D. Disruption of synaptosomal calcium channel function by Lambert-Eaton myasthenic immunoglobulin is serum-dependent. Brain Res. 1992 Dec 25;599(2):317–323. doi: 10.1016/0006-8993(92)90407-z. [DOI] [PubMed] [Google Scholar]
- Hewett S. J., Atchison W. D. Serum and plasma from patients with Lambert-Eaton Myasthenic Syndrome reduce depolarization-dependent uptake of 45Ca2+ into rat cortical synaptosomes. Brain Res. 1991 Dec 6;566(1-2):320–324. doi: 10.1016/0006-8993(91)91717-f. [DOI] [PubMed] [Google Scholar]
- Hewett S. J., Atchison W. D. Specificity of Lambert-Eaton myasthenic syndrome immunoglobulin for nerve terminal calcium channels. Brain Res. 1992 Dec 25;599(2):324–332. doi: 10.1016/0006-8993(92)90408-2. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Hsu B. L., Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991 Feb;28(2):254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Hughes C. C., Male D. K., Lantos P. L. Adhesion of lymphocytes to cerebral microvascular cells: effects of interferon-gamma, tumour necrosis factor and interleukin-1. Immunology. 1988 Aug;64(4):677–681. [PMC free article] [PubMed] [Google Scholar]
- Ingalls T. H. Endemic clustering of multiple sclerosis in time and place, 1934-1984. Confirmation of a hypothesis. Am J Forensic Med Pathol. 1986 Mar;7(1):3–8. doi: 10.1097/00000433-198603000-00002. [DOI] [PubMed] [Google Scholar]
- Ingalls T. H. Epidemiology, etiology, and prevention of multiple sclerosis. Hypothesis and fact. Am J Forensic Med Pathol. 1983 Mar;4(1):55–61. doi: 10.1097/00000433-198303000-00006. [DOI] [PubMed] [Google Scholar]
- Irani D. N., Griffin D. E. Isolation of brain parenchymal lymphocytes for flow cytometric analysis. Application to acute viral encephalitis. J Immunol Methods. 1991 Jun 3;139(2):223–231. doi: 10.1016/0022-1759(91)90192-i. [DOI] [PubMed] [Google Scholar]
- Irvine D. G., Schiefer H. B., Hader W. J. Geotoxicology of multiple sclerosis: the Henribourg, Saskatchewan, cluster focus. II. The soil. Sci Total Environ. 1988 Dec;77(2-3):175–188. doi: 10.1016/0048-9697(88)90054-x. [DOI] [PubMed] [Google Scholar]
- Kajiwara K., Ito H., Fukumoto T. Lymphocyte infiltration into normal rat brain following hyperosmotic blood-brain barrier opening. J Neuroimmunol. 1990 May;27(2-3):133–140. doi: 10.1016/0165-5728(90)90062-r. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Neher E. IgG from patients with Lambert-Eaton syndrome blocks voltage-dependent calcium channels. Science. 1988 Jan 22;239(4838):405–408. doi: 10.1126/science.2447652. [DOI] [PubMed] [Google Scholar]
- Koller L. D., Exon J. H., Roan J. G. Humoral antibody response in mice after single dose exposure to lead or cadmium. Proc Soc Exp Biol Med. 1976 Feb;151(2):339–342. doi: 10.3181/00379727-151-39205. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A. Heavy metal modulation of lymphocyte activities--II. Lead, an in vitro mediator of B-cell activation. Int J Immunopharmacol. 1981;3(2):153–161. doi: 10.1016/0192-0561(81)90006-0. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A. Heavy metal modulation of lymphocyte activities. 1. In vitro effects of heavy metals on primary humoral immune responses. Toxicol Appl Pharmacol. 1981 Mar 15;57(3):439–451. doi: 10.1016/0041-008x(81)90241-6. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A. In vivo and in vitro effects of lead on humoral and cell-mediated immunity. Infect Immun. 1981 Jan;31(1):136–143. doi: 10.1128/iai.31.1.136-143.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Liu W., Dickson D. W., Brosnan C. F., Berman J. W. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993 Apr 1;150(7):2659–2667. [PubMed] [Google Scholar]
- Lewis S. A., Balcarek J. M., Krek V., Shelanski M., Cowan N. J. Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: structural conservation of intermediate filaments. Proc Natl Acad Sci U S A. 1984 May;81(9):2743–2746. doi: 10.1073/pnas.81.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Cheng L., Tu Y., Zhang J., Dong J., Li S., Tian Y., Peng Y. Mechanism of anti-beta-adrenoceptor antibody mediated myocardial damage in dilated cardiomyopathy. J Tongji Med Univ. 1997;17(1):5–8. doi: 10.1007/BF02887992. [DOI] [PubMed] [Google Scholar]
- Lieberman A. P., Pitha P. M., Shin H. S., Shin M. L. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limas C. J., Limas C. Immune-mediated modulation of sarcoplasmic reticulum function in human dilated cardiomyopathy. Basic Res Cardiol. 1992;87 (Suppl 1):269–276. doi: 10.1007/978-3-642-72474-9_22. [DOI] [PubMed] [Google Scholar]
- Little A. R., Jr, Gong Z., Singh U., El-Fawal H., Evans H. L. Decreases in brain glial fibrillary acidic protein (GFAP) are associated with increased serum corticosterone following inhalation exposure to toluene. Neurotoxicology. 1998 Aug-Oct;19(4-5):739–747. [PubMed] [Google Scholar]
- Livneh A., Sarova I., Michaeli D., Pras M., Wagner K., Zakut H., Soreq H. Antibodies against acetylcholinesterase and low levels of cholinesterases in a patient with an atypical neuromuscular disorder. Clin Immunol Immunopathol. 1988 Aug;48(2):119–131. doi: 10.1016/0090-1229(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Lowenthal A., Noppe M., Gheuens J., Karcher D. alpha-Albumin (glial fibrillary acidic protein) in normal and pathological human brain and cerebrospinal fluid. J Neurol. 1978 Oct 25;219(2):87–91. doi: 10.1007/BF00314391. [DOI] [PubMed] [Google Scholar]
- Lévi-Strauss M., Mallat M. Primary cultures of murine astrocytes produce C3 and factor B, two components of the alternative pathway of complement activation. J Immunol. 1987 Oct 1;139(7):2361–2366. [PubMed] [Google Scholar]
- Male D., Pyrce G., Hughes C., Lantos P. Lymphocyte migration into brain modelled in vitro: control by lymphocyte activation, cytokines, and antigen. Cell Immunol. 1990 Apr 15;127(1):1–11. doi: 10.1016/0008-8749(90)90109-5. [DOI] [PubMed] [Google Scholar]
- Mano Y., Takayanagi T., Abe T., Takizawa Y. [Amyotrophic lateral sclerosis and mercury--preliminary report]. Rinsho Shinkeigaku. 1990 Nov;30(11):1275–1277. [PubMed] [Google Scholar]
- Markovac J., Goldstein G. W. Picomolar concentrations of lead stimulate brain protein kinase C. Nature. 1988 Jul 7;334(6177):71–73. doi: 10.1038/334071a0. [DOI] [PubMed] [Google Scholar]
- Matsiota P., Blancher A., Doyon B., Guilbert B., Clanet M., Kouvelas E. D., Avrameas S. Comparative study of natural autoantibodies in the serum and cerebrospinal fluid of normal individuals and patients with multiple sclerosis and other neurological diseases. Ann Inst Pasteur Immunol. 1988 Jan-Feb;139(1):99–108. doi: 10.1016/0769-2625(88)90134-1. [DOI] [PubMed] [Google Scholar]
- McCabe M. J., Jr, Lawrence D. A. Lead, a major environmental pollutant, is immunomodulatory by its differential effects on CD4+ T cells subsets. Toxicol Appl Pharmacol. 1991 Oct;111(1):13–23. doi: 10.1016/0041-008X(91)90129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R., Delgado-Téllez E., Cuadra R., Tórres E., Keifer M., Almendárez J., Miranda J., El-Fawal H. A., Wolff M., Simpson D. Organophosphate neuropathy due to methamidophos: biochemical and neurophysiological markers. Arch Toxicol. 1999 Aug;73(6):296–300. doi: 10.1007/s002040050621. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., Kawamata T., Walker D. G., Akiyama H., Tooyama I., McGeer E. G. Microglia in degenerative neurological disease. Glia. 1993 Jan;7(1):84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- Merrill J. E., Koyanagi Y., Zack J., Thomas L., Martin F., Chen I. S. Induction of interleukin-1 and tumor necrosis factor alpha in brain cultures by human immunodeficiency virus type 1. J Virol. 1992 Apr;66(4):2217–2225. doi: 10.1128/jvi.66.4.2217-2225.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrová E., Mayer V. Antibodies to axonal neurofilaments in Creutzfeldt-Jakob disease and other organic dementias. Acta Virol. 1989 Aug;33(4):371–374. [PubMed] [Google Scholar]
- Moszczyński P., Lisiewicz J., Bartuś R., Bem S. The serum immunoglobulins in workers after prolonged occupational exposure to the mercury vapors. Med Interne. 1990 Jan-Mar;28(1):25–30. [PubMed] [Google Scholar]
- Mucke L., Eddleston M. Astrocytes in infectious and immune-mediated diseases of the central nervous system. FASEB J. 1993 Oct;7(13):1226–1232. doi: 10.1096/fasebj.7.13.8405808. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Takeda M., Tanaka T., Tanaka J., Kato Y., Nishinuma K., Nishimura T. Glial fibrillary acidic protein stimulates proliferation and immunoglobulin synthesis of lymphocytes from Alzheimer's disease patients. Methods Find Exp Clin Pharmacol. 1992 Mar;14(2):141–149. [PubMed] [Google Scholar]
- Narahashi T. Nerve membrane ion channels as the target site of environmental toxicants. Environ Health Perspect. 1987 Apr;71:25–29. doi: 10.1289/ehp.877125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppe M., Crols R., Andries D., Lowenthal A. Determination in human cerebrospinal fluid of glial fibrillary acidic protein, S-100 and myelin basic protein as indices of non-specific or specific central nervous tissue pathology. Clin Chim Acta. 1986 Mar 16;155(2):143–150. doi: 10.1016/0009-8981(86)90275-5. [DOI] [PubMed] [Google Scholar]
- O'Callaghan J. P. Neurotypic and gliotypic proteins as biochemical markers of neurotoxicity. Neurotoxicol Teratol. 1988 Sep-Oct;10(5):445–452. doi: 10.1016/0892-0362(88)90006-2. [DOI] [PubMed] [Google Scholar]
- Ogawa N., Dang H., Talal N. Apoptosis and autoimmunity. J Autoimmun. 1995 Feb;8(1):1–19. doi: 10.1006/jaut.1995.0001. [DOI] [PubMed] [Google Scholar]
- Peers C., Johnston I., Lang B., Wray D. Cross-linking of presynaptic calcium channels: a mechanism of action for Lambert-Eaton myasthenic syndrome antibodies at the mouse neuromuscular junction. Neurosci Lett. 1993 Apr 16;153(1):45–48. doi: 10.1016/0304-3940(93)90073-t. [DOI] [PubMed] [Google Scholar]
- Perin M. S., Fried V. A., Mignery G. A., Jahn R., Südhof T. C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990 May 17;345(6272):260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Plata-Salamán C. R. Immunoregulators in the nervous system. Neurosci Biobehav Rev. 1991 Summer;15(2):185–215. doi: 10.1016/s0149-7634(05)80001-6. [DOI] [PubMed] [Google Scholar]
- Prochiantz A., Mallat M. Astrocyte diversity. Ann N Y Acad Sci. 1988;540:52–63. doi: 10.1111/j.1749-6632.1988.tb27051.x. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Cannella B., Duijvestijn A. M., Cross A. H. Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab Invest. 1990 Oct;63(4):476–489. [PubMed] [Google Scholar]
- Raivich G., Gehrmann J., Kreutzberg G. W. Increase of macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor receptors in the regenerating rat facial nucleus. J Neurosci Res. 1991 Dec;30(4):682–686. doi: 10.1002/jnr.490300412. [DOI] [PubMed] [Google Scholar]
- Reuveny E., Narahashi T. Potent blocking action of lead on voltage-activated calcium channels in human neuroblastoma cells SH-SY5Y. Brain Res. 1991 Apr 5;545(1-2):312–314. doi: 10.1016/0006-8993(91)91304-j. [DOI] [PubMed] [Google Scholar]
- Ristic H., Srinivasan S., Hall K. E., Sima A. A., Wiley J. W. Serum from diabetic BB/W rats enhances calcium currents in primary sensory neurons. J Neurophysiol. 1998 Sep;80(3):1236–1244. doi: 10.1152/jn.1998.80.3.1236. [DOI] [PubMed] [Google Scholar]
- Rowles T. K., Womac C., Bratton G. R., Tiffany-Castiglioni E. Interaction of lead and zinc in cultured astroglia. Metab Brain Dis. 1989 Sep;4(3):187–201. doi: 10.1007/BF01000295. [DOI] [PubMed] [Google Scholar]
- Rönnbäck L., Hansson E. Chronic encephalopathies induced by mercury or lead: aspects of underlying cellular and molecular mechanisms. Br J Ind Med. 1992 Apr;49(4):233–240. doi: 10.1136/oem.49.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M., Suzumura A., Marunouchi T. TNF alpha induces IL-6 production by astrocytes but not by microglia. Brain Res. 1992 Jun 26;583(1-2):296–299. doi: 10.1016/s0006-8993(10)80037-x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R., Ingvar M. Loss of immunoreactivity for glial fibrillary acidic protein (GFAP) in astrocytes as a marker for profound tissue damage in substantia nigra and basal cortical areas after status epilepticus induced by pilocarpine in rat. Glia. 1994 Nov;12(3):165–172. doi: 10.1002/glia.440120302. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Shafit-Zagardo B., Aquino D. A., Farooq M., Raine C. S., Norton W. T., Brosnan C. F. Tumor necrosis factor-induced proliferation of astrocytes from mature brain is associated with down-regulation of glial fibrillary acidic protein mRNA. J Neurochem. 1991 Sep;57(3):823–830. doi: 10.1111/j.1471-4159.1991.tb08225.x. [DOI] [PubMed] [Google Scholar]
- Selvín-Testa A., Lopez-Costa J. J., Nessi de Aviñon A. C., Pecci Saavedra J. Astroglial alterations in rat hippocampus during chronic lead exposure. Glia. 1991;4(4):384–392. doi: 10.1002/glia.440040406. [DOI] [PubMed] [Google Scholar]
- Shafer T. J., Atchison W. D. Methylmercury blocks N- and L-type Ca++ channels in nerve growth factor-differentiated pheochromocytoma (PC12) cells. J Pharmacol Exp Ther. 1991 Jul 1;258(1):149–157. [PubMed] [Google Scholar]
- Sheffield W. D., Kim S. U. Myelin basic protein causes proliferation of lymphocytes and astrocytes in vitro. Brain Res. 1977 Sep 2;132(3):580–584. doi: 10.1016/0006-8993(77)90208-6. [DOI] [PubMed] [Google Scholar]
- Sienko D. G., Davis J. P., Taylor J. A., Brooks B. R. Amyotrophic lateral sclerosis. A case-control study following detection of a cluster in a small Wisconsin community. Arch Neurol. 1990 Jan;47(1):38–41. doi: 10.1001/archneur.1990.00530010046017. [DOI] [PubMed] [Google Scholar]
- Sindhuphak R., Karlsson E., Conradi S., Ronnevi L. O. Immunoglobulins from patients with amyotrophic lateral sclerosis affect human erythrocyte acetylcholinesterase. J Neurol Sci. 1988 Sep;86(2-3):195–202. doi: 10.1016/0022-510x(88)90098-6. [DOI] [PubMed] [Google Scholar]
- Smith D. O., Conklin M. W., Jensen P. J., Atchison W. D. Decreased calcium currents in motor nerve terminals of mice with Lambert-Eaton myasthenic syndrome. J Physiol. 1995 Aug 15;487(1):115–123. doi: 10.1113/jphysiol.1995.sp020865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. G., Hamilton S., Hofmann F., Schneider T., Nastainczyk W., Birnbaumer L., Stefani E., Appel S. H. Serum antibodies to L-type calcium channels in patients with amyotrophic lateral sclerosis. N Engl J Med. 1992 Dec 10;327(24):1721–1728. doi: 10.1056/NEJM199212103272405. [DOI] [PubMed] [Google Scholar]
- Stefansson K., Marton L. S., Dieperink M. E., Molnar G. K., Schlaepfer W. W., Helgason C. M. Circulating autoantibodies to the 200,000-dalton protein of neurofilaments in the serum of healthy individuals. Science. 1985 May 31;228(4703):1117–1119. doi: 10.1126/science.4039466. [DOI] [PubMed] [Google Scholar]
- Suzumura A., Mezitis S. G., Gonatas N. K., Silberberg D. H. MHC antigen expression on bulk isolated macrophage-microglia from newborn mouse brain: induction of Ia antigen expression by gamma-interferon. J Neuroimmunol. 1987 Jul-Aug;15(3):263–278. doi: 10.1016/0165-5728(87)90121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabira T. Autoimmune demyelination in the central nervous system. Ann N Y Acad Sci. 1988;540:187–201. doi: 10.1111/j.1749-6632.1988.tb27061.x. [DOI] [PubMed] [Google Scholar]
- Tanaka J., Nakamura K., Takeda M., Tada K., Suzuki H., Morita H., Okado T., Hariguchi S., Nishimura T. Enzyme-linked immunosorbent assay for human autoantibody to glial fibrillary acidic protein: higher titer of the antibody is detected in serum of patients with Alzheimer's disease. Acta Neurol Scand. 1989 Dec;80(6):554–560. doi: 10.1111/j.1600-0404.1989.tb03926.x. [DOI] [PubMed] [Google Scholar]
- Tiffany-Castiglioni E., Sierra E. M., Wu J. N., Rowles T. K. Lead toxicity in neuroglia. Neurotoxicology. 1989 Fall;10(3):417–443. [PubMed] [Google Scholar]
- Toh B. H., Gibbs C. J., Jr, Gajdusek D. C., Goudsmit J., Dahl D. The 200- and 150-kDa neurofilament proteins react with IgG autoantibodies from patients with kuru, Creutzfeldt-Jakob disease, and other neurologic diseases. Proc Natl Acad Sci U S A. 1985 May;82(10):3485–3489. doi: 10.1073/pnas.82.10.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchitel O. D., Appel S. H., Crawford F., Sczcupak L. Immunoglobulins from amyotrophic lateral sclerosis patients enhance spontaneous transmitter release from motor-nerve terminals. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7371–7374. doi: 10.1073/pnas.85.19.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedeler C. A., Matre R., Nyland H. Class and IgG subclass distribution of antibodies against peripheral nerve myelin in sera from patients with inflammatory demyelinating polyradiculoneuropathy. Acta Neurol Scand. 1988 Nov;78(5):401–407. doi: 10.1111/j.1600-0404.1988.tb03676.x. [DOI] [PubMed] [Google Scholar]
- Vlajković S., Janković B. D. Experimental epilepsy in vitro: neuromodulating activity of anti-brain autoantibodies from rats exposed to electroconvulsive shock. Int J Neurosci. 1991 Jul;59(1-3):205–211. doi: 10.3109/00207459108985464. [DOI] [PubMed] [Google Scholar]
- Walls A. F., Suckling A. J., Rumsby M. G. Autoantibody responses in the cerebrospinal fluid of guinea pigs with chronic relapsing experimental allergic encephalomyelitis. Acta Neurol Scand. 1988 Nov;78(5):422–428. doi: 10.1111/j.1600-0404.1988.tb03680.x. [DOI] [PubMed] [Google Scholar]
- Warner G. L., Lawrence D. A. The effect of metals on IL-2-related lymphocyte proliferation. Int J Immunopharmacol. 1988;10(5):629–637. doi: 10.1016/0192-0561(88)90082-3. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Catz I. A myelin basic protein antibody cascade in purified IgG from cerebrospinal fluid of multiple sclerosis patients. J Neurol Sci. 1990 Apr;96(1):19–27. doi: 10.1016/0022-510x(90)90053-p. [DOI] [PubMed] [Google Scholar]
- Waterman S. J., el-Fawal H. A., Snyder C. A. Lead alters the immunogenicity of two neural proteins: a potential mechanism for the progression of lead-induced neurotoxicity. Environ Health Perspect. 1994 Dec;102(12):1052–1056. doi: 10.1289/ehp.941021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells M. R., Racis S. P., Jr, Vaidya U. Changes in plasma cytokines associated with peripheral nerve injury. J Neuroimmunol. 1992 Aug;39(3):261–268. doi: 10.1016/0165-5728(92)90260-r. [DOI] [PubMed] [Google Scholar]
- Woodroofe M. N., Sarna G. S., Wadhwa M., Hayes G. M., Loughlin A. J., Tinker A., Cuzner M. L. Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J Neuroimmunol. 1991 Sep;33(3):227–236. doi: 10.1016/0165-5728(91)90110-s. [DOI] [PubMed] [Google Scholar]
- Xu Y. F., Hewett S. J., Atchison W. D. Passive transfer of Lambert-Eaton myasthenic syndrome induces dihydropyridine sensitivity of ICa in mouse motor nerve terminals. J Neurophysiol. 1998 Sep;80(3):1056–1069. doi: 10.1152/jn.1998.80.3.1056. [DOI] [PubMed] [Google Scholar]
- Yong V. W., Moumdjian R., Yong F. P., Ruijs T. C., Freedman M. S., Cashman N., Antel J. P. Gamma-interferon promotes proliferation of adult human astrocytes in vitro and reactive gliosis in the adult mouse brain in vivo. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7016–7020. doi: 10.1073/pnas.88.16.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Atchison W. D. Disruption by methylmercury of membrane excitability and synaptic transmission of CA1 neurons in hippocampal slices of the rat. Toxicol Appl Pharmacol. 1993 Jun;120(2):203–215. doi: 10.1006/taap.1993.1104. [DOI] [PubMed] [Google Scholar]
- de la Porte S., Ragueh F., Eymard B., Courbin P., Chapron J., Koenig J. Effect of sera from myasthenia gravis patients and of alpha-bungarotoxin on acetylcholinesterase during in vitro neuromuscular synaptogenesis. J Neurol Sci. 1993 Jul;117(1-2):92–102. doi: 10.1016/0022-510x(93)90160-z. [DOI] [PubMed] [Google Scholar]
- el-Fawal H. A., Gong Z., Little A. R., Evans H. L. Exposure to methylmercury results in serum autoantibodies to neurotypic and gliotypic proteins. Neurotoxicology. 1996 Summer;17(2):531–539. [PubMed] [Google Scholar]