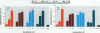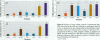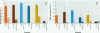Abstract
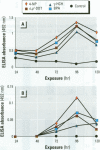
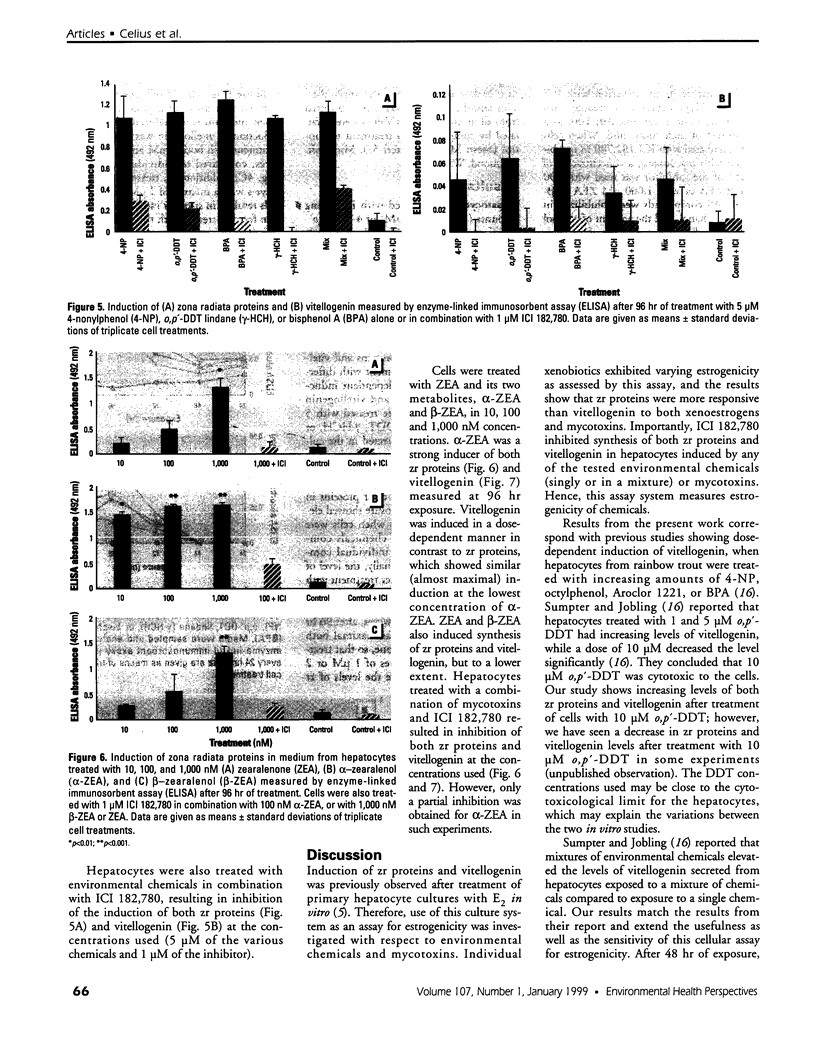
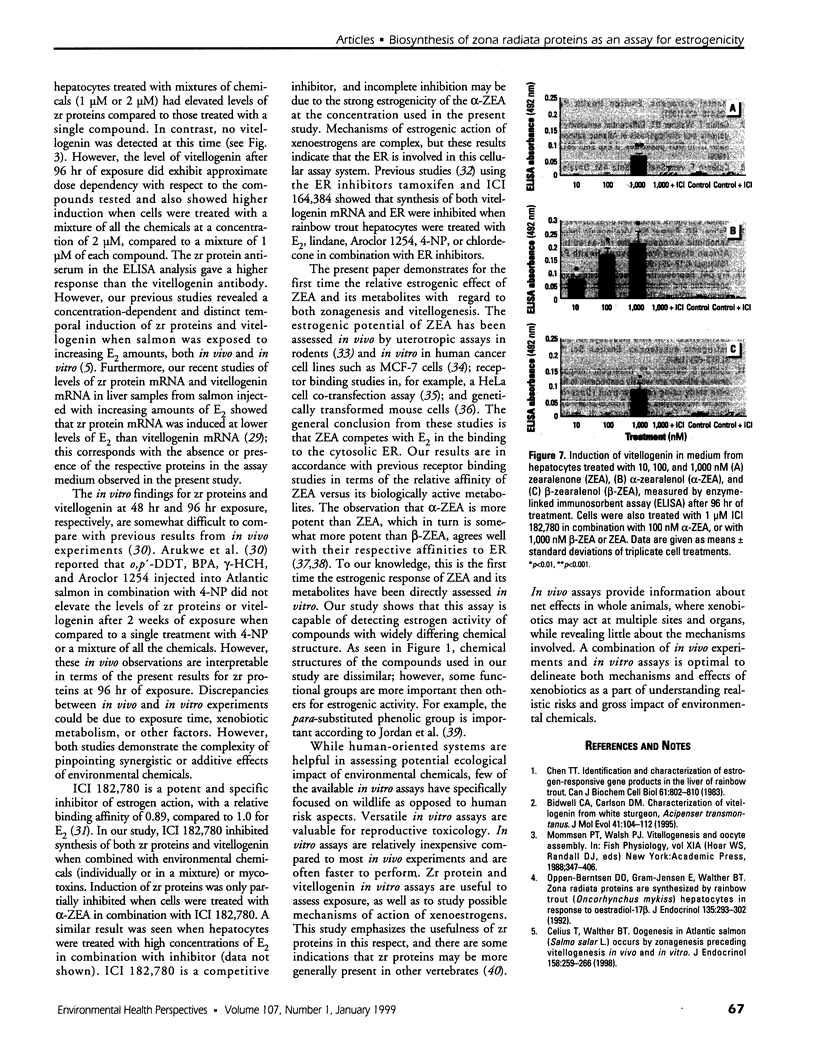
Mounting evidence confirms that hepatic biosynthetic processes are essential for female sexual maturation in fish, which is directly controlled by estrogens. These oogenetic events (zonagenesis and vitellogenesis) are induced in both sexes by estrogens. In this paper, we report the induction of zona radiata (zr) proteins and vitellogenin in primary hepatocytes from Atlantic salmon (Salmo salar L.) exposed to xenoestrogens and mycotoxins. Cells were treated with doses of 1, 5, and 10 microM 4-nonylphenol (4-NP), o, p'-DDT, lindane ([gamma]-HCH), and bisphenol A (BPA), which all induced zr proteins and vitellogenin in an approximate dose-dependent manner. Hepatocytes were also treated with combinations of xenoestrogens at 1 or 2 microM, resulting in elevated levels of both zr proteins and vitellogenin, compared to single treatment. The estrogenic activity of the mycotoxin zearalenone (ZEA) and its metabolites [alpha]-ZEA) and ss-zearalenol (ss-ZEA)], with regard to zonagenesis and vitellogenesis, was assessed in this assay system. Mycotoxins were used at concentrations of 10, 100, or 1,000 nM. All induced zr proteins and vitellogenin, with [alpha]-ZEA being the strongest inducer. When cells were treated with xenoestrogens or mycotoxins in combination with an estrogen receptor inhibitor (ICI 182,780), the induction of both zr proteins and vitellogenin was inhibited in all cases. Thus, the reported estrogen effects are bonafide estrogen responses. Zona radiata proteins were more responsive than vitellogenin to both xenoestrogens and mycotoxins. The versatility and sensitivity of the hepatocyte assay demonstrates that biosynthesis of zr proteins provides a new supplementary method for estimating xenoestrogenicity and mycotoxin action.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson T., Förlin L., Hansson T. Biotransformation of 7-ethoxycoumarin in isolated perfused rainbow trout liver. Drug Metab Dispos. 1983 Sep-Oct;11(5):494–498. [PubMed] [Google Scholar]
- Arukwe A., Knudsen F. R., Goksøyr A. Fish zona radiata (eggshell) protein: a sensitive biomarker for environmental estrogens. Environ Health Perspect. 1997 Apr;105(4):418–422. doi: 10.1289/ehp.97105418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell C. A., Carlson D. M. Characterization of vitellogenin from white sturgeon, Acipenser transmontanus. J Mol Evol. 1995 Jul;41(1):104–112. doi: 10.1007/BF00174046. [DOI] [PubMed] [Google Scholar]
- Celius T., Walther B. T. Differential sensitivity of zonagenesis and vitellogenesis in Atlantic salmon (Salmo salar L) to DDT pesticides. J Exp Zool. 1998 Jul 1;281(4):346–353. doi: 10.1002/(sici)1097-010x(19980701)281:4<346::aid-jez9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Celius T., Walther B. T. Oogenesis in Atlantic salmon (Salmo salar L.) occurs by zonagenesis preceding vitellogenesis in vivo and in vitro. J Endocrinol. 1998 Aug;158(2):259–266. doi: 10.1677/joe.0.1580259. [DOI] [PubMed] [Google Scholar]
- Chen T. T. Identification and characterization of estrogen-responsive gene products in the liver of rainbow trout. Can J Biochem Cell Biol. 1983 Jul;61(7):802–810. doi: 10.1139/o83-102. [DOI] [PubMed] [Google Scholar]
- Coe J. E., Ishak K. G., Ward J. M., Ross M. J. Tamoxifen prevents induction of hepatic neoplasia by zeranol, an estrogenic food contaminant. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1085–1089. doi: 10.1073/pnas.89.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T., vom Saal F. S., Soto A. M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993 Oct;101(5):378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facemire C. F., Gross T. S., Guillette L. J., Jr Reproductive impairment in the Florida panther: nature or nurture? Environ Health Perspect. 1995 May;103 (Suppl 4):79–86. doi: 10.1289/ehp.103-1519283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D. W., Picken C. A., Murphy L. C., Buhr M. M. Measurement of the relative binding affinity of zearalenone, alpha-zearalenol and beta-zearalenol for uterine and oviduct estrogen receptors in swine, rats and chickens: an indicator of estrogenic potencies. Comp Biochem Physiol C. 1989;94(2):691–694. doi: 10.1016/0742-8413(89)90133-3. [DOI] [PubMed] [Google Scholar]
- Flouriot G., Pakdel F., Ducouret B., Valotaire Y. Influence of xenobiotics on rainbow trout liver estrogen receptor and vitellogenin gene expression. J Mol Endocrinol. 1995 Oct;15(2):143–151. doi: 10.1677/jme.0.0150143. [DOI] [PubMed] [Google Scholar]
- Fry D. M. Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ Health Perspect. 1995 Oct;103 (Suppl 7):165–171. doi: 10.1289/ehp.95103s7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan V. C., Mittal S., Gosden B., Koch R., Lieberman M. E. Structure-activity relationships of estrogens. Environ Health Perspect. 1985 Sep;61:97–110. doi: 10.1289/ehp.856197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Katzenellenbogen J. A., Mordecai D. Zearalenones: characterization of the estrogenic potencies and receptor interactions of a series of fungal beta-resorcylic acid lactones. Endocrinology. 1979 Jul;105(1):33–40. doi: 10.1210/endo-105-1-33. [DOI] [PubMed] [Google Scholar]
- Kuiper-Goodman T., Scott P. M., Watanabe H. Risk assessment of the mycotoxin zearalenone. Regul Toxicol Pharmacol. 1987 Sep;7(3):253–306. doi: 10.1016/0273-2300(87)90037-7. [DOI] [PubMed] [Google Scholar]
- Mayr U. E. Estrogen-controlled gene expression in tissue culture cells by zearalenone. FEBS Lett. 1988 Nov 7;239(2):223–226. doi: 10.1016/0014-5793(88)80921-9. [DOI] [PubMed] [Google Scholar]
- Miksicek R. J. Interaction of naturally occurring nonsteroidal estrogens with expressed recombinant human estrogen receptor. J Steroid Biochem Mol Biol. 1994 Jun;49(2-3):153–160. doi: 10.1016/0960-0760(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Mirocha C. J., Pathre S. V., Robison T. S. Comparative metabolism of zearalenone and transmission into bovine milk. Food Cosmet Toxicol. 1981 Feb;19(1):25–30. doi: 10.1016/0015-6264(81)90299-6. [DOI] [PubMed] [Google Scholar]
- Oppen-Berntsen D. O., Gram-Jensen E., Walther B. T. Zona radiata proteins are synthesized by rainbow trout (Oncorhynchus mykiss) hepatocytes in response to oestradiol-17 beta. J Endocrinol. 1992 Nov;135(2):293–302. doi: 10.1677/joe.0.1350293. [DOI] [PubMed] [Google Scholar]
- Palmer B. D., Palmer S. K. Vitellogenin induction by xenobiotic estrogens in the red-eared turtle and African clawed frog. Environ Health Perspect. 1995 May;103 (Suppl 4):19–25. doi: 10.1289/ehp.95103s419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelissero C., Flouriot G., Foucher J. L., Bennetau B., Dunoguès J., Le Gac F., Sumpter J. P. Vitellogenin synthesis in cultured hepatocytes; an in vitro test for the estrogenic potency of chemicals. J Steroid Biochem Mol Biol. 1993 Mar;44(3):263–272. doi: 10.1016/0960-0760(93)90086-c. [DOI] [PubMed] [Google Scholar]
- Powell-Jones W., Raeford S., Lucier G. W. Binding properties of zearalenone mycotoxins to hepatic estrogen receptors. Mol Pharmacol. 1981 Jul;20(1):35–42. [PubMed] [Google Scholar]
- Schoental R. Trichothecenes, zearalenone, and other carcinogenic metabolites of Fusarium and related microfungi. Adv Cancer Res. 1985;45:217–290. doi: 10.1016/s0065-230x(08)60270-5. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Sonnenschein C., Chung K. L., Fernandez M. F., Olea N., Serrano F. O. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995 Oct;103 (Suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter J. P. Feminized responses in fish to environmental estrogens. Toxicol Lett. 1995 Dec;82-83:737–742. doi: 10.1016/0378-4274(95)03517-6. [DOI] [PubMed] [Google Scholar]
- Sumpter J. P., Jobling S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect. 1995 Oct;103 (Suppl 7):173–178. doi: 10.1289/ehp.95103s7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sándor G., Ványi A. Mycotoxin research in the Hungarian Central Veterinary Institute. Acta Vet Hung. 1990;38(1-2):61–68. [PubMed] [Google Scholar]
- Thigpen J. E., Li L. A., Richter C. B., Lebetkin E. H., Jameson C. W. The mouse bioassay for the detection of estrogenic activity in rodent diets: I. A standardized method for conducting the mouse bioassay. Lab Anim Sci. 1987 Oct;37(5):596–601. [PubMed] [Google Scholar]
- Wakeling A. E., Dukes M., Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991 Aug 1;51(15):3867–3873. [PubMed] [Google Scholar]