Abstract
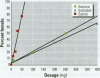
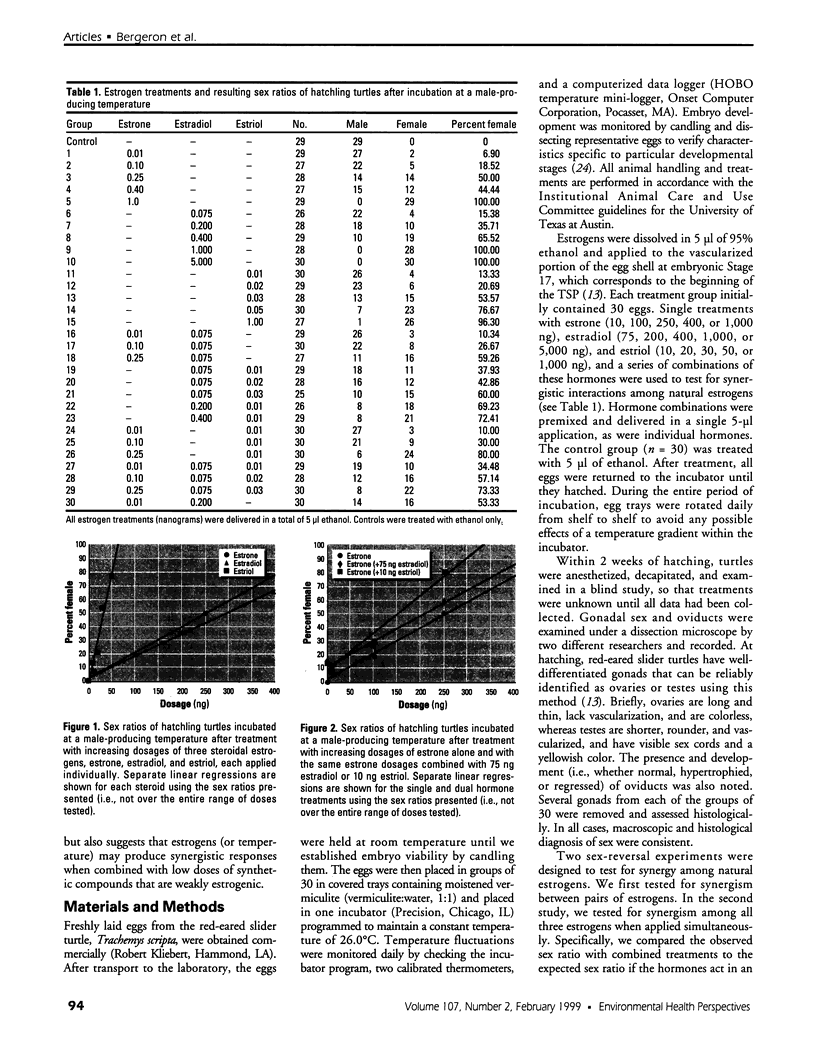
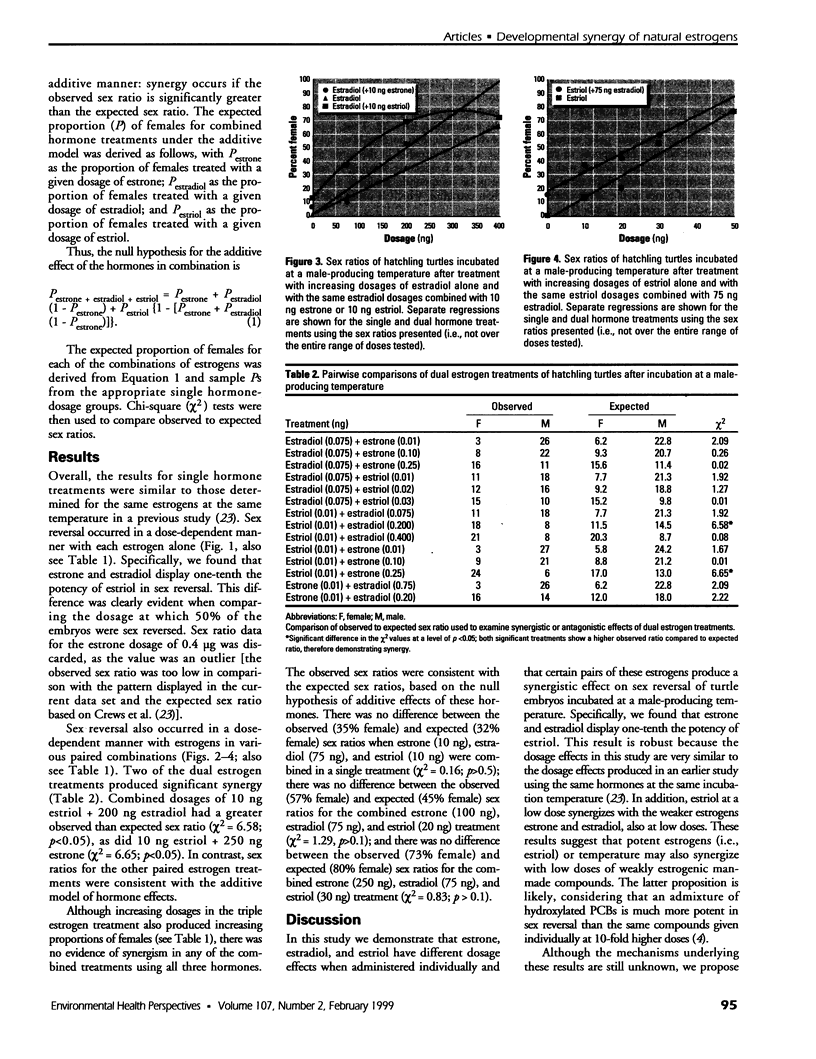
Gonadal sex in the red-eared slider turtle, Trachemys scripta, is determined by incubation temperature during embryonic development. Evidence suggests that temperature determines sex by influencing steroid hormone metabolism and/or sensitivity: steroidogenic enzyme inhibitors or exogenous sex steroid hormones and their man-made analogs override (or enhance) temperature effects on sex determination. Specifically, nonaromatizable androgens and aromatase inhibitors induce testis differentiation at female-producing temperatures, whereas aromatizable androgens and estrogens induce ovary differentiation at male-producing temperatures. Moreover, natural estrogens and temperature synergize to produce more females than would be expected if estrogens and temperature had purely additive effects on sex determination. In this study, we use sex reversal of turtle embryos incubated at a male-producing temperature to examine synergism among steroidal estrogens: estrone, 17ss-estradiol, and estriol. A low dose of 17ss-estradiol (200 ng) showed significant synergism when administered with a single low dose of estriol (10 ng). Likewise, a single low dose of estrone (250 ng) had a synergistic effect when combined with the same low dose of estriol (10 ng). We conclude that the weak natural estrogens estrone and 17ss-estradiol synergize with a low dose of the more potent estriol to reverse gonadal sex during the critical period of sexual differentiation. These results suggest that weak environmental estrogens may also synergize with stronger natural estrogens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold S. F., Klotz D. M., Collins B. M., Vonier P. M., Guillette L. J., Jr, McLachlan J. A. Synergistic activation of estrogen receptor with combinations of environmental chemicals. Science. 1996 Jun 7;272(5267):1489–1492. doi: 10.1126/science.272.5267.1489. [DOI] [PubMed] [Google Scholar]
- Ashby J., Lefevre P. A., Odum J., Harris C. A., Routledge E. J., Sumpter J. P. Synergy between synthetic oestrogens? Nature. 1997 Feb 6;385(6616):494–494. doi: 10.1038/385494a0. [DOI] [PubMed] [Google Scholar]
- Bergeron J. M., Crews D., McLachlan J. A. PCBs as environmental estrogens: turtle sex determination as a biomarker of environmental contamination. Environ Health Perspect. 1994 Sep;102(9):780–781. doi: 10.1289/ehp.94102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. M., Gahr M., Horan K., Wibbels T., Crews D. Cloning and in situ hybridization analysis of estrogen receptor in the developing gonad of the red-eared slider turtle, a species with temperature-dependent sex determination. Dev Growth Differ. 1998 Apr;40(2):243–254. doi: 10.1046/j.1440-169x.1998.00013.x. [DOI] [PubMed] [Google Scholar]
- Bull J. J., Gutzke W. H., Crews D. Sex reversal by estradiol in three reptilian orders. Gen Comp Endocrinol. 1988 Jun;70(3):425–428. doi: 10.1016/0016-6480(88)90117-7. [DOI] [PubMed] [Google Scholar]
- Colborn T., vom Saal F. S., Soto A. M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993 Oct;101(5):378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D., Bergeron J. M., Bull J. J., Flores D., Tousignant A., Skipper J. K., Wibbels T. Temperature-dependent sex determination in reptiles: proximate mechanisms, ultimate outcomes, and practical applications. Dev Genet. 1994;15(3):297–312. doi: 10.1002/dvg.1020150310. [DOI] [PubMed] [Google Scholar]
- Crews D., Bergeron J. M. Role of reductase and aromatase in sex determination in the red-eared slider (Trachemys scripta), a turtle with temperature-dependent sex determination. J Endocrinol. 1994 Nov;143(2):279–289. doi: 10.1677/joe.0.1430279. [DOI] [PubMed] [Google Scholar]
- Crews D., Bull J. J., Wibbels T. Estrogen and sex reversal in turtles: a dose-dependent phenomenon. Gen Comp Endocrinol. 1991 Mar;81(3):357–364. doi: 10.1016/0016-6480(91)90162-y. [DOI] [PubMed] [Google Scholar]
- Crews D., Cantú A. R., Bergeron J. M., Rhen T. The relative effectiveness of androstenedione, testosterone, and estrone, precursors to estradiol, in sex reversal in the red-eared slider (Trachemys scripta), a turtle with temperature-dependent sex determination. Gen Comp Endocrinol. 1995 Oct;100(1):119–127. doi: 10.1006/gcen.1995.1140. [DOI] [PubMed] [Google Scholar]
- Crews D., Cantú A. R., Bergeron J. M. Temperature and non-aromatizable androgens: a common pathway in male sex determination in a turtle with temperature-dependent sex determination? J Endocrinol. 1996 Jun;149(3):457–463. doi: 10.1677/joe.0.1490457. [DOI] [PubMed] [Google Scholar]
- Crews D., Cantú A. R., Rhen T., Vohra R. The relative effectiveness of estrone, estradiol-17 beta, and estriol in sex reversal in the red-eared slider (Trachemys scripta), a turtle with temperature-dependent sex determination. Gen Comp Endocrinol. 1996 Jun;102(3):317–326. doi: 10.1006/gcen.1996.0075. [DOI] [PubMed] [Google Scholar]
- Crews D. Temperature-dependent sex determination: the interplay of steroid hormones and temperature. Zoolog Sci. 1996 Feb;13(1):1–13. doi: 10.2108/zsj.13.1. [DOI] [PubMed] [Google Scholar]
- Desvages G., Pieau C. Aromatase activity in gonads of turtle embryos as a function of the incubation temperature of eggs. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):851–853. doi: 10.1016/0960-0760(92)90437-n. [DOI] [PubMed] [Google Scholar]
- Desvages G., Pieau C. Steroid metabolism in gonads of turtle embryos as a function of the incubation temperature of eggs. J Steroid Biochem Mol Biol. 1991 Aug;39(2):203–213. doi: 10.1016/0960-0760(91)90064-c. [DOI] [PubMed] [Google Scholar]
- Gahr M., Wibbels T., Crews D. Sites of estrogen uptake in embryonic Trachemys scripta, a turtle with temperature-dependent sex determination. Biol Reprod. 1992 Mar;46(3):458–463. doi: 10.1095/biolreprod46.3.458. [DOI] [PubMed] [Google Scholar]
- Gasc J. M. Estrogen target cells in gonads of the chicken embryo during sexual differentiation. J Embryol Exp Morphol. 1980 Feb;55:331–342. [PubMed] [Google Scholar]
- Gimeno S., Gerritsen A., Bowmer T., Komen H. Feminization of male carp. Nature. 1996 Nov 21;384(6606):221–222. doi: 10.1038/384221a0. [DOI] [PubMed] [Google Scholar]
- Johnston S. D., Liu X., Zuo F., Eisenbraun T. L., Wiley S. R., Kraus R. J., Mertz J. E. Estrogen-related receptor alpha 1 functionally binds as a monomer to extended half-site sequences including ones contained within estrogen-response elements. Mol Endocrinol. 1997 Mar;11(3):342–352. doi: 10.1210/mend.11.3.9897. [DOI] [PubMed] [Google Scholar]
- Korach K. S., Sarver P., Chae K., McLachlan J. A., McKinney J. D. Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: conformationally restricted structural probes. Mol Pharmacol. 1988 Jan;33(1):120–126. [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- McLachlan J. A. Synergistic effect of environmental estrogens: report withdrawn. Science. 1997 Jul 25;277(5325):462–463. doi: 10.1126/science.277.5325.459d. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy K., Wang F., Chen I. C., Safe S., Norris J. D., McDonnell D. P., Gaido K. W., Bocchinfuso W. P., Korach K. S. Potency of combined estrogenic pesticides. Science. 1997 Jan 17;275(5298):405–406. doi: 10.1126/science.275.5298.405. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy K., Wang F., Chen I. C., Safe S., Norris J. D., McDonnell D. P., Gaido K. W., Bocchinfuso W. P., Korach K. S. Potency of combined estrogenic pesticides. Science. 1997 Jan 17;275(5298):405–406. doi: 10.1126/science.275.5298.405. [DOI] [PubMed] [Google Scholar]
- Rhen T., Lang J. W. Temperature-dependent sex determination in the snapping turtle: manipulation of the embryonic sex steroid environment. Gen Comp Endocrinol. 1994 Nov;96(2):243–254. doi: 10.1006/gcen.1994.1179. [DOI] [PubMed] [Google Scholar]
- Safe S. H. Environmental and dietary estrogens and human health: is there a problem? Environ Health Perspect. 1995 Apr;103(4):346–351. doi: 10.1289/ehp.95103346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhanick A. C., Callard I. P. A sex-steroid-binding protein in the plasma of the freshwater turtle, Chrysemys picta. Gen Comp Endocrinol. 1980 Oct;42(2):163–166. doi: 10.1016/0016-6480(80)90183-5. [DOI] [PubMed] [Google Scholar]
- Sasson S., Notides A. C. Estriol and estrone interaction with the estrogen receptor. I. Temperature-induced modulation of the cooperative binding of [3H]estriol and [3H]estrone to the estrogen receptor. J Biol Chem. 1983 Jul 10;258(13):8113–8117. [PubMed] [Google Scholar]
- Scheib D. Effects and role of estrogens in avian gonadal differentiation. Differentiation. 1983;23 (Suppl):S87–S92. doi: 10.1007/978-3-642-69150-8_15. [DOI] [PubMed] [Google Scholar]
- Simons S. S., Jr Environmental estrogens: can two "alrights" make a wrong? Science. 1996 Jun 7;272(5267):1451–1451. doi: 10.1126/science.272.5267.1451. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Chung K. L., Sonnenschein C. The pesticides endosulfan, toxaphene, and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environ Health Perspect. 1994 Apr;102(4):380–383. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay G. B., Tremblay A., Copeland N. G., Gilbert D. J., Jenkins N. A., Labrie F., Giguère V. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol. 1997 Mar;11(3):353–365. doi: 10.1210/mend.11.3.9902. [DOI] [PubMed] [Google Scholar]
- Webster N. J., Green S., Jin J. R., Chambon P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell. 1988 Jul 15;54(2):199–207. doi: 10.1016/0092-8674(88)90552-1. [DOI] [PubMed] [Google Scholar]
- Wibbels T., Bull J. J., Crews D. Chronology and morphology of temperature-dependent sex determination. J Exp Zool. 1991 Dec;260(3):371–381. doi: 10.1002/jez.1402600311. [DOI] [PubMed] [Google Scholar]
- Wibbels T., Bull J. J., Crews D. Synergism between temperature and estradiol: a common pathway in turtle sex determination? J Exp Zool. 1991 Oct;260(1):130–134. doi: 10.1002/jez.1402600117. [DOI] [PubMed] [Google Scholar]
- Wibbels T., Crews D. Putative aromatase inhibitor induces male sex determination in a female unisexual lizard and in a turtle with temperature-dependent sex determination. J Endocrinol. 1994 May;141(2):295–299. doi: 10.1677/joe.0.1410295. [DOI] [PubMed] [Google Scholar]
- Wibbels T., Gideon P., Bull J. J., Crews D. Estrogen- and temperature-induced medullary cord regression during gonadal differentiation in a turtle. Differentiation. 1993 Jul;53(3):149–154. doi: 10.1111/j.1432-0436.1993.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Yntema C. L. A series of stages in the embryonic development of Chelydra serpentina. J Morphol. 1968 Jun;125(2):219–251. doi: 10.1002/jmor.1051250207. [DOI] [PubMed] [Google Scholar]
- vom Saal F. S., Timms B. G., Montano M. M., Palanza P., Thayer K. A., Nagel S. C., Dhar M. D., Ganjam V. K., Parmigiani S., Welshons W. V. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]