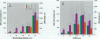Abstract
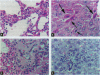
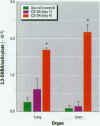
To examine the toxicity of cyclosiloxanes (CSs), the predominant low molecular weight cyclic silicones found in breast implants, we injected female CD-1 mice intraperitoneally with different doses of distillate (3.5-35 g/kg body weight) containing cyclosiloxane D3 (hexamethylcyclotrisiloxane; CS-D3), cyclosiloxane D4 (octamethylcyclotetrasiloxane; CS-D4), cyclosiloxane D5 (decamethylcyclopentasiloxane; CS-D5), and cyclosiloxane D6 (dodecamethylcyclohexasiloxane; CS-D6). The distillate was found to be lethal and all the mice injected with 35 g/kg died within 5-8 days. The median lethal dose (LD50) for distillate was estimated to be approximately 28 g/kg. These mice developed inflammatory lesions of the lung and liver as well as liver cell necrosis with elevated serum levels of alanine aminotransferase, aspartate aminotransferase, and lactic acid dehydrogenase. Administration of CS-D4 alone also produced lethality in these mice with an LD50 of 6-7 g/kg. CS-D4-treated mice also exhibited pulmonary and hepatic lesions and elevated serum enzymes. Analysis of LD50 data indicates that CS-D4 is about as toxic as carbon tetrachloride or trichloroethylene. We measured hydroxyl radical formation in CS-D4-treated mice and found increases of approximately 20-fold in liver and approximately 7-fold in lung on day 4 following injection. Our findings are significant because in vitro experiments have demonstrated that CSs can migrate out of breast implants, and in mouse experiments CSs have been shown to be widely distributed in many organs after a single subcutaneous injection and to persist for at least a year.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHLEY F. L., REES T. D., BALLANTYNE D. L., Jr, GALLOWAY D., MACHIDA R., GRAZER F., MCCONNELL D. V., EDGINGTON T., KISKADDEN W. AN INJECTION TECHNIQUE FOR THE TREATMENT OF FACIAL HEMIATROPHY. Plast Reconstr Surg. 1965 Jun;35:640–648. doi: 10.1097/00006534-196506000-00009. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Chen J., Crow J. P., Ye Y. Z. Reactions of nitric oxide, superoxide and peroxynitrite with superoxide dismutase in neurodegeneration. Prog Brain Res. 1994;103:371–380. doi: 10.1016/s0079-6123(08)61151-6. [DOI] [PubMed] [Google Scholar]
- Berman E., Schlicht M., Moser V. C., MacPhail R. C. A multidisciplinary approach to toxicological screening: I. Systemic toxicity. J Toxicol Environ Health. 1995 Jun;45(2):127–143. doi: 10.1080/15287399509531986. [DOI] [PubMed] [Google Scholar]
- Bradley S. G., Munson A. E., McCay J. A., Brown R. D., Musgrove D. L., Wilson S., Stern M., Luster M. I., White K. L., Jr Subchronic 10 day immunotoxicity of polydimethylsiloxane (silicone) fluid, gel and elastomer and polyurethane disks in female B6C3F1 mice. Drug Chem Toxicol. 1994;17(3):175–220. doi: 10.3109/01480549409017860. [DOI] [PubMed] [Google Scholar]
- Cojocel C., Beuter W., Müller W., Mayer D. Lipid peroxidation: a possible mechanism of trichloroethylene-induced nephrotoxicity. Toxicology. 1989 Apr;55(1-2):131–141. doi: 10.1016/0300-483x(89)90180-7. [DOI] [PubMed] [Google Scholar]
- Cook R. R., Perkins L. L. The prevalence of breast implants among women in the United States. Curr Top Microbiol Immunol. 1996;210:419–425. doi: 10.1007/978-3-642-85226-8_45. [DOI] [PubMed] [Google Scholar]
- Deapen D. M., Pike M. C., Casagrande J. T., Brody G. S. The relationship between breast cancer and augmentation mammaplasty: an epidemiologic study. Plast Reconstr Surg. 1986 Mar;77(3):361–368. doi: 10.1097/00006534-198603000-00001. [DOI] [PubMed] [Google Scholar]
- Destouet J. M., Monsees B. S., Oser R. F., Nemecek J. R., Young V. L., Pilgram T. K. Screening mammography in 350 women with breast implants: prevalence and findings of implant complications. AJR Am J Roentgenol. 1992 Nov;159(5):973–981. doi: 10.2214/ajr.159.5.1414810. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Henderson R., Watson J. J., Wong P. K. Use of salicylate with high pressure liquid chromatography and electrochemical detection (LCED) as a sensitive measure of hydroxyl free radicals in adriamycin treated rats. J Free Radic Biol Med. 1986;2(1):13–18. doi: 10.1016/0748-5514(86)90118-2. [DOI] [PubMed] [Google Scholar]
- Garrido L., Pfleiderer B., Jenkins B. G., Hulka C. A., Kopans D. B. Migration and chemical modification of silicone in women with breast prostheses. Magn Reson Med. 1994 Mar;31(3):328–330. doi: 10.1002/mrm.1910310314. [DOI] [PubMed] [Google Scholar]
- Kala S. V., Lykissa E. D., Lebovitz R. M. Detection and characterization of poly(dimethylsiloxane)s in biological tissues by GC/AED and GC/MS. Anal Chem. 1997 Apr 1;69(7):1267–1272. doi: 10.1021/ac961235p. [DOI] [PubMed] [Google Scholar]
- Kala S. V., Lykissa E. D., Neely M. W., Lieberman M. W. Low molecular weight silicones are widely distributed after a single subcutaneous injection in mice. Am J Pathol. 1998 Mar;152(3):645–649. [PMC free article] [PubMed] [Google Scholar]
- Lane T. H., Burns S. A. Silica, silicon and silicones ... unraveling the mystery. Curr Top Microbiol Immunol. 1996;210:3–12. doi: 10.1007/978-3-642-85226-8_1. [DOI] [PubMed] [Google Scholar]
- Lykissa E. D., Kala S. V., Hurley J. B., Lebovitz R. M. Release of low molecular weight silicones and platinum from silicone breast implants. Anal Chem. 1997 Dec 1;69(23):4912–4916. doi: 10.1021/ac970710w. [DOI] [PubMed] [Google Scholar]
- May D. S., Stroup N. E. The incidence of sarcomas of the breast among women in the United States, 1973-1986. Plast Reconstr Surg. 1991 Jan;87(1):193–194. doi: 10.1097/00006534-199101000-00045. [DOI] [PubMed] [Google Scholar]
- Minns R. J., Walsh W. K. Preliminary design and experimental studies of a novel soft implant for correcting sagittal plane instability in the lumbar spine. Spine (Phila Pa 1976) 1997 Aug 15;22(16):1819–1827. doi: 10.1097/00007632-199708150-00004. [DOI] [PubMed] [Google Scholar]
- Nakamura A., Kawasaki Y., Takada K., Aida Y., Kurokama Y., Kojima S., Shintani H., Matsui M., Nohmi T., Matsuoka A. Difference in tumor incidence and other tissue responses to polyetherurethanes and polydimethylsiloxane in long-term subcutaneous implantation into rats. J Biomed Mater Res. 1992 May;26(5):631–650. doi: 10.1002/jbm.820260506. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Refojo M. F., Crabtree D. V., Pastor J., Leong F. L. Ocular toxicity of low-molecular-weight components of silicone and fluorosilicone oils. Invest Ophthalmol Vis Sci. 1991 Nov;32(12):3007–3020. [PubMed] [Google Scholar]
- Robinson O. G., Jr, Bradley E. L., Wilson D. S. Analysis of explanted silicone implants: a report of 300 patients. Ann Plast Surg. 1995 Jan;34(1):1–7. doi: 10.1097/00000637-199501000-00001. [DOI] [PubMed] [Google Scholar]
- Sipes I. G., el Sisi A. E., Sim W. W., Mobley S. A., Earnest D. L. Reactive oxygen species in the progression of CCl4-induced liver injury. Adv Exp Med Biol. 1991;283:489–497. doi: 10.1007/978-1-4684-5877-0_65. [DOI] [PubMed] [Google Scholar]
- Yu L. T., Latorre G., Marotta J., Batich C., Hardt N. S. In vitro measurement of silicone bleed from breast implants. Plast Reconstr Surg. 1996 Apr;97(4):756–764. doi: 10.1097/00006534-199604000-00011. [DOI] [PubMed] [Google Scholar]
- elSisi A. E., Earnest D. L., Sipes I. G. Vitamin A potentiation of carbon tetrachloride hepatotoxicity: role of liver macrophages and active oxygen species. Toxicol Appl Pharmacol. 1993 Apr;119(2):295–301. doi: 10.1006/taap.1993.1072. [DOI] [PubMed] [Google Scholar]