Abstract
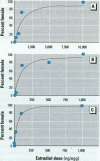
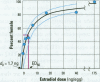
Risk assessments for nongenotoxic chemicals assume a threshold below which no adverse outcomes are seen. However, when an endogenous chemical, such as 17ss-estradiol (E2), occurs at a concentration sufficient to cause an effect, the threshold is already exceeded. Under these circumstances, exogenous estradiol is not expected to provide a threshold dose. This principle is demonstrated for E2 in the red-eared slider, a turtle with temperature-dependent sex determination. In this species, gonadal sex is determined by egg incubation temperature; female development requires endogenous estrogen produced by elevated temperature. While normal production of females by endogenous estrogens is not an adverse effect, exogenous estrogens can sex reverse presumptive males, which can be an adverse effect. A large dose-response study was conducted using seven doses and a vehicle control (starting n = 300/group); a single E2 dose was applied to the eggshell of recently laid eggs. Animals were sexed after hatching. The incubation temperature chosen, 28.6 degrees C, generates a minority of females. Thus, the criteria for testing the threshold hypothesis were met, i.e., there is evidence that there is endogenous estrogen and that it generates an irreversible response. The lowest E2 dose tested, 400 pg/egg (40 ng/kg), sex reversed 14.4% of the animals, demonstrating very low dose sensitivity. The data were fit with a modified Michaelis-Menten equation, which provided an estimate of 1.7 ng/egg for endogenous estradiol. The median effective dose (ED50) was 5.0 +/- 2.0 ng/egg (95% confidence limits), of which 1.7 ng/egg was endogenous estradiol and 3.3 ng/egg came from the applied estradiol. There was no apparent threshold dose for E2. A smaller replication confirmed these results. These results provide a simple biologically based dose-response model and suggest that chemicals which act mechanistically like E2 may also show no threshold dose. If so, even low environmental concentrations of such chemicals may carry risk for sex reversal.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BICKOFF E. M., LIVINGSTON A. L., BOOTH A. N. Estrogenic activity of coumestrol and related compounds. Arch Biochem Biophys. 1960 Jun;88:262–266. doi: 10.1016/0003-9861(60)90232-0. [DOI] [PubMed] [Google Scholar]
- Bergeron J. M., Crews D., McLachlan J. A. PCBs as environmental estrogens: turtle sex determination as a biomarker of environmental contamination. Environ Health Perspect. 1994 Sep;102(9):780–781. doi: 10.1289/ehp.94102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitman J., Cecil H. C., Harris S. J., Fries G. F. Estrogenic activity of o,p'-DDT in the mammalian uterus and avian oviduct. Science. 1968 Oct 18;162(3851):371–372. doi: 10.1126/science.162.3851.371. [DOI] [PubMed] [Google Scholar]
- Branham W. S., Sheehan D. M. Ovarian and adrenal contributions to postnatal growth and differentiation of the rat uterus. Biol Reprod. 1995 Oct;53(4):863–872. doi: 10.1095/biolreprod53.4.863. [DOI] [PubMed] [Google Scholar]
- Crews D., Bergeron J. M., Bull J. J., Flores D., Tousignant A., Skipper J. K., Wibbels T. Temperature-dependent sex determination in reptiles: proximate mechanisms, ultimate outcomes, and practical applications. Dev Genet. 1994;15(3):297–312. doi: 10.1002/dvg.1020150310. [DOI] [PubMed] [Google Scholar]
- Crews D., Bull J. J., Wibbels T. Estrogen and sex reversal in turtles: a dose-dependent phenomenon. Gen Comp Endocrinol. 1991 Mar;81(3):357–364. doi: 10.1016/0016-6480(91)90162-y. [DOI] [PubMed] [Google Scholar]
- Crews D. Temperature-dependent sex determination: the interplay of steroid hormones and temperature. Zoolog Sci. 1996 Feb;13(1):1–13. doi: 10.2108/zsj.13.1. [DOI] [PubMed] [Google Scholar]
- DeRosa C., Richter P., Pohl H., Jones D. E. Environmental exposures that affect the endocrine system: public health implications. J Toxicol Environ Health B Crit Rev. 1998 Jan-Mar;1(1):3–26. doi: 10.1080/10937409809524541. [DOI] [PubMed] [Google Scholar]
- Fry D. M., Toone C. K. DDT-induced feminization of gull embryos. Science. 1981 Aug 21;213(4510):922–924. doi: 10.1126/science.7256288. [DOI] [PubMed] [Google Scholar]
- Gaylor D. W. Risk estimation--an overview: disease risk estimation based upon animal bioassays. Drug Metab Rev. 1996 Feb-May;28(1-2):9–27. doi: 10.3109/03602539608993989. [DOI] [PubMed] [Google Scholar]
- Gaylor D. W., Sheehan D. M., Young J. F., Mattison D. R. The threshold dose question in teratogenesis. Teratology. 1988 Oct;38(4):389–391. doi: 10.1002/tera.1420380410. [DOI] [PubMed] [Google Scholar]
- Gimeno S., Gerritsen A., Bowmer T., Komen H. Feminization of male carp. Nature. 1996 Nov 21;384(6606):221–222. doi: 10.1038/384221a0. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr, Ostby J., Marshall R., Andrews J. Reproductive and thyroid effects of low-level polychlorinated biphenyl (Aroclor 1254) exposure. Fundam Appl Toxicol. 1993 Apr;20(3):288–294. doi: 10.1006/faat.1993.1038. [DOI] [PubMed] [Google Scholar]
- Hoel D. G. Incorporation of background in dose-response models. Fed Proc. 1980 Jan;39(1):73–75. [PubMed] [Google Scholar]
- Kohn M. C., Portier C. J. Effects of the mechanism of receptor-mediated gene expression on the shape of the dose-response curve. Risk Anal. 1993 Oct;13(5):565–572. doi: 10.1111/j.1539-6924.1993.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Nelson C. J., Holson J. F., Gaines T. B., LaBorde J. B., McCallum W. F., Wolff G. L., Sheehan D. M., Young J. F. Developmental toxicity of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T). II. Multireplicated dose-response studies with technical and analytical grades of 2,4,5-T in four-way outcross mice. Fundam Appl Toxicol. 1992 Aug;19(2):298–306. doi: 10.1016/0272-0590(92)90164-d. [DOI] [PubMed] [Google Scholar]
- Portier C., Tritscher A., Kohn M., Sewall C., Clark G., Edler L., Hoel D., Lucier G. Ligand/receptor binding for 2,3,7,8-TCDD: implications for risk assessment. Fundam Appl Toxicol. 1993 Jan;20(1):48–56. doi: 10.1006/faat.1993.1006. [DOI] [PubMed] [Google Scholar]
- Sexton K., Reiter L. W., Zenick H. Research to strengthen the scientific basis for health risk assessment: a survey of the context and rationale for mechanistically based methods and models. Toxicology. 1995 Sep 1;102(1-2):3–20. doi: 10.1016/0300-483x(95)03033-c. [DOI] [PubMed] [Google Scholar]
- Wibbels T., Bull J. J., Crews D. Chronology and morphology of temperature-dependent sex determination. J Exp Zool. 1991 Dec;260(3):371–381. doi: 10.1002/jez.1402600311. [DOI] [PubMed] [Google Scholar]
- Wibbels T., Crews D. Steroid-induced sex determination at incubation temperatures producing mixed sex ratios in a turtle with TSD. Gen Comp Endocrinol. 1995 Oct;100(1):53–60. doi: 10.1006/gcen.1995.1132. [DOI] [PubMed] [Google Scholar]
- vom Saal F. S., Timms B. G., Montano M. M., Palanza P., Thayer K. A., Nagel S. C., Dhar M. D., Ganjam V. K., Parmigiani S., Welshons W. V. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]