Abstract
Background
To determine whether the dose-volume histograms (DVH's) for the rectum and bladder constructed using biological-effective dose (BED-DVH's) better correlate with late gastrointestinal (GI) and genitourinary (GU) toxicity after treatment with external beam radiotherapy for prostate cancer than conventional DVH's (C-DVH's).
Methods
The charts of 190 patients treated with external beam radiotherapy with a minimum follow-up of 2 years were reviewed. Six patients (3.2%) were found to have RTOG grade 3 GI toxicity, and similarly 6 patients (3.2%) were found to have RTOG grade 3 GU toxicity. Average late C-DVH's and BED-DVH's of the bladder and rectum were computed for these patients as well as for matched-pair control patients. For each matched pair the following measures of normalized difference in the DVH's were computed: (a) δAUC = (Area Under Curve [AUC] in grade 3 patient – AUC in grade 0 patient)/(AUC in grade 0 patient) and (b) δV60 = (Percent volume receiving = 60 Gy [V60] in grade 3 patient – V60 in grade 0 patient)/(V60 in grade 0 patient).
Results
As expected, the grade 3 curve is to the right of and above the grade 0 curve for all four sets of average DVH's – suggesting that both the C-DVH and the BED-DVH can be used for predicting late toxicity. δAUC was higher for the BED-DVH's than for the C-DVH's – 0.27 vs 0.23 (p = 0.036) for the rectum and 0.24 vs 0.20 (p = 0.065) for the bladder. δV60 was also higher for the BED-DVH's than for the C-DVH's – 2.73 vs 1.49 for the rectum (p = 0.021) and 1.64 vs 0.71 (p = 0.021) for the bladder.
Conclusions
When considering well-established dosimetric endpoints used in evaluating treatment plans, BED-DVH's for the rectum and bladder correlate better with late toxicity than C-DVH's and should be considered when attempting to minimize late GI and GU toxicity after external beam radiotherapy for prostate cancer.
Keywords: Prostate Cancer, Dose Volume Histogram, Biological Effective Dose
Background
Radiotherapy treatment planning is, in broad terms, the process of planning the delivery of a prescribed dose to a tumor or target volume while minimizing dose to the surrounding critical structures. Prior to the modern radiotherapy treatment planning era, the 'optimum' plan was decided upon using qualitative criteria, or by estimating the inhomogeneity of the dose in the target or in critical regions. With the advent of CT-based planning, the planning process could be done with increased precision, as 3-dimensional structures representing the target and critical structures could be defined more accurately and reproducibly. One of the most useful tools which emerged in the 3-dimensional planning era that could be used in establishing the acceptability or unacceptability of a treatment plan is the dose-volume histogram (DVH) [1].
The DVH is used ubiquitously and plots delivered dose on the x-axis and percent volume of the structure of interest on the y-axis. The general shape and area under the DVH curve is instrumental in determining adequate coverage and homogeneity of dose in the target volume as well as in determining acceptable dose to critical structures. Indeed, the DVH has occupied a central role in modern treatment planning [2-5].
The DVH is not without its limitations, however. First, there is an inherent loss of spatial information with the construction of a DVH plot. Second, the dose plotted on the x-axis of a conventional DVH (C-DVH) is conventional dose, which does not take into account many factors influencing the effects of the dose on the given tissue. Among them are the α/β of the structure (which can vary considerably for early vs late reacting tissues and for early vs late reactions within the same tissue), time taken to deliver treatment, fraction size, and length of treatment interruptions. For this reason, the concept of biological-effective dose (BED) was developed [6,7] and has been studied extensively by the radiobiology community. Because the BED incorporates factors related to delivery of treatment and tissue factors, it is a better representation of the dose delivered to a given tissue than conventional dose [6-8].
The application of BED has particular importance to radiation treatment planning for prostate cancer. The prostate, more so than any other disease site, has been the focus of advances in treatment delivery technique. The prostate has been the paradigm site for treatment planning efforts related to conformal therapy [9-12] and intensity modulated radiotherapy (IMRT)/inverse planning [13,14]. Also, the prostate has been the leading disease site for efforts related to improving daily setup [15] and incorporating organ motion into the delivery strategy [16]. These above efforts have allowed for dose escalation of the prostate while minimizing early and late treatment toxicity [9-14,17].
In view of the lead role the prostate has taken in understanding treatment planning, patient setup, and dose escalation, incorporation of treatment-related information into the DVH – to further facilitate advances in reduction of rectal or urinary toxicity – is highly appropriate. The purpose of the current investigation is to compare C-DVH's of the bladder and rectum in those patients who developed late treatment toxicity versus those patients who did not develop such toxicity, and, using the same groups of patients, to determine whether the biological-effective DVH (BED-DVH) can potentially serve as a better tool for such toxicity comparisons than the C-DVH.
Methods
A generalized theoretical model for determination of biological effective dose has been developed and reported extensively [6-8]. Recently, our group has applied this model for use with any combination of short-lived interstitial brachytherapy and external beam radiotherapy for prostate cancer (unpublished/submitted data, 2003). For external beam radiotherapy, the generalized BED model reduces to the familiar equation:
BED = [n*d(1 + d/(α/β))] - [(0.693*T)/(α*Tp)] (1)
where n = number of treatments, d = dose per fraction, T = treatment time, α/β = 3 Gy(for late effects), and Tp (potential doubling time) and α (linear component of cell killing) were estimated from the published literature [6-8],[18,19] to be 34 days and 0.3/Gy, respectively. The first term in Equation (1) accounts for dose fractionation and the second term accounts for repopulation. The authors recognize that there is considerable controversy in the exact values used for α/β, α, and Tp for prostate cancer in the reported literature.
The charts of 190 consecutive patients with T1 or T2 prostate cancer treated with external beam radiotherapy at our institution between 1996–1999 were reviewed. During this period, external beam radiotherapy was delivered using 6-field technique, once daily, 5 times a week, using 180 cGy or 200 cGy fractions, to a final dose of 7000–7200 cGy (minimum PTV dose). The conformal techniques at our institution have been previously reported [20] – the prostate, seminal vesicles, rectum, and bladder were outlined routinely on all patients, and uniform guidelines were adhered to in order to minimize inter-observer variability in the definition of these structures. In particular, with regard to the rectum, our institution followed routine Radiation Therapy Oncology Group (RTOG) guidelines for definition – from the anus at the level of the ischial tuberosities {lower border} to the rectosigmoid junction {upper border} [21]. The bladder was contoured from the apex to the dome, again as outlined per RTOG guidelines. The planning target volume (PTV) was the prostate + seminal vesicles + 1 cm margin for the initial phase; after 4500–5000 cGy, the PTV was the prostate + 1 cm margin. Corresponding DVH's were computed for the PTV, entire rectum (i.e., not the rectal wall or rectal surface), and entire bladder (again, not the bladder wall or bladder surface). The dose-fractionation and total treatment time for each patient were recorded to obtain values for n, d, and T in Equation (1) above.
No charts for those patients treated after 1999 were reviewed to ensure long minimum follow-up. At each routine follow-up, the GI and GU complications using definitions and grading as stated by the RTOG [22] were recorded. Thus, the charts were reviewed to identify those patients with significant (RTOG grade 3 or grade 4) late GI (large bowel) or GU (bladder) complications.
Of the 190 patients, 6 patients (3.2%) were found to have late grade 3 rectal toxicity and 6 patients (3.2%) were found to have late grade 3 urinary toxicity. No patients were found to have grade 4 GI or GU toxicity. A matched-pair group of patients (6 GI and 6 GU) with grade 0 toxicity having similar pretreatment (age, stage, grade, and PSA) and treatment (technique and dose) characteristics were identified from the same patient database. The characteristics of the groups are shown in comparison with the groups of patients demonstrating toxicity in Tables 1 and 2. The limitations and potential biases inherent to a matched-pair comparison are well-understood by the authors; however, as these tables demonstrate, there is no difference in the pretreatment and treatment characteristics between the groups demonstrating and not demonstrating GI [Table 1] and GU [Table 2] toxicity.
Table 1.
Characteristics of groups having grade 3 vs grade 0 rectal (GI) toxicity
| Grade 3 group | Grade 0 group | |
| Total Number | 6 | 6 |
| T-Stage (number) | ||
| T1c | 2 | 2 |
| T2a/b | 4 | 4 |
| Mean Age (yr) | 70.8 | 69.4 |
| Mean PSA (ng/dl) | 16.8 | 18.0 |
| Race (number) | ||
| AA | 2 | 2 |
| White | 4 | 4 |
| Fraction Size (number) | ||
| 180 cGy | 1 | 1 |
| 200 cGy | 5 | 5 |
| Technique (number) | ||
| 6-Field | 6 | 6 |
| Mean Dose (cGy) | 7170 | 7167 |
Table 2.
Characteristics of groups having grade 3 vs grade 0 urinary (GU) toxicity
| Grade 3 group | Grade 0 group | |
| Total Number | 6 | 6 |
| T-Stage (number) | ||
| T1c | 2 | 2 |
| T2a/b | 4 | 4 |
| Mean Age (yr) | 70.4 | 69.7 |
| Mean PSA (ng/dl) | 17.9 | 18.3 |
| Race (number) | ||
| AA | 3 | 3 |
| White | 3 | 3 |
| Fraction Size (number) | ||
| 180 cGy | 0 | 0 |
| 200 cGy | 6 | 6 |
| Technique (number) | ||
| 6-Field | 6 | 6 |
| Mean Dose (cGy) | 7170 | 7200 |
The C-DVH's were already constructed (prior to undertaking the current investigation) for the patients in each of the four groups (grade 0 – rectal, grade 3 – rectal, grade 0 – bladder, grade 3 – bladder) – these are the C-DVH's that were approved by the attending radiation oncologist prior to delivering the external beam treatment. Then, these C-DVH's derived from each of the individual dose distributions were averaged over the six patients in each of the four groups (grade 0 – rectal, grade 3 – rectal, grade 0 – bladder, and grade 3 – bladder) to produce average C-DVH's.
Using equation (1), the time-dose fractionation parameters were incorporated to transform the conventional dose to BED at each conventional dose level. This allowed for the construction of the BED-DVH's for all of the patients. Then, in a process similar to that for the C-DVH's, the BED-DVH's derived from each of the individual dose distributions were averaged over the six patients in each of the four groups (grade 0 – rectal, grade 3 – rectal, grade 0 – bladder, and grade 3 – bladder) to produce average BED-DVH's. The authors are aware of the recent efforts regarding advanced modeling concepts for evaluating the relationship between physical irradiation and biological damage on normal structures – in particular, BED provides a measure of biological effect at a point at which it is calculated – measuring the absolute biological effect over a volume having a non-uniform dose distribution formally requires the computation of the equivalent uniform dose (EUD) [23]. However, this caveat does not apply directly to the current task, because the goal of the current investigation is to use the DVH (constructed using either conventional dose or BED) as a relative measure of biological response, not as an absolute measure, and in this context constructing a DVH from BED computations and averaging the DVH's (both C-DVH's and BED-DVH's) over a patient population was felt to be an appropriate methodology.
After construction of the C-DVH's and BED-DVH's, it was clear that the general shape of the two were slightly different, as would be expected by the non-linear transformation in Equation (1) above. In order to perform comparison of the individual matched pairs in an objective, reproducible, and systematic manner, the following measure of normalized difference in the average DVH was computed:
![]()
Equation (2) represents an attempt to compress the DVH difference information into a single quantity, and was felt to be a valid quantity as the DVH's did not 'cross' – that is, the grade 3 DVH's were above and to the right of the grade 0 DVH's at all dose (or BED) levels. Furthermore, this approach was felt to be valid biologically, as integral dose to critical structures has been correlated by many investigators with late treatment toxicity [24-30]. The authors are also aware of the special problems related to applying the DVH for tubular and surface structures (such as the rectum and bladder) [28,31]. However, because C-DVH's of the whole organs were used as the basis for the original clinical decision making on the patient population under consideration (i.e., those treated before 1999), it was felt that integral dose to the entire rectum and entire bladder would be appropriate as one measure for clinical comparison of the use of BED-DVH's versus C-DVH's.
Equation (2) was applied to compute the δAUC's (both for the C-DVH's and for the BED-DVH's) for each of the six matched-patient pairs in the rectal toxicity category and to each of the six matched-patient pairs in the bladder toxicity category. In order to perform a statistical comparison between these matched-pair δAUC's obtained using C-DVH's and those obtained using BED-DVH's, the student's t-test was used.
Although the treatment decisions were made with knowledge of the integral dose delivered to the critical structures, the authors recognize that there is controversy with regard to the classification of biological response of the normal structures under study in the current investigation. In particular, many recent reports (e.g., [32]) suggest that the bladder and rectum may be serial rather than parallel structures with regard to their biological response to radiation injury. Thus, an alternative measure of merit was chosen to complement the AUC analysis above. Specifically, the percent volume receiving above a set high dose level (in this study, 60 Gy) was analyzed. The analogous expression to Equation (2) is the following:
![]()
Equation (3) was applied in exactly the same manner as above to compute δV60's for the C-DVH's and BED-DVH's for both the bladder and rectum. It should be noted that when applying Equation (3) to the BED-DVH's, the 60 Gy conventional dose transforms [using Equation (1), assuming once-daily 2 Gy fractions] to approximately 100 Gy; thus, V60 on the C-DVH's corresponds to V100 on the BED-DVH's. The statistical comparison between the matched-pair δV60's obtained between C-DVH's and BED-DVH's was performed using the student's t-test.
Results
As expected, the DVH's (both C-DVH's and BED-DVH's) were predictive of rectal and urinary toxicity – that is, those that developed late GI or GU toxicity had DVH's that demonstrated more integral dose to the rectum or bladder than those patients who did not develop such toxicity. This reinforces and validates the well-known and widespread use of DVH's in evaluating external beam [5,24-29] and interstitial [33] treatment plans for prostate cancer patients.
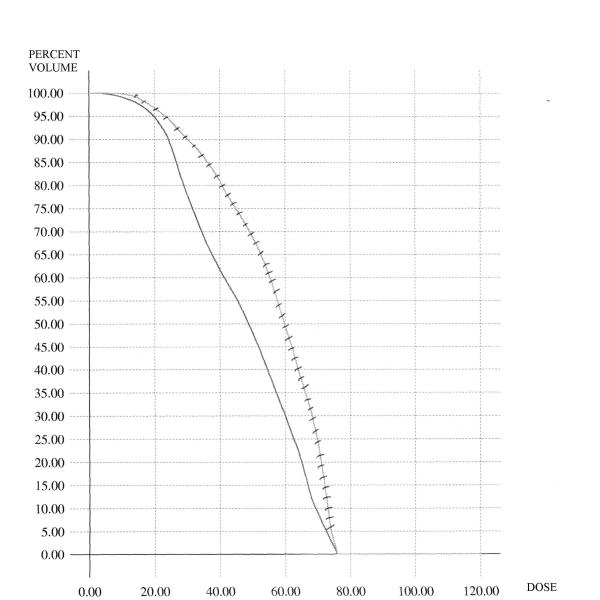
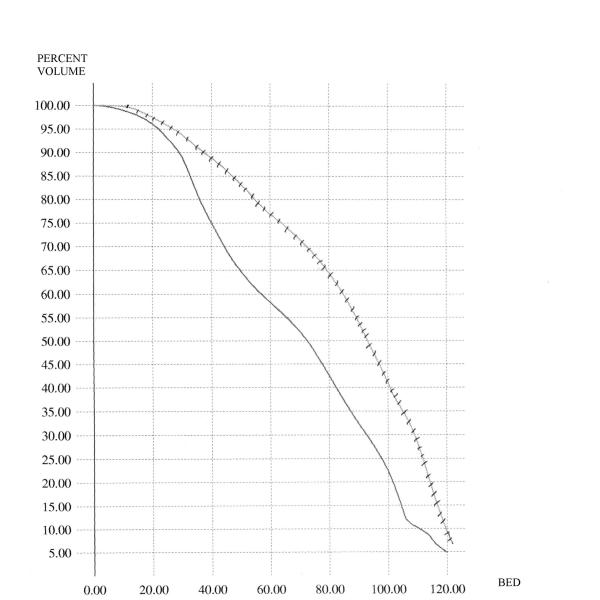
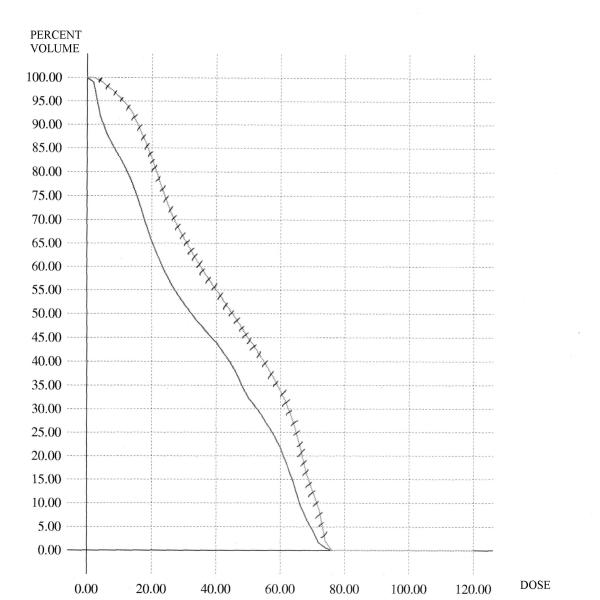
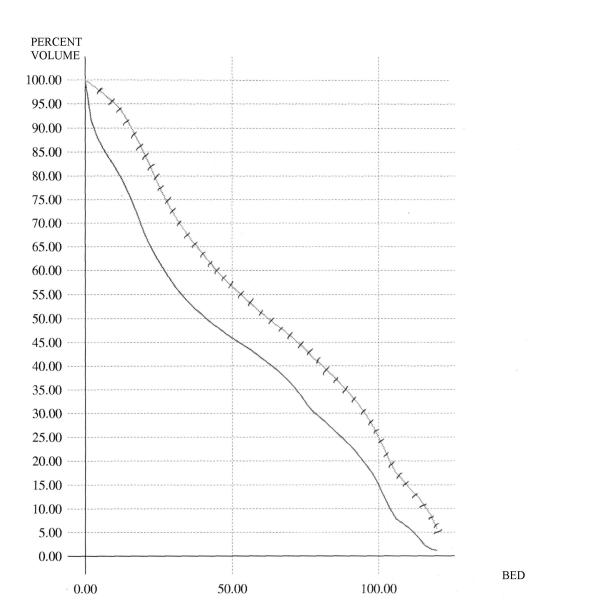
Figure 1 shows the comparison for the case of the conventional DVH's of the rectum. In this figure, the average C-DVH of the rectum was computed for the 6 patients in the grade 3 group as well as the 6 patients in the grade 0 group. The average DVH's are displayed – as is demonstrated, the grade 3 curve is to the right of and above the grade 0 curve. Similar analyses were done for the BED-DVH-Rectum (as demonstrated in Figure 2), for the C-DVH-Bladder (as demonstrated in Figure 3) and for the BED-DVH-Bladder (as demonstrated in Figure 4). It should be noted that in all cases, the average grade 3 DVH's – conventional or BED – were consistently above and to the right of the corresponding grade 0 curve at each dose (or BED) level from zero to maximum, again validating the uniform comparison approach summarized in Equations (2) and (3).
Figure 1.
Average Conventional DVH's for the rectum. Solid = grade 0 toxicity; hashed = grade 3 toxicity.
Figure 2.
Average Biological-Effective DVH's for the rectum. Solid = grade 0 toxicity; hashed = grade 3 toxicity.
Figure 3.
Average Conventional DVH's for the bladder. Solid = grade 0 toxicity; hashed = grade 3 toxicity.
Figure 4.
Average Biological-Effective DVH's for the bladder. Solid = grade 0 toxicity; hashed = grade 3 toxicity.
Table 3 shows the results of the detailed matched-pair AUC analysis. In this table, the δAUC's obtained using the C-DVH's and BED-DVH's for each of the matched-patient pairs is displayed. The students' t-test was used to obtain a p-value for each of the rectal toxicity and bladder toxicity categories. As Table 3 displays, the δAUC analysis demonstrates a p-value of 0.036 for the rectal toxicity comparison, and a p-value of 0.065 for the bladder toxicity comparison, suggesting that [when using AUC as the measure of merit] the BED-DVH's were significantly better-correlated (than the corresponding C-DVH's) with rectal toxicity, and marginally better-correlated with bladder toxicity.
Table 3.
Matched-pair AUC Analyses using C-DVH and BED-DVH.
| δAUC (C-DVH) | δAUC (BED-DVH) | p-value* | |
| Rectum | |||
| Matched Pair 1 | 0.14 | 0.11 | |
| Matched Pair 2 | 0.47 | 0.55 | |
| Matched Pair 3 | 0.11 | 0.18 | |
| Matched Pair 4 | 0.38 | 0.46 | |
| Matched Pair 5 | 0.23 | 0.29 | |
| Matched Pair 6 | 0.03 | 0.03 | |
| Mean | 0.23 | 0.27 | 0.036 |
| Bladder | |||
| Matched Pair 1 | 0.22 | 0.23 | |
| Matched Pair 2 | 0.28 | 0.37 | |
| Matched Pair 3 | 0.19 | 0.30 | |
| Matched Pair 4 | 0.15 | 0.23 | |
| Matched Pair 5 | 0.18 | 0.19 | |
| Matched Pair 6 | 0.18 | 0.14 | |
| Mean | 0.20 | 0.24 | 0.065 |
Table 4 displays the results of the matched-pair V60 analysis. In this table, the δV60's obtained using the C-DVH's and BED-DVH's for each of the matched-patient pairs is displayed. This table demonstrates a p-value of 0.021 for the rectal toxicity comparison, and a p-value of 0.021 for the bladder toxicity comparison, suggesting that [when using V60 as the measure of merit] the BED-DVH's were significantly better-correlated (than the corresponding C-DVH's) with rectal toxicity and with bladder toxicity.
Table 4.
Matched-pair V60 Analyses using C-DVH and BED-DVH.
| δV60 (C-DVH) | δV60 (BED-DVH) | p-value* | |
| Rectum | |||
| Matched Pair 1 | 1.17 | 1.99 | |
| Matched Pair 2 | 0.87 | 1.73 | |
| Matched Pair 3 | 1.88 | 2.73 | |
| Matched Pair 4 | 1.06 | 1.21 | |
| Matched Pair 5 | 2.17 | 3.53 | |
| Matched Pair 6 | 1.81 | 5.21 | |
| Mean | 1.49 | 2.73 | 0.021 |
| Bladder | |||
| Matched Pair 1 | 1.10 | 3.13 | |
| Matched Pair 2 | 0.90 | 2.13 | |
| Matched Pair 3 | 0.92 | 2.01 | |
| Matched Pair 4 | 0.40 | 0.27 | |
| Matched Pair 5 | 0.38 | 1.78 | |
| Matched Pair 6 | 0.57 | 0.55 | |
| Mean | 0.71 | 1.64 | 0.021 |
*p-values obtained using a students' t-test
Conclusions
The values for δAUC and δV60 are higher for the BED-DVH's of the rectum and bladder than for the counterpart C-DVH's. Because the AUC and V60 are dosimetric endpoints used routinely for the evaluation of radiation treatment plans, this analysis suggests that BED-DVH's for the rectum and bladder correlate better with late toxicity than C-DVH's and should be considered when attempting to minimize late GI and GU toxicity after radiotherapy for prostate cancer. Because this analysis was performed with two independent measures of merit of the DVH's – the AUC and the V60, the early results reported herein appear promising for the use of BED-DVH's.
Because the patient population having late grade 3/4 toxicity (which is a small population but was chosen as the target population that is most likely to benefit from the incorporation of BED-DVH's into the process of treatment planning) was the focus of this initial investigation, the number of patients in the current study is understandably small. Six cases of late grade 3/4 GI toxicity represent approximately 3% of the study population – this low incidence also applies to the six cases of late grade 3/4 GU toxicity. Assuming that the same low rate of late toxicity is maintained, the number of cases of such late complications will not increase significantly with a significant increase in size of the study population. Thus, while the results are encouraging for the correlation of BED-DVH's to rectal toxicity, the correlation to bladder toxicity using the integral dose/AUC endpoint did not reach statistical significance, likely due to the limitations in sample size. However, because the integral dose/AUC analysis did reach significance for the rectum, and because the V60 analysis did reach statistical significance for both the bladder and rectum, the results of the current investigation represent initial success with the application of the BED-DVH concept.
As one means of avoiding the statistical problems posed by evaluating late toxicity, analyzing early grade 3/4 toxicity, which has a higher incidence, may allow for greater statistical power in validating the BED-DVH concept. However, because the modeling for the Tp, alpha, and alpha/beta is altogether different for early vs late structures and because the transformation between late to early parameters in Equations (1–2) above are nonlinear, the results of the current analysis (and corresponding conclusions) may be completely different from such an early toxicity analysis. For these reasons, the use of BED-DVH's for evaluating and predicting early GI and GU toxicity will be the subject of a separate communication.
The results of the current investigation are encouraging – the well-known conventional DVH can be successfully expanded to include treatment delivery, tissue biology, and time/fractionation-related information that better represents the true effect of the treatment on critical structures. Other investigators have expanded the C-DVH to include functional and spatial information [4,34,35], but the current investigation is the first report correlating a DVH which has been expanded to include BED parameters with late treatment toxicity for prostate cancer irradiation.
Although the initial focus of this investigation was the study of late radiation effects on the rectum and bladder, there is no conceptual limitation to the use of the techniques described herein to study early effects of treatment (for acute toxicity assessment) or to study target structures (for outcome/survival analysis) at any anatomic site. The results of the current investigation, while encouraging, are preliminary and require validation by other investigators – because of the low incidence of late grade 3/4 complications, such an analysis might be better undertaken at a multi-institutional level.
Competing interests
None Declared
Authors' contributions
ABJ designed and coordinated the investigation and drafted the manuscript. CMH, CAP, and JCR constructed and implemented the software code for which the dose volume histograms could be computed and averaged. LK and SV were responsible for maintaining the patient database – in addition SV provided general methodological and clinical guidance. All authors have read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Ashesh B Jani, Email: jani_1969@yahoo.com.
Christopher M Hand, Email: cdrradiation@comcast.net.
Charles A Pelizzari, Email: chuck@rover.uchicago.edu.
John C Roeske, Email: roeske@rover.uchicago.edu.
Lani Krauz, Email: lani@rover.uchicago.edu.
Srinivasan Vijayakumar, Email: svijayakum@aol.com.
References
- Drzymala RE, et al. Dose-volume histograms. Int J Radiat Oncol Biol Phys. 1991;21:71–78. doi: 10.1016/0360-3016(91)90168-4. [DOI] [PubMed] [Google Scholar]
- Hart KB, Porter AT. A rational approach to the treatment of prostate cancer with radiation therapy: lessons for the future. Semin Oncol. 1997;24:745–755. [PubMed] [Google Scholar]
- Lawrence TS, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781–788. doi: 10.1016/0360-3016(92)90651-w. [DOI] [PubMed] [Google Scholar]
- Marks LB, et al. The role of three dimensional functional lung imaging in radiation treatment planning: the functional dose-volume histogram. Int J Radiat Oncol Biol Phys. 1995;33:65–75. doi: 10.1016/0360-3016(95)00091-C. [DOI] [PubMed] [Google Scholar]
- Ting JY, et al. Dose-volume histograms for bladder and rectum. Int J Radiat Oncol Biol Phys. 1997;38:1105–1111. doi: 10.1016/S0360-3016(97)00312-X. [DOI] [PubMed] [Google Scholar]
- Dale RG. The application of the linear quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiology. 1984;58:515–528. doi: 10.1259/0007-1285-58-690-515. [DOI] [PubMed] [Google Scholar]
- Hall EJ. Radiobiology for the Radiologist. Philadephia, PA: JB Lippincott & Company. 1994.
- Lee SP, et al. Biologically effective dose distribution based on the linear quadratic model and its clinical relevance. Int J Radiat Oncol Biol Phys. 1995;33:375–389. doi: 10.1016/0360-3016(95)00162-R. [DOI] [PubMed] [Google Scholar]
- Michalski JM, et al. Preliminary report of toxicity following 3D radiation therapy for prostate cancer on 3DOG/RTOG 9406. Int J Radiat Oncol Biol Phys. 2000;46:391–402. doi: 10.1016/S0360-3016(99)00443-5. [DOI] [PubMed] [Google Scholar]
- Neal AJ, Oldham M, Dearnaley DP. Comparison of treatment techniques for conformal radiotherapy of the prostate using dose-volume histograms and normal tissue complication probabilities. Radiother Oncol. 1995;37:29–34. doi: 10.1016/0167-8140(95)01619-R. [DOI] [PubMed] [Google Scholar]
- Pollack A, et al. Conventional vs. conformal radiotherapy for prostate cancer: preliminary results of dosimetry and acute toxicity. Int J Radiat Oncol Biol Phys. 1996;34:555–564. doi: 10.1016/0360-3016(95)02103-5. [DOI] [PubMed] [Google Scholar]
- Zelefsky MJ, Leibel SA, Kutcher GJ, Fuks Z. Three-dimensional conformal radiotherapy and dose escalation: where do we stand? Semin Radiat Oncol. 1998;8:107–114. doi: 10.1016/s1053-4296(98)80006-4. [DOI] [PubMed] [Google Scholar]
- Ling CC, et al. Conformal radiation treatment of prostate cancer using inversely-planned intensity-modulated photon beams produced with dynamic multileaf collimation. Int J Radiat Oncol Biol Phys. 1996;35:721–730. doi: 10.1016/0360-3016(96)00174-5. [DOI] [PubMed] [Google Scholar]
- Zelefsky MJ, et al. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166:876–881. doi: 10.1097/00005392-200109000-00017. [DOI] [PubMed] [Google Scholar]
- Roeske JC, et al. Evaluation of changes in the size and location of the prostate, seminal vesicles, bladder, and rectum during a course of external beam radiation therapy. Int J Radiat Oncol Biol Phys. 1995;33:1321–1329. doi: 10.1016/0360-3016(95)00225-1. [DOI] [PubMed] [Google Scholar]
- Mohan DS, Kupelian PA, Willoughby TR. Short-course intensity-modulated radiotherapy for localized prostate cancer with daily transabdominal ultrasound localization of the prostate gland. Int J Radiat Oncol Biol Phys. 2000;46:575–580. doi: 10.1016/S0360-3016(99)00454-X. [DOI] [PubMed] [Google Scholar]
- Storey MR, et al. Complications from radiotherapy dose escalation in prostate cancer: preliminary results of a randomized trial. Int J Radiat Oncol Biol Phys. 2000;48:635–642. doi: 10.1016/S0360-3016(00)00700-8. [DOI] [PubMed] [Google Scholar]
- King CR, DiPetrillo TA, Wazer DE. Optimal radiotherapy for prostate cancer: predictions for conventional external beam, IMRT, and brachytherapy from radiobiologic models. Int J Radiat Oncol Biol Phys. 2000;46:165–172. doi: 10.1016/S0360-3016(99)00406-X. [DOI] [PubMed] [Google Scholar]
- King CR. What is the T(pot) for prostate cancer? Radiobiological implications of the equivalent outcome with (125)I or (103)Pd. Int J Radiat Oncol Biol Phys. 2000;47:1165–1167. doi: 10.1016/S0360-3016(00)00543-5. [DOI] [PubMed] [Google Scholar]
- Vijayakumar S, et al. In the radiotherapy of prostate cancer, technique determines the doses to the penile structures. Br J Radiol. 1999;72:882–888. doi: 10.1259/bjr.72.861.10645194. [DOI] [PubMed] [Google Scholar]
- Michalski JM, et al. Preliminary report of toxicity following 3D radiation therapy for prostate cancer on 3DOG/RTOG 9406. Int J Radiat Oncol Biol Phys. 2000;46:391–402. doi: 10.1016/S0360-3016(99)00443-5. [DOI] [PubMed] [Google Scholar]
- Lawton CA, et al. Long-term treatment sequelae following external beam irradiation for adenocarcinoma of the prostate: analysis of RTOG studies 7506 and 7706. Int J Radiat Oncol Biol Phys. 1991;21:935–939. doi: 10.1016/0360-3016(91)90732-j. [DOI] [PubMed] [Google Scholar]
- Niemerko A. Reporting and analyzing dose distributions: a concept of equivalent uniform dose. Med Phys. 1997;24:103–110. doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- Boersma LJ, et al. Estimation of the incidence of late bladder and rectum complications after high-dose (70–78 GY) conformal radiotherapy for prostate cancer, using dose-volume histograms. Int J Radiat Oncol Biol Phys. 1998;41:83–92. doi: 10.1016/S0360-3016(98)00037-6. [DOI] [PubMed] [Google Scholar]
- Hanks GE, Schultheiss TE, Hunt MA, Epstein B. Factors influencing incidence of acute grade 2 morbidity in conformal and standard radiation treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 1995;31:25–29. doi: 10.1016/0360-3016(94)00366-S. [DOI] [PubMed] [Google Scholar]
- Jackson A, et al. Late rectal bleeding after conformal radiotherapy of prostate cancer. II. Volume effects and dose-volume histograms. Int J Radiat Oncol Biol Phys. 2001;49:685–698. doi: 10.1016/S0360-3016(00)01414-0. [DOI] [PubMed] [Google Scholar]
- Kitamura K, et al. The relationship between technical parameters of external beam radiation therapy and for localized prostate cancer. Jpn J Clin Oncol. 2000;30:225–229. doi: 10.1093/jjco/hyd058. [DOI] [PubMed] [Google Scholar]
- MacKay RI, et al. Predicting late rectal complications following prostate conformal radiotherapy using biologically effective doses and normalized dose-surface histograms. Br J Radiol. 1997;70:517–526. doi: 10.1259/bjr.70.833.9227235. [DOI] [PubMed] [Google Scholar]
- Schultheiss TE, Hanks GE, Hunt MA, Lee WR. Incidence of and factors related to late complications in conformal and conventional radiation treatment of cancer of the prostate. Int J Radiat Oncol Biol Phys. 1995;32:643–649. doi: 10.1016/0360-3016(95)00149-S. [DOI] [PubMed] [Google Scholar]
- Wolbarst AB, Chin LM, Svensson GK. Optimization of radiation therapy: integral-response of a model biological system. Int J Radiat Oncol Biol Phys. 1982;8:1761–1769. doi: 10.1016/0360-3016(82)90299-1. [DOI] [PubMed] [Google Scholar]
- Fenwick JD, et al. Correlations between dose-surface histograms and the incidence of long-term rectal bleeding following conformal or conventional radiotherapy treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 2001;49:473–480. doi: 10.1016/S0360-3016(00)01496-6. [DOI] [PubMed] [Google Scholar]
- Dale E, Olsen DR, Fossa SD. Normal tissue complication probabilities correlated with late effects in the rectum after prostate conformal radiotherapy. Int J Radiat Oncol Biol Phys. 1999;43:385–91. doi: 10.1016/S0360-3016(98)00400-3. [DOI] [PubMed] [Google Scholar]
- Snyder KM, et al. Defining the risk of developing Grade 2 proctitis following (125)I prostate brachytherapy using a rectal dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2001;50:335–341. doi: 10.1016/S0360-3016(01)01442-0. [DOI] [PubMed] [Google Scholar]
- Cheng CW, Das IJ. Treatment plan evaluation using dose-volume histogram (DVH) and spatial dose-volume histogram (zDVH) Int J Radiat Oncol Biol Phys. 1999;43:1143–1150. doi: 10.1016/S0360-3016(98)00492-1. [DOI] [PubMed] [Google Scholar]
- Mohan R, Brewster LJ, Barest GD. A technique for computing dose volume histograms for structure combinations. Med Phys. 1987;14:1048–1052. doi: 10.1118/1.595984. [DOI] [PubMed] [Google Scholar]