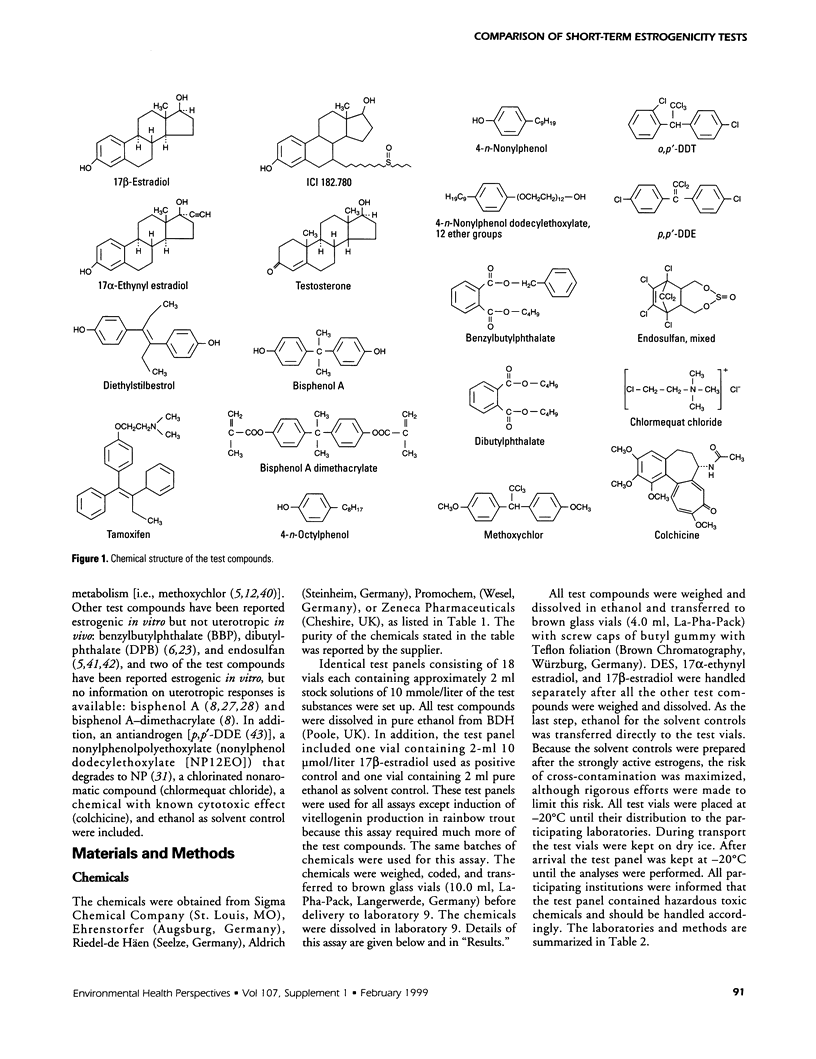
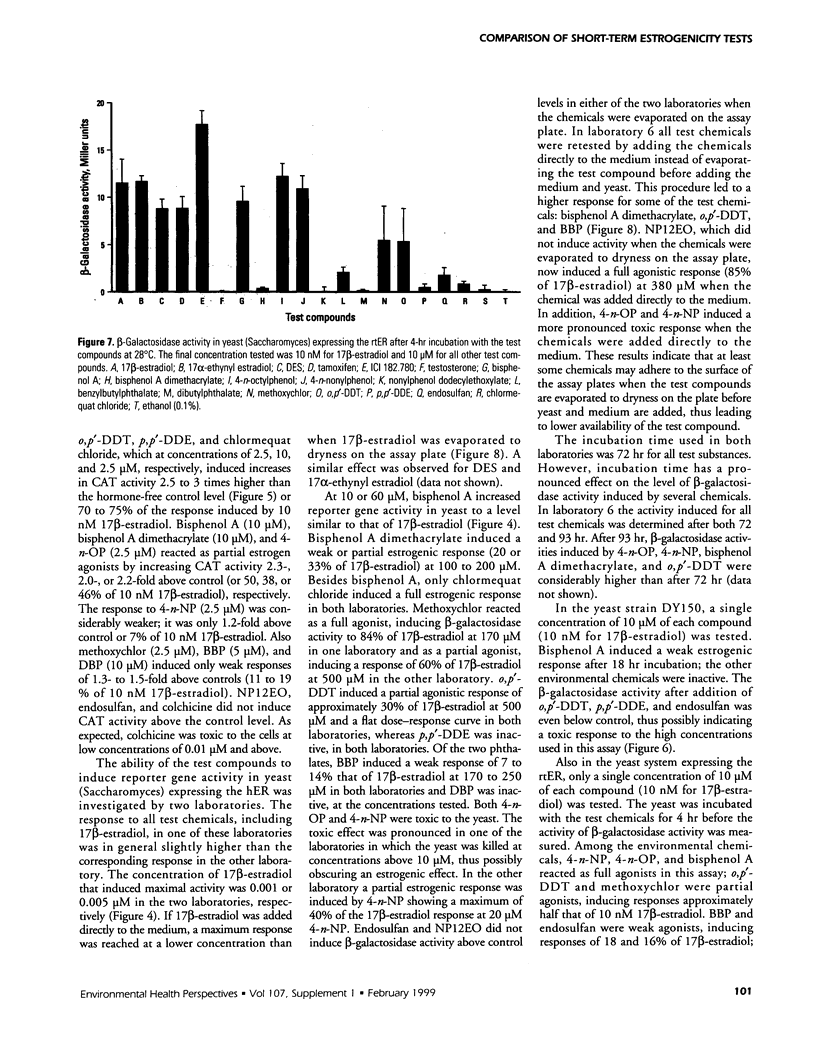
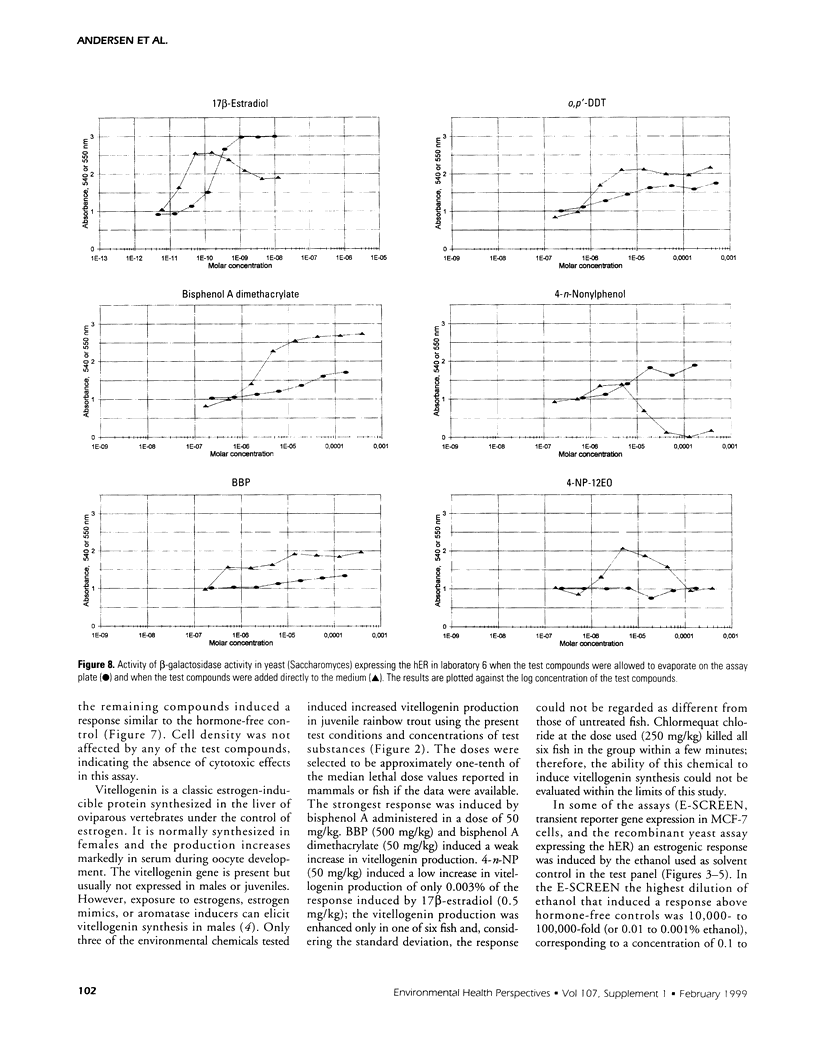
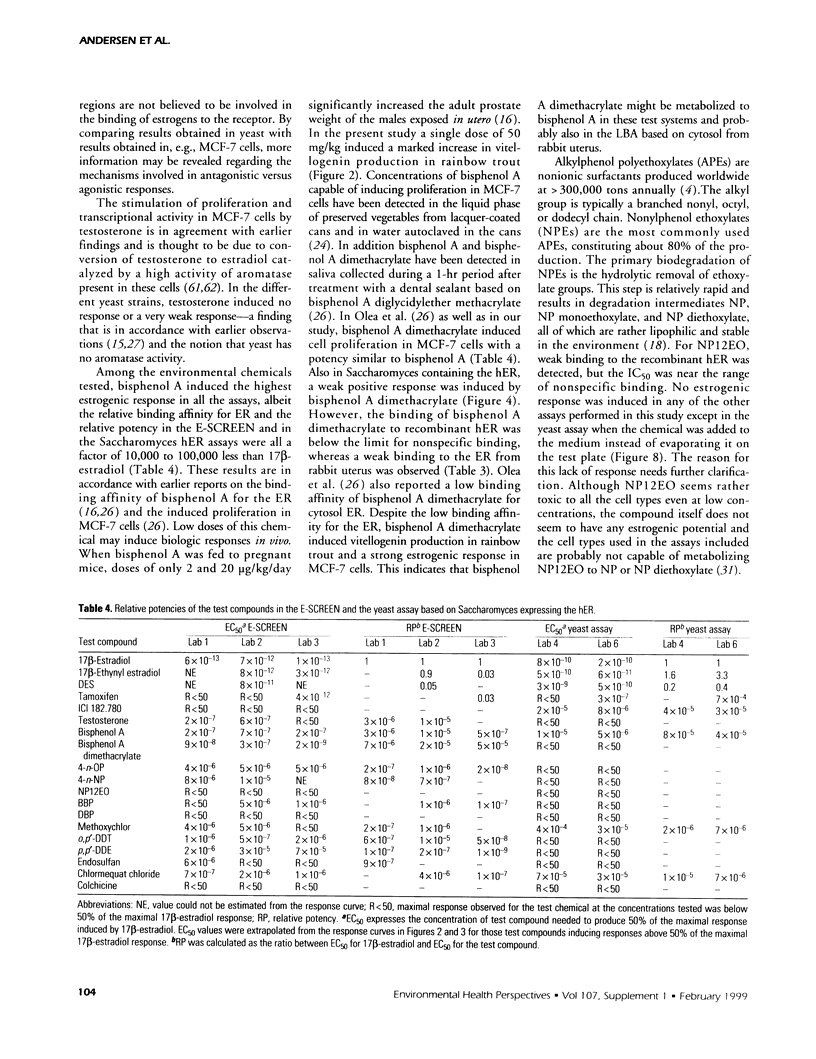
Abstract
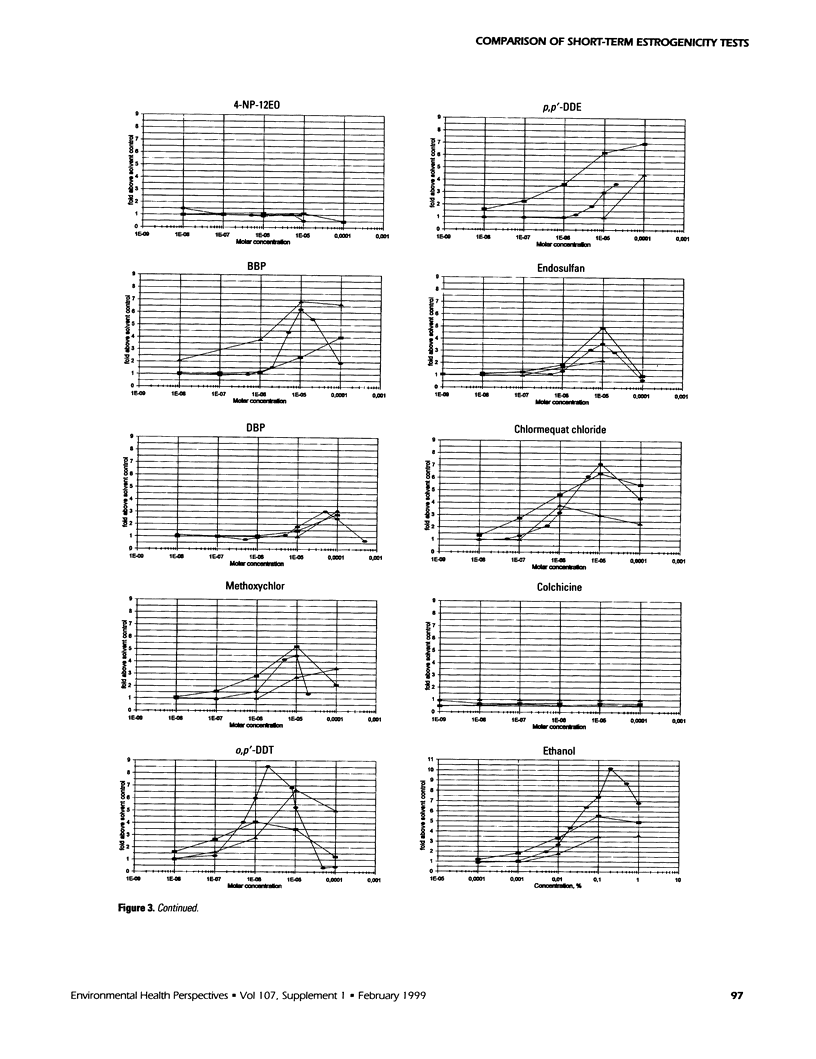
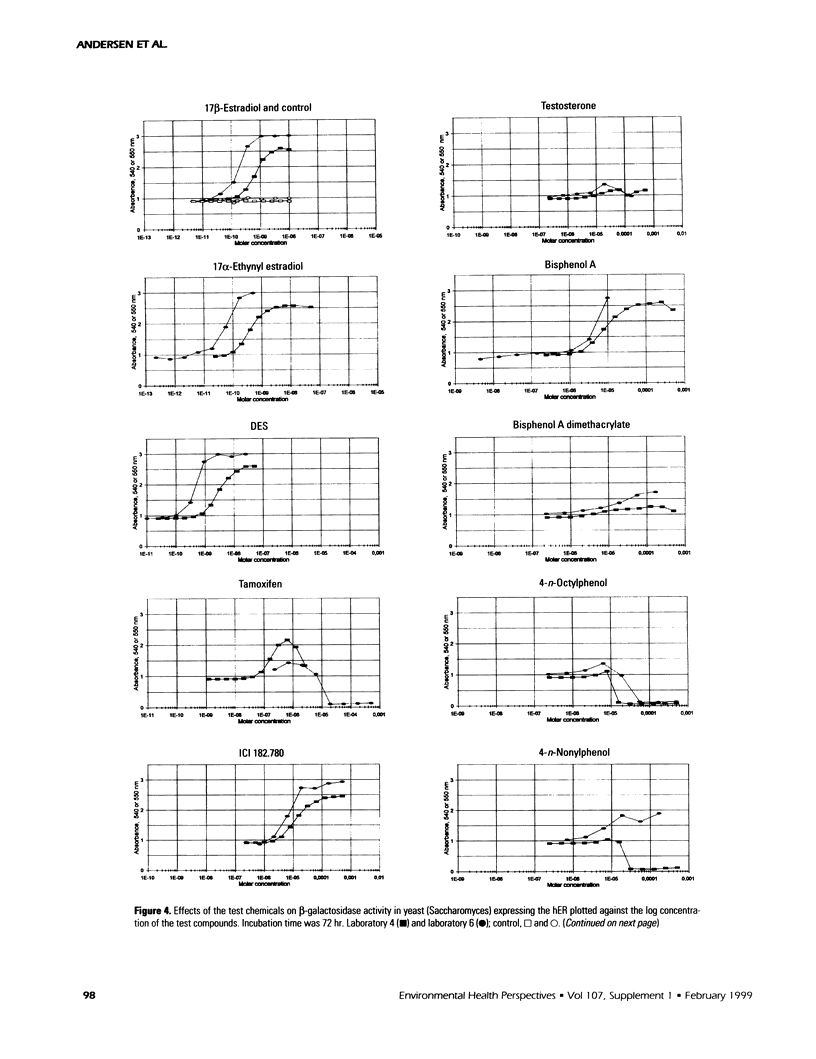
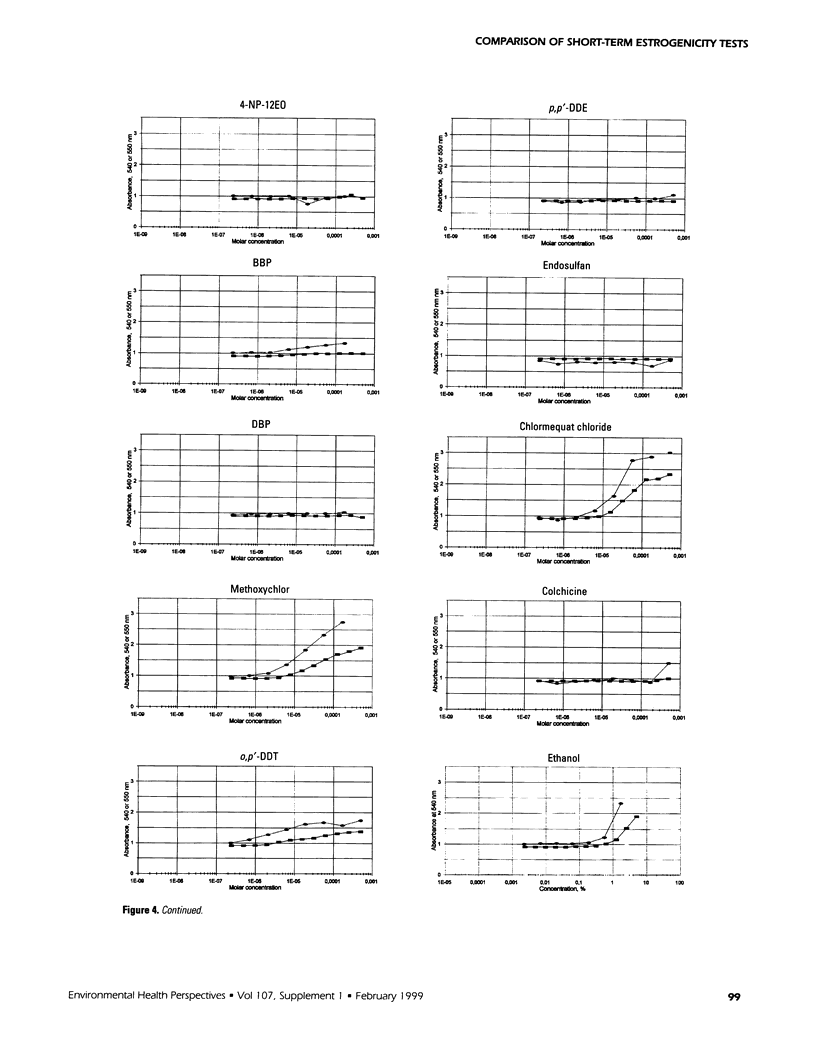
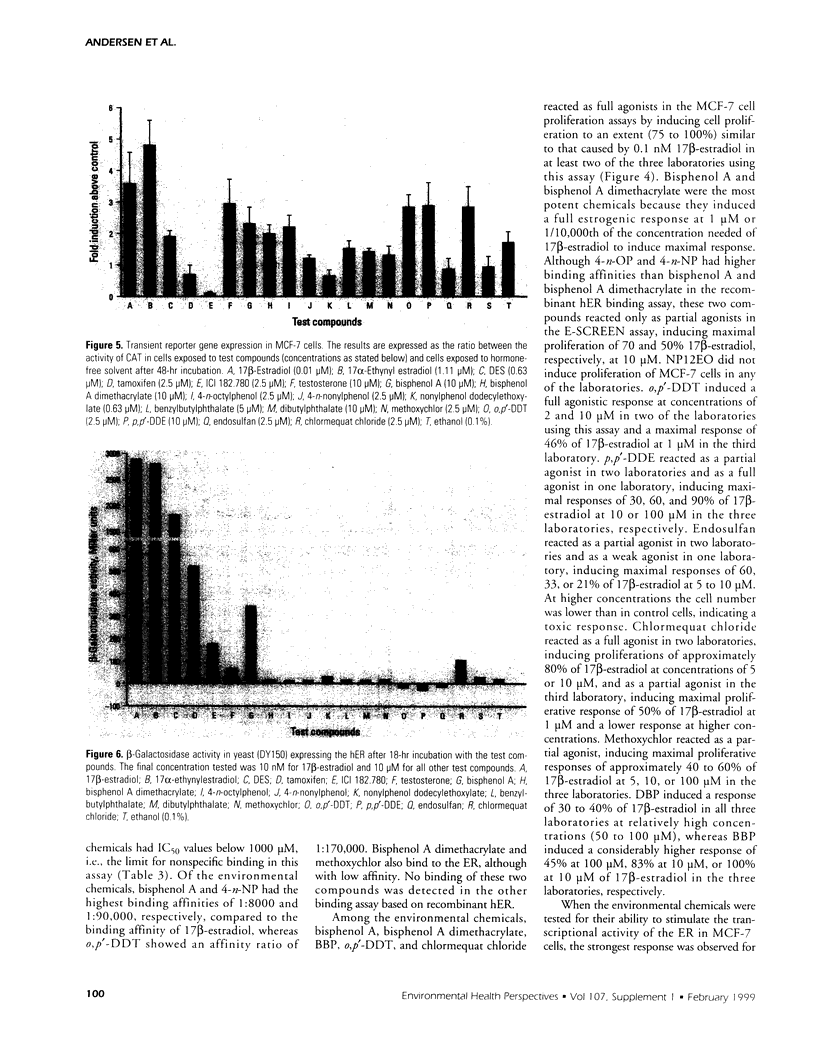
The aim of this study was to compare results obtained by eight different short-term assays of estrogenlike actions of chemicals conducted in 10 different laboratories in five countries. Twenty chemicals were selected to represent direct-acting estrogens, compounds with estrogenic metabolites, estrogenic antagonists, and a known cytotoxic agent. Also included in the test panel were 17β-estradiol as a positive control and ethanol as solvent control. The test compounds were coded before distribution. Test methods included direct binding to the estrogen receptor (ER), proliferation of MCF-7 cells, transient reporter gene expression in MCF-7 cells, reporter gene expression in yeast strains stably transfected with the human ER and an estrogen-responsive reporter gene, and vitellogenin production in juvenile rainbow trout. 17β-Estradiol, 17α-ethynyl estradiol, and diethylstilbestrol induced a strong estrogenic response in all test systems. Colchicine caused cytotoxicity only. Bisphenol A induced an estrogenic response in all assays. The results obtained for the remaining test compounds—tamoxifen, ICI 182.780, testosterone, bisphenol A dimethacrylate, 4-n-octylphenol, 4-n-nonylphenol, nonylphenol dodecylethoxylate, butylbenzylphthalate, dibutylphthalate, methoxychlor, o,p′-DDT, p,p′-DDE, endosulfan, chlomequat chloride, and ethanol—varied among the assays. The results demonstrate that careful standardization is necessary to obtain a reasonable degree of reproducibility. Also, similar methods vary in their sensitivity to estrogenic compounds. Thus, short-term tests are useful for screening purposes, but the methods must be further validated by additional interlaboratory and interassay comparisons to document the reliability of the methods.
Keywords: estrogenic chemicals, estrogens, antiestrogens, estrogenicity tests, binding assay, yeast, MCF-7, vitellogenin
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold S. F., Klotz D. M., Collins B. M., Vonier P. M., Guillette L. J., Jr, McLachlan J. A. Synergistic activation of estrogen receptor with combinations of environmental chemicals. Science. 1996 Jun 7;272(5267):1489–1492. doi: 10.1126/science.272.5267.1489. [DOI] [PubMed] [Google Scholar]
- Arnold S. F., Robinson M. K., Notides A. C., Guillette L. J., Jr, McLachlan J. A. A yeast estrogen screen for examining the relative exposure of cells to natural and xenoestrogens. Environ Health Perspect. 1996 May;104(5):544–548. doi: 10.1289/ehp.96104544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R. J., Herbison A. E., Sumpter J. P. Oestrogenic activity of an environmentally persistent alkylphenol in the reproductive tract but not the brain of rodents. J Steroid Biochem Mol Biol. 1995 Jul;54(1-2):7–9. doi: 10.1016/0960-0760(95)00118-j. [DOI] [PubMed] [Google Scholar]
- Bigsby R. M., Caperell-Grant A., Madhukar B. V. Xenobiotics released from fat during fasting produce estrogenic effects in ovariectomized mice. Cancer Res. 1997 Mar 1;57(5):865–869. [PubMed] [Google Scholar]
- Bitman J., Cecil H. C., Harris S. J., Fries G. F. Estrogenic activity of o,p'-DDT in the mammalian uterus and avian oviduct. Science. 1968 Oct 18;162(3851):371–372. doi: 10.1126/science.162.3851.371. [DOI] [PubMed] [Google Scholar]
- Bonefeld Jørgensen E. C., Autrup H., Hansen J. C. Effect of toxaphene on estrogen receptor functions in human breast cancer cells. Carcinogenesis. 1997 Aug;18(8):1651–1654. doi: 10.1093/carcin/18.8.1651. [DOI] [PubMed] [Google Scholar]
- Borrás M., Laios I., el Khissiin A., Seo H. S., Lempereur F., Legros N., Leclercq G. Estrogenic and antiestrogenic regulation of the half-life of covalently labeled estrogen receptor in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1996 Feb;57(3-4):203–213. doi: 10.1016/0960-0760(95)00272-3. [DOI] [PubMed] [Google Scholar]
- Brotons J. A., Olea-Serrano M. F., Villalobos M., Pedraza V., Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995 Jun;103(6):608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger W. H., Muccitelli R. M., Kupfer D. Studies on the in vivo and in vitro estrogenic activities of methoxychlor and its metabolites. Role of hepatic mono-oxygenase in methoxychlor activation. Biochem Pharmacol. 1978;27(20):2417–2423. doi: 10.1016/0006-2952(78)90354-4. [DOI] [PubMed] [Google Scholar]
- Chakravorty S., Lal B., Singh T. P. Effect of endosulfan (thiodan) on vitellogenesis and its modulation by different hormones in the vitellogenic catfish Clarias batrachus. Toxicology. 1992 Nov 15;75(3):191–198. doi: 10.1016/0300-483x(92)90001-u. [DOI] [PubMed] [Google Scholar]
- Chen C. W., Hurd C., Vorojeikina D. P., Arnold S. F., Notides A. C. Transcriptional activation of the human estrogen receptor by DDT isomers and metabolites in yeast and MCF-7 cells. Biochem Pharmacol. 1997 Apr 25;53(8):1161–1172. doi: 10.1016/s0006-2952(97)00097-x. [DOI] [PubMed] [Google Scholar]
- Coldham N. G., Dave M., Sivapathasundaram S., McDonnell D. P., Connor C., Sauer M. J. Evaluation of a recombinant yeast cell estrogen screening assay. Environ Health Perspect. 1997 Jul;105(7):734–742. doi: 10.1289/ehp.97105734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmar L. C., Denslow N. D., Rao V., Chow M., Crain D. A., Enblom J., Marcino J., Guillette L. J., Jr Vitellogenin induction and reduced serum testosterone concentrations in feral male carp (Cyprinus carpio) captured near a major metropolitan sewage treatment plant. Environ Health Perspect. 1996 Oct;104(10):1096–1101. doi: 10.1289/ehp.961041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. M. Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ Health Perspect. 1995 Oct;103 (Suppl 7):165–171. doi: 10.1289/ehp.95103s7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D. M., Toone C. K. DDT-induced feminization of gull embryos. Science. 1981 Aug 21;213(4510):922–924. doi: 10.1126/science.7256288. [DOI] [PubMed] [Google Scholar]
- Gradishar W. J., Jordan V. C. Clinical potential of new antiestrogens. J Clin Oncol. 1997 Feb;15(2):840–852. doi: 10.1200/JCO.1997.15.2.840. [DOI] [PubMed] [Google Scholar]
- Harris C. A., Henttu P., Parker M. G., Sumpter J. P. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997 Aug;105(8):802–811. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T. H., Jensen A., Kjems J. Tools for the production and purification of full-length, N- or C-terminal 32P-labeled protein, applied to HIV-1 Gag and Rev. Gene. 1995 Sep 11;162(2):235–237. doi: 10.1016/0378-1119(95)00328-4. [DOI] [PubMed] [Google Scholar]
- Jobling S., Reynolds T., White R., Parker M. G., Sumpter J. P. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995 Jun;103(6):582–587. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen E. C., Autrup H. Autoregulation of human CYP1A1 gene promotor activity in HepG2 and MCF-7 cells. Carcinogenesis. 1996 Mar;17(3):435–441. doi: 10.1093/carcin/17.3.435. [DOI] [PubMed] [Google Scholar]
- Jørgensen E. C., Autrup H. Effect of a negative regulatory element (NRE) on the human CYP1A1 gene expression in breast carcinoma MCF-7 and hepatoma HepG2 cells. FEBS Lett. 1995 May 29;365(2-3):101–107. doi: 10.1016/0014-5793(95)00456-j. [DOI] [PubMed] [Google Scholar]
- Kelce W. R., Stone C. R., Laws S. C., Gray L. E., Kemppainen J. A., Wilson E. M. Persistent DDT metabolite p,p'-DDE is a potent androgen receptor antagonist. Nature. 1995 Jun 15;375(6532):581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- Klotz D. M., Beckman B. S., Hill S. M., McLachlan J. A., Walters M. R., Arnold S. F. Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ Health Perspect. 1996 Oct;104(10):1084–1089. doi: 10.1289/ehp.961041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno H., Gandini O., Curtis S. W., Korach K. S. Anti-estrogen activity in the yeast transcription system: estrogen receptor mediated agonist response. Steroids. 1994 Oct;59(10):572–578. doi: 10.1016/0039-128x(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Korach K. S., McLachlan J. A. Techniques for detection of estrogenicity. Environ Health Perspect. 1995 Oct;103 (Suppl 7):5–8. doi: 10.1289/ehp.95103s75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lech J. J., Lewis S. K., Ren L. In vivo estrogenic activity of nonylphenol in rainbow trout. Fundam Appl Toxicol. 1996 Apr;30(2):229–232. [PubMed] [Google Scholar]
- Levin W., Welch R. M., Conney A. H. Effect of carbon tetrachloride and other inhibitors of drug metabolism on the metabolism and action of estradiol-17 beta and estrone in the rat. J Pharmacol Exp Ther. 1970 Jun;173(2):247–255. [PubMed] [Google Scholar]
- Mellanen P., Petänen T., Lehtimäki J., Mäkelä S., Bylund G., Holmbom B., Mannila E., Oikari A., Santti R. Wood-derived estrogens: studies in vitro with breast cancer cell lines and in vivo in trout. Toxicol Appl Pharmacol. 1996 Feb;136(2):381–388. doi: 10.1006/taap.1996.0046. [DOI] [PubMed] [Google Scholar]
- Metzger D., Ali S., Bornert J. M., Chambon P. Characterization of the amino-terminal transcriptional activation function of the human estrogen receptor in animal and yeast cells. J Biol Chem. 1995 Apr 21;270(16):9535–9542. doi: 10.1074/jbc.270.16.9535. [DOI] [PubMed] [Google Scholar]
- Nagel S. C., vom Saal F. S., Thayer K. A., Dhar M. G., Boechler M., Welshons W. V. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ Health Perspect. 1997 Jan;105(1):70–76. doi: 10.1289/ehp.9710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimrod A. C., Benson W. H. Environmental estrogenic effects of alkylphenol ethoxylates. Crit Rev Toxicol. 1996 May;26(3):335–364. doi: 10.3109/10408449609012527. [DOI] [PubMed] [Google Scholar]
- Odum J., Lefevre P. A., Tittensor S., Paton D., Routledge E. J., Beresford N. A., Sumpter J. P., Ashby J. The rodent uterotrophic assay: critical protocol features, studies with nonyl phenols, and comparison with a yeast estrogenicity assay. Regul Toxicol Pharmacol. 1997 Apr;25(2):176–188. doi: 10.1006/rtph.1997.1100. [DOI] [PubMed] [Google Scholar]
- Olea N., Pulgar R., Pérez P., Olea-Serrano F., Rivas A., Novillo-Fertrell A., Pedraza V., Soto A. M., Sonnenschein C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996 Mar;104(3):298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousterhout J., Struck R. F., Nelson J. A. Estrogenic activities on methoxychlor metabolites. Biochem Pharmacol. 1981 Oct;30(20):2869–2871. doi: 10.1016/0006-2952(81)90429-9. [DOI] [PubMed] [Google Scholar]
- Perez P., Pulgar R., Olea-Serrano F., Villalobos M., Rivas A., Metzler M., Pedraza V., Olea N. The estrogenicity of bisphenol A-related diphenylalkanes with various substituents at the central carbon and the hydroxy groups. Environ Health Perspect. 1998 Mar;106(3):167–174. doi: 10.1289/ehp.98106167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit F., Valotaire Y., Pakdel F. Differential functional activities of rainbow trout and human estrogen receptors expressed in the yeast Saccharomyces cerevisiae. Eur J Biochem. 1995 Oct 15;233(2):584–592. doi: 10.1111/j.1432-1033.1995.584_2.x. [DOI] [PubMed] [Google Scholar]
- Przylipiak A., Rabe T., Hafner J., Przylipiak M., Runnebaum R. Influence of ethanol on in vitro growth of human mammary carcinoma cell line MCF-7. Arch Gynecol Obstet. 1996;258(3):137–140. doi: 10.1007/s004040050114. [DOI] [PubMed] [Google Scholar]
- Reel J. R., Lamb IV J. C., Neal B. H. Survey and assessment of mammalian estrogen biological assays for hazard characterization. Fundam Appl Toxicol. 1996 Dec;34(2):288–305. doi: 10.1006/faat.1996.0198. [DOI] [PubMed] [Google Scholar]
- Routledge E. J., Sumpter J. P. Structural features of alkylphenolic chemicals associated with estrogenic activity. J Biol Chem. 1997 Feb 7;272(6):3280–3288. doi: 10.1074/jbc.272.6.3280. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Fisher J. S., Millar M. M., Jobling S., Sumpter J. P. Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environ Health Perspect. 1995 Dec;103(12):1136–1143. doi: 10.1289/ehp.951031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby M. D., Newbold R. R., Tully D. B., Chae K., Davis V. L. Assessing environmental chemicals for estrogenicity using a combination of in vitro and in vivo assays. Environ Health Perspect. 1996 Dec;104(12):1296–1300. doi: 10.1289/ehp.961041296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A. M., Chung K. L., Sonnenschein C. The pesticides endosulfan, toxaphene, and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environ Health Perspect. 1994 Apr;102(4):380–383. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A. M., Justicia H., Wray J. W., Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect. 1991 May;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A. M., Murai J. T., Siiteri P. K., Sonnenschein C. Control of cell proliferation: evidence for negative control on estrogen-sensitive T47D human breast cancer cells. Cancer Res. 1986 May;46(5):2271–2275. [PubMed] [Google Scholar]
- Soto A. M., Sonnenschein C., Chung K. L., Fernandez M. F., Olea N., Serrano F. O. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995 Oct;103 (Suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter J. P. Feminized responses in fish to environmental estrogens. Toxicol Lett. 1995 Dec;82-83:737–742. doi: 10.1016/0378-4274(95)03517-6. [DOI] [PubMed] [Google Scholar]
- Sumpter J. P., Jobling S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect. 1995 Oct;103 (Suppl 7):173–178. doi: 10.1289/ehp.95103s7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada A., Sasaki H., Nakamura J., Yoshihama M., Terashima Y. Aromatase activity and the effect of estradiol and testosterone on DNA synthesis in endometrial carcinoma cell lines. J Steroid Biochem Mol Biol. 1993 Mar;44(4-6):661–666. doi: 10.1016/0960-0760(93)90276-3. [DOI] [PubMed] [Google Scholar]
- Thorpe S. M. Steroid receptors in breast cancer: sources of inter-laboratory variation in dextran-charcoal assays. Breast Cancer Res Treat. 1987;9(3):175–189. doi: 10.1007/BF01806378. [DOI] [PubMed] [Google Scholar]
- Tiemann U., Schneider F., Tuchscherer A. Effects of organochlorine pesticides on DNA synthesis of cultured oviductal and uterine cells and on estrogen receptor of uterine tissue from heifers. Arch Toxicol. 1996;70(8):490–496. doi: 10.1007/s002040050303. [DOI] [PubMed] [Google Scholar]
- Toppari J., Larsen J. C., Christiansen P., Giwercman A., Grandjean P., Guillette L. J., Jr, Jégou B., Jensen T. K., Jouannet P., Keiding N. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996 Aug;104 (Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos M., Olea N., Brotons J. A., Olea-Serrano M. F., Ruiz de Almodovar J. M., Pedraza V. The E-screen assay: a comparison of different MCF7 cell stocks. Environ Health Perspect. 1995 Sep;103(9):844–850. doi: 10.1289/ehp.95103844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade M. G., Desaulniers D., Leingartner K., Foster W. G. Interactions between endosulfan and dieldrin on estrogen-mediated processes in vitro and in vivo. Reprod Toxicol. 1997 Nov-Dec;11(6):791–798. doi: 10.1016/s0890-6238(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Welch R. M., Levin W., Conney A. H. Estrogenic action of DDT and its analogs. Toxicol Appl Pharmacol. 1969 Mar;14(2):358–367. doi: 10.1016/0041-008x(69)90117-3. [DOI] [PubMed] [Google Scholar]
- White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994 Jul;135(1):175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- Wiley H. S., Opresko L., Wallace R. A. New methods for the purification of vertebrate vitellogenin. Anal Biochem. 1979 Aug;97(1):145–152. doi: 10.1016/0003-2697(79)90338-5. [DOI] [PubMed] [Google Scholar]
- vom Saal F. S., Nagel S. C., Palanza P., Boechler M., Parmigiani S., Welshons W. V. Estrogenic pesticides: binding relative to estradiol in MCF-7 cells and effects of exposure during fetal life on subsequent territorial behaviour in male mice. Toxicol Lett. 1995 May;77(1-3):343–350. doi: 10.1016/0378-4274(95)03316-5. [DOI] [PubMed] [Google Scholar]