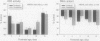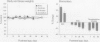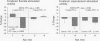Abstract
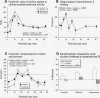
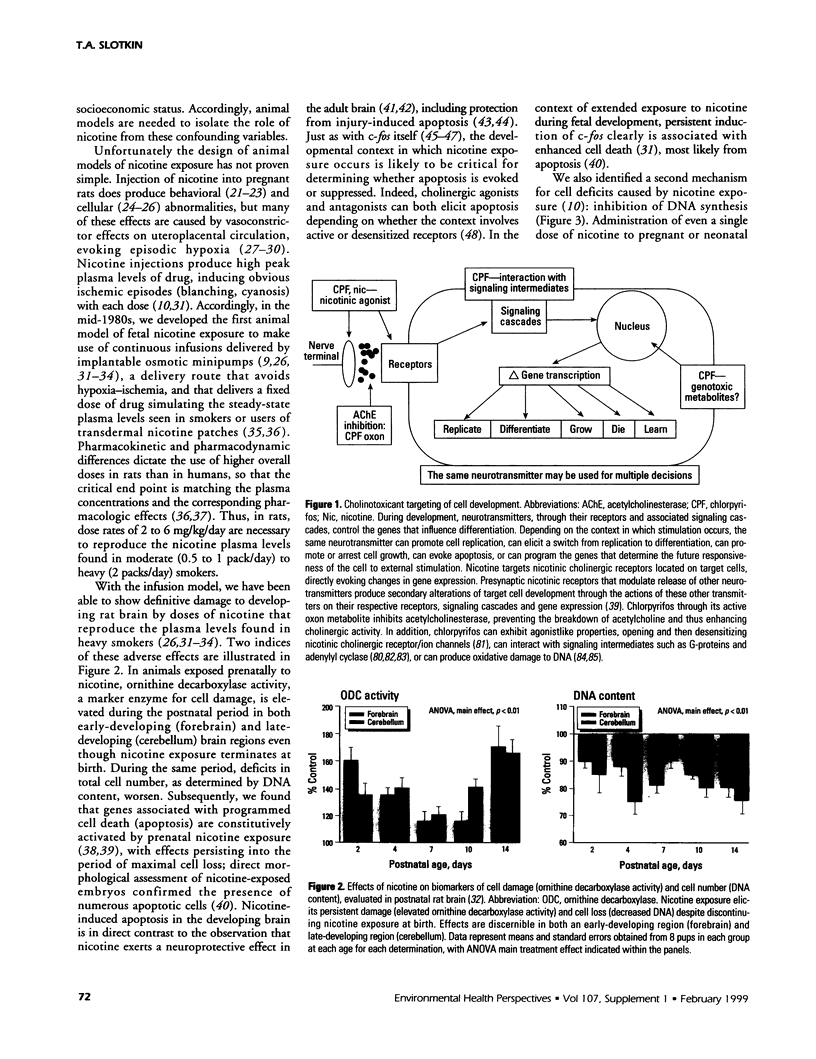
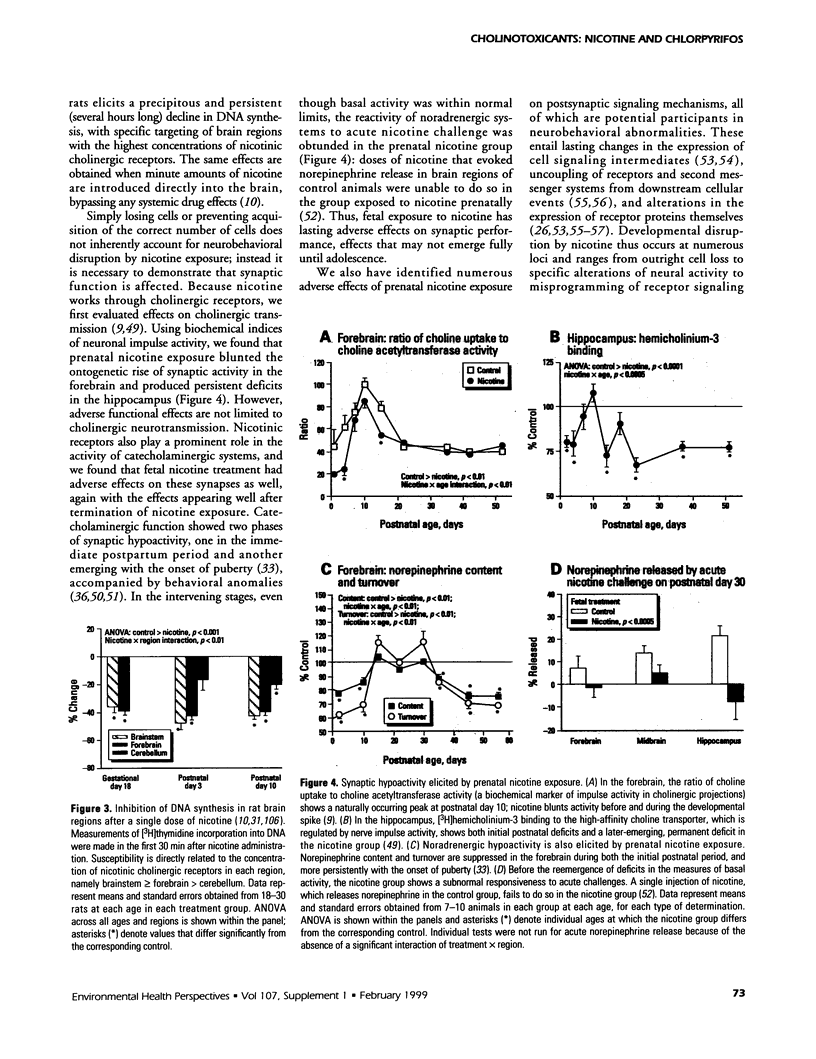
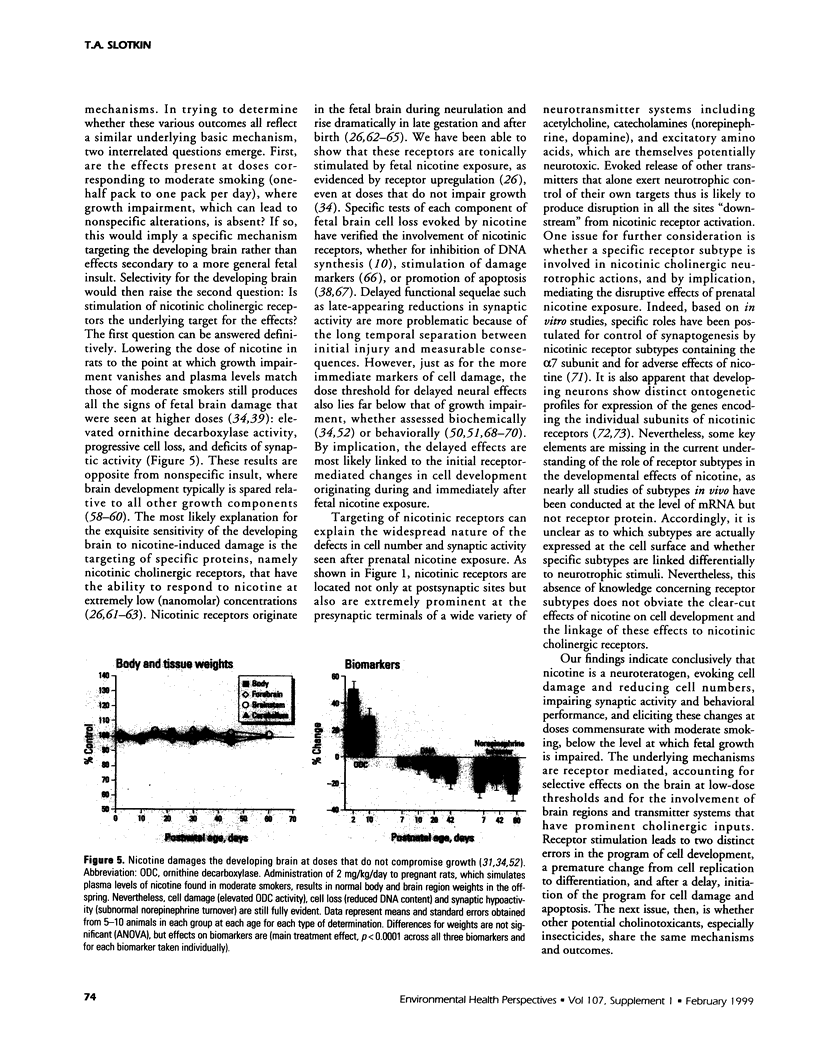
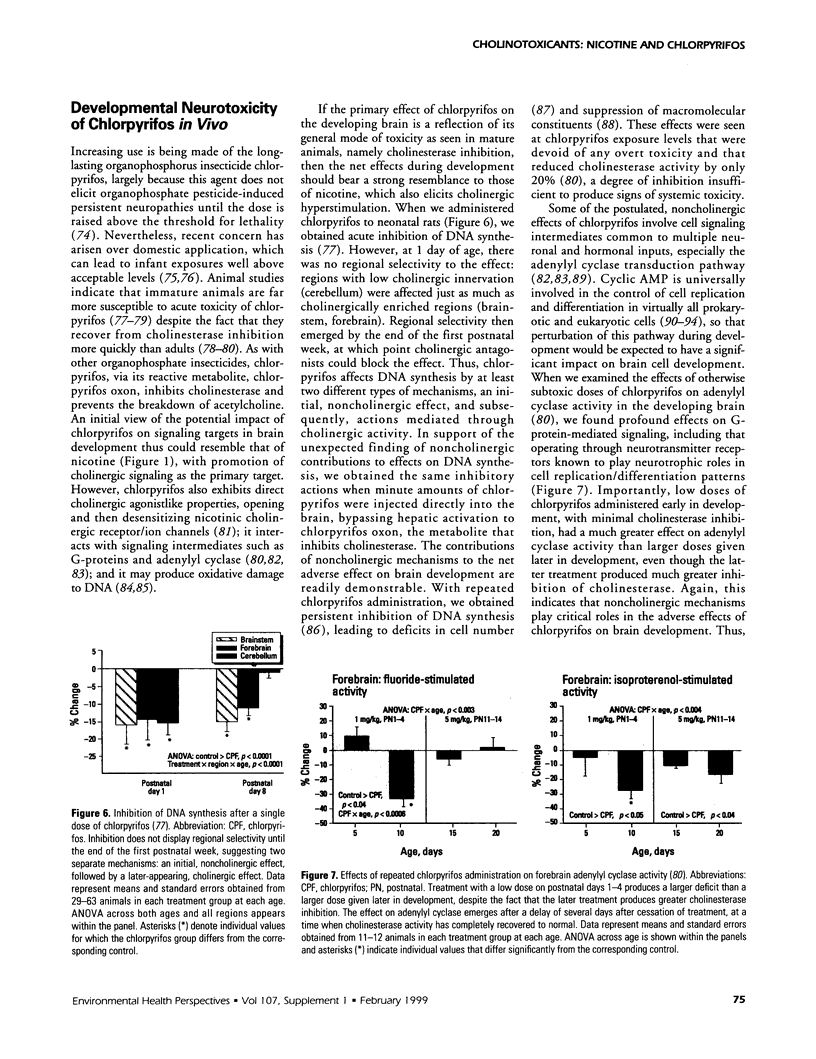
The stimulation of cholinergic receptors in target cells during a critical developmental period provides signals that influence cell replication and differentiation. Accordingly, environmental agents that promote cholinergic activity evoke neurodevelopmental damage because of the inappropriate timing or intensity of stimulation. Nicotine evokes mitotic arrest in brain cells possessing high concentrations of nicotinic cholinergic receptors. In addition, the cholinergic overstimulation programs the expression of genes that evoke apoptosis and delayed cell loss. Effects of cholinesterase inhibitors exhibit many similarities to those of nicotine. Chlorpyrifos administered to developing rats in doses that do not evoke signs of overt toxicity decreased DNA synthesis and caused shortfalls in cell numbers in brain regions enriched in cholinergic innervation. In embryo cultures, chlorpyrifos also evoked apoptosis during neurulation. However, chlorpyrifos also evokes noncholinergic disruption of cell development by interfering with cell signaling via adenylyl cyclase, leading to widespread disruption that is not limited to cholinergic systems. We have tested this hypothesis in vitro with PC12 cells, which lack the enzymes necessary to produce chlorpyrifos oxon, the metabolite that inhibits cholinesterase. Chlorpyrifos inhibited DNA synthesis in undifferentiated PC12 cells, which have relatively few cholinergic receptors. Furthermore, chlorpyrifos was more effective than nicotine and its effects were not blocked by cholinergic antagonists. When cells were allowed to differentiate in the presence of chlorpyrifos, cell replication was inhibited even more profoundly and cell acquisition was arrested. At higher concentrations, chlorpyrifos also inhibited neuritic outgrowth. Thus, chlorpyrifos elicits damage by both noncholinergic and cholinergic mechanisms extending from early stages of neural cell replication through late stages of axonogenesis and terminal differentiation. Accordingly, the window of developmental vulnerability to chlorpyrifos is likely to extend from the embryonic period into postnatal life.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUZNIKOV G. A., CHUDAKOVA I. V., ZVEZDINA N. D. THE R OLE OF NEUROHUMOURS IN EARLY EMBRYOGENESIS. I. SEROTONIN CONTENT OF DEVELOPING EMBRYOS OF SEA URCHIN AND LOACH. J Embryol Exp Morphol. 1964 Dec;12:563–573. [PubMed] [Google Scholar]
- Bachman E. S., Berger-Sweeney J., Coyle J. T., Hohmann C. F. Developmental regulation of adult cortical morphology and behavior: an animal model for mental retardation. Int J Dev Neurosci. 1994 Jun;12(4):239–253. doi: 10.1016/0736-5748(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Bagchi D., Bagchi M., Hassoun E. A., Stohs S. J. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995 Dec 15;104(1-3):129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- Bagchi D., Bhattacharya G., Stohs S. J. In vitro and in vivo induction of heat shock (stress) protein (Hsp) gene expression by selected pesticides. Toxicology. 1996 Aug 1;112(1):57–68. doi: 10.1016/0300-483x(96)03350-1. [DOI] [PubMed] [Google Scholar]
- Bardy A. H., Seppälä T., Lillsunde P., Kataja J. M., Koskela P., Pikkarainen J., Hiilesmaa V. K. Objectively measured tobacco exposure during pregnancy: neonatal effects and relation to maternal smoking. Br J Obstet Gynaecol. 1993 Aug;100(8):721–726. doi: 10.1111/j.1471-0528.1993.tb14262.x. [DOI] [PubMed] [Google Scholar]
- Bell G. L., Lau K. Perinatal and neonatal issues of substance abuse. Pediatr Clin North Am. 1995 Apr;42(2):261–281. doi: 10.1016/s0031-3955(16)38946-5. [DOI] [PubMed] [Google Scholar]
- Bell J. M., Lundberg P. K. Effects of a commercial soy lecithin preparation on development of sensorimotor behavior and brain biochemistry in the rat. Dev Psychobiol. 1985 Jan;18(1):59–66. doi: 10.1002/dev.420180105. [DOI] [PubMed] [Google Scholar]
- Bell J. M., Whitmore W. L., Barnes G., Seidler F. J., Slotkin T. A. Perinatal dietary exposure to soy lecithin: altered sensitivity to central cholinergic stimulation. Int J Dev Neurosci. 1986;4(6):497–501. doi: 10.1016/0736-5748(86)90001-8. [DOI] [PubMed] [Google Scholar]
- Bell J. M., Whitmore W. L., Queen K. L., Orband-Miller L., Slotkin T. A. Biochemical determinants of growth sparing during neonatal nutritional deprivation or enhancement: ornithine decarboxylase, polyamines, and macromolecules in brain regions and heart. Pediatr Res. 1987 Nov;22(5):599–604. doi: 10.1203/00006450-198711000-00024. [DOI] [PubMed] [Google Scholar]
- Berse B., Blusztajn J. K. Modulation of cholinergic locus expression by glucocorticoids and retinoic acid is cell-type specific. FEBS Lett. 1997 Jun 30;410(2-3):175–179. doi: 10.1016/s0014-5793(97)00568-1. [DOI] [PubMed] [Google Scholar]
- Bhat N. R., Shanker G., Pieringer R. A. Cell proliferation in growing cultures of dissociated embryonic mouse brain: macromolecule and ornithine decarboxylase synthesis and regulation by hormones and drugs. J Neurosci Res. 1983;10(2):221–230. doi: 10.1002/jnr.490100210. [DOI] [PubMed] [Google Scholar]
- Bushnell P. J., Pope C. N., Padilla S. Behavioral and neurochemical effects of acute chlorpyrifos in rats: tolerance to prolonged inhibition of cholinesterase. J Pharmacol Exp Ther. 1993 Aug;266(2):1007–1017. [PubMed] [Google Scholar]
- Butler N. R., Goldstein H. Smoking in pregnancy and subsequent child development. Br Med J. 1973 Dec 8;4(5892):573–575. doi: 10.1136/bmj.4.5892.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buznikov G. A., Kost A. N., Kucherova N. F., Mndzhoyan A. L., Suvorov N. N., Berdysheva L. V. The role of neurohumours in early embryogenesis. 3. Pharmacological analysis of the role of neurohumours in cleavage divisions. J Embryol Exp Morphol. 1970 Jun;23(3):549–569. [PubMed] [Google Scholar]
- Campbell C. G., Seidler F. J., Slotkin T. A. Chlorpyrifos interferes with cell development in rat brain regions. Brain Res Bull. 1997;43(2):179–189. doi: 10.1016/s0361-9230(96)00436-4. [DOI] [PubMed] [Google Scholar]
- Carlos R. Q., Seidler F. J., Lappi S. E., Slotkin T. A. Fetal dexamethasone exposure affects basal ornithine decarboxylase activity in developing rat brain regions and alters acute responses to hypoxia and maternal separation. Biol Neonate. 1991;59(2):69–77. doi: 10.1159/000243325. [DOI] [PubMed] [Google Scholar]
- Chakraborti T. K., Farrar J. D., Pope C. N. Comparative neurochemical and neurobehavioral effects of repeated chlorpyrifos exposures in young and adult rats. Pharmacol Biochem Behav. 1993 Sep;46(1):219–224. doi: 10.1016/0091-3057(93)90344-s. [DOI] [PubMed] [Google Scholar]
- Claycomb W. C. Biochemical aspects of cardiac muscle differentiation. Possible control of deoxyribonucleic acid synthesis and cell differentiation by adrenergic innervation and cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1976 Oct 10;251(19):6082–6089. [PubMed] [Google Scholar]
- Cosenza M. E., Bidanset J. Effects of chlorpyrifos on neuronal development in rat embryo midbrain micromass cultures. Vet Hum Toxicol. 1995 Apr;37(2):118–121. [PubMed] [Google Scholar]
- Curran T., Abate C., Cohen D. R., Macgregor P. F., Rauscher F. J., 3rd, Sonnenberg J. L., Connor J. A., Morgan J. I. Inducible proto-oncogene transcription factors: third messengers in the brain? Cold Spring Harb Symp Quant Biol. 1990;55:225–234. doi: 10.1101/sqb.1990.055.01.024. [DOI] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Fos: an immediate-early transcription factor in neurons. J Neurobiol. 1995 Mar;26(3):403–412. doi: 10.1002/neu.480260312. [DOI] [PubMed] [Google Scholar]
- Cutler A. R., Wilkerson A. E., Gingras J. L., Levin E. D. Prenatal cocaine and/or nicotine exposure in rats: preliminary findings on long-term cognitive outcome and genital development at birth. Neurotoxicol Teratol. 1996 Nov-Dec;18(6):635–643. doi: 10.1016/s0892-0362(96)00125-0. [DOI] [PubMed] [Google Scholar]
- Dam K., Seidler F. J., Slotkin T. A. Developmental neurotoxicity of chlorpyrifos: delayed targeting of DNA synthesis after repeated administration. Brain Res Dev Brain Res. 1998 Jun 15;108(1-2):39–45. doi: 10.1016/s0165-3806(98)00028-5. [DOI] [PubMed] [Google Scholar]
- DiFranza J. R., Lew R. A. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. J Fam Pract. 1995 Apr;40(4):385–394. [PubMed] [Google Scholar]
- Dunn H. G., McBurney A. K. Cigarette smoking and the fetus and child. Pediatrics. 1977 Nov;60(5):772–772. [PubMed] [Google Scholar]
- Fenske R. A., Black K. G., Elkner K. P., Lee C. L., Methner M. M., Soto R. Potential exposure and health risks of infants following indoor residential pesticide applications. Am J Public Health. 1990 Jun;80(6):689–693. doi: 10.2105/ajph.80.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Rukenstein A. Regulation of acetylcholinesterase activity by nerve growth factor. Role of transcription and dissociation from effects on proliferation and neurite outgrowth. J Biol Chem. 1981 Jun 25;256(12):6363–6367. [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Weiss B., Costa E. Adenosine 3',5'-monophosphate concentrations and isoproterenol-induced synthesis of deoxyribonucleic acid in mouse parotid gland. Mol Pharmacol. 1972 Sep;8(5):521–530. [PubMed] [Google Scholar]
- Gurunathan S., Robson M., Freeman N., Buckley B., Roy A., Meyer R., Bukowski J., Lioy P. J. Accumulation of chlorpyrifos on residential surfaces and toys accessible to children. Environ Health Perspect. 1998 Jan;106(1):9–16. doi: 10.1289/ehp.981069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström-Lindahl E., Gorbounova O., Seiger A., Mousavi M., Nordberg A. Regional distribution of nicotinic receptors during prenatal development of human brain and spinal cord. Brain Res Dev Brain Res. 1998 Jun 15;108(1-2):147–160. doi: 10.1016/s0165-3806(98)00046-7. [DOI] [PubMed] [Google Scholar]
- Hohmann C. F., Brooks A. R., Coyle J. T. Neonatal lesions of the basal forebrain cholinergic neurons result in abnormal cortical development. Brain Res. 1988 Aug 1;470(2):253–264. doi: 10.1016/0165-3806(88)90244-1. [DOI] [PubMed] [Google Scholar]
- Huff R. A., Abou-Donia M. B. In vitro effect of chlorpyrifos oxon on muscarinic receptors and adenylate cyclase. Neurotoxicology. 1995 Summer;16(2):281–290. [PubMed] [Google Scholar]
- Huff R. A., Corcoran J. J., Anderson J. K., Abou-Donia M. B. Chlorpyrifos oxon binds directly to muscarinic receptors and inhibits cAMP accumulation in rat striatum. J Pharmacol Exp Ther. 1994 Apr;269(1):329–335. [PubMed] [Google Scholar]
- Höhmann C. F., Wilson L., Coyle J. T. Efferent and afferent connections of mouse sensory-motor cortex following cholinergic deafferentation at birth. Cereb Cortex. 1991 Mar-Apr;1(2):158–172. doi: 10.1093/cercor/1.2.158. [DOI] [PubMed] [Google Scholar]
- Janson A. M., Fuxe K., Agnati L. F., Kitayama I., Härfstrand A., Andersson K., Goldstein M. Chronic nicotine treatment counteracts the disappearance of tyrosine-hydroxylase-immunoreactive nerve cell bodies, dendrites and terminals in the mesostriatal dopamine system of the male rat after partial hemitransection. Brain Res. 1988 Jul 12;455(2):332–345. doi: 10.1016/0006-8993(88)90092-3. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Seidler F. J., Slotkin T. A. Early biochemical detection of delayed neurotoxicity resulting from developmental exposure to chloropyrifos. Brain Res Bull. 1998;45(2):143–147. doi: 10.1016/s0361-9230(97)00329-8. [DOI] [PubMed] [Google Scholar]
- Jonsson G., Hallman H. Effects of neonatal nicotine administration on the postnatal development of central noradrenaline neurons. Acta Physiol Scand Suppl. 1980;479:25–26. [PubMed] [Google Scholar]
- Kaneko S., Maeda T., Kume T., Kochiyama H., Akaike A., Shimohama S., Kimura J. Nicotine protects cultured cortical neurons against glutamate-induced cytotoxicity via alpha7-neuronal receptors and neuronal CNS receptors. Brain Res. 1997 Aug 8;765(1):135–140. doi: 10.1016/s0006-8993(97)00556-8. [DOI] [PubMed] [Google Scholar]
- Katz E. J., Cortes V. I., Eldefrawi M. E., Eldefrawi A. T. Chlorpyrifos, parathion, and their oxons bind to and desensitize a nicotinic acetylcholine receptor: relevance to their toxicities. Toxicol Appl Pharmacol. 1997 Oct;146(2):227–236. doi: 10.1006/taap.1997.8201. [DOI] [PubMed] [Google Scholar]
- Lauder J. M. Roles for neurotransmitters in development: possible interaction with drugs during the fetal and neonatal periods. Prog Clin Biol Res. 1985;163C:375–380. [PubMed] [Google Scholar]
- Levin E. D., Briggs S. J., Christopher N. C., Rose J. E. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol Teratol. 1993 Jul-Aug;15(4):251–260. doi: 10.1016/0892-0362(93)90006-a. [DOI] [PubMed] [Google Scholar]
- Levin E. D., Wilkerson A., Jones J. P., Christopher N. C., Briggs S. J. Prenatal nicotine effects on memory in rats: pharmacological and behavioral challenges. Brain Res Dev Brain Res. 1996 Dec 23;97(2):207–215. doi: 10.1016/s0165-3806(96)00144-7. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W., Ribary U., Schlumpf M., Odermatt B., Widmer H. R. Prenatal adverse effects of nicotine on the developing brain. Prog Brain Res. 1988;73:137–157. doi: 10.1016/S0079-6123(08)60502-6. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W., Schlumpf M. Prenatal nicotine affects fetal testosterone and sexual dimorphism of saccharin preference. Pharmacol Biochem Behav. 1985 Sep;23(3):439–444. doi: 10.1016/0091-3057(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W., Schlumpf M., Ribary U. Modifications pharmacologiques de l'ontogenèse neuroendocrine. D'eveloppement de récepteurs, nicotine et catécholamines. Ann Endocrinol (Paris) 1987;48(5):393–399. [PubMed] [Google Scholar]
- Mapoles J., Berthou F., Alexander A., Simon F., Ménez J. F. Mammalian PC-12 cell genetically engineered for human cytochrome P450 2E1 expression. Eur J Biochem. 1993 Jun 15;214(3):735–745. doi: 10.1111/j.1432-1033.1993.tb17975.x. [DOI] [PubMed] [Google Scholar]
- Martin J. C., Becker R. F. The effects of maternal nicotine absorption or hypoxic episodes upon appetitive behavior of rat offspring. Dev Psychobiol. 1971;4(2):133–147. doi: 10.1002/dev.420040205. [DOI] [PubMed] [Google Scholar]
- Martino-Barrows A. M., Kellar K. J. [3H]acetylcholine and [3H](-)nicotine label the same recognition site in rat brain. Mol Pharmacol. 1987 Feb;31(2):169–174. [PubMed] [Google Scholar]
- McFarland B. J., Seidler F. J., Slotkin T. A. Inhibition of DNA synthesis in neonatal rat brain regions caused by acute nicotine administration. Brain Res Dev Brain Res. 1991 Feb 22;58(2):223–229. doi: 10.1016/0165-3806(91)90008-7. [DOI] [PubMed] [Google Scholar]
- Murrin L. C., Ferrer J. R., Zeng W. Y., Haley N. J. Nicotine administration to rats: methodological considerations. Life Sci. 1987 Apr 27;40(17):1699–1708. doi: 10.1016/0024-3205(87)90020-8. [DOI] [PubMed] [Google Scholar]
- Muscarella D. E., Keown J. F., Bloom S. E. Evaluation of the genotoxic and embryotoxic potential of chlorpyrifos and its metabolites in vivo and in vitro. Environ Mutagen. 1984;6(1):13–23. doi: 10.1002/em.2860060103. [DOI] [PubMed] [Google Scholar]
- Naeye R. L. Cognitive and behavioral abnormalities in children whose mothers smoked cigarettes during pregnancy. J Dev Behav Pediatr. 1992 Dec;13(6):425–428. [PubMed] [Google Scholar]
- Naeye R. L. Effects of maternal cigarette smoking on the fetus and placenta. Br J Obstet Gynaecol. 1978 Oct;85(10):732–737. doi: 10.1111/j.1471-0528.1978.tb15593.x. [DOI] [PubMed] [Google Scholar]
- Naeye R. L., Peters E. C. Mental development of children whose mothers smoked during pregnancy. Obstet Gynecol. 1984 Nov;64(5):601–607. [PubMed] [Google Scholar]
- Nasrat H. A., Al-Hachim G. M., Mahmood F. A. Perinatal effects of nicotine. Biol Neonate. 1986;49(1):8–14. doi: 10.1159/000242503. [DOI] [PubMed] [Google Scholar]
- Navarro H. A., Mills E., Seidler F. J., Baker F. E., Lappi S. E., Tayyeb M. I., Spencer J. R., Slotkin T. A. Prenatal nicotine exposure impairs beta-adrenergic function: persistent chronotropic subsensitivity despite recovery from deficits in receptor binding. Brain Res Bull. 1990 Aug;25(2):233–237. doi: 10.1016/0361-9230(90)90066-9. [DOI] [PubMed] [Google Scholar]
- Navarro H. A., Seidler F. J., Eylers J. P., Baker F. E., Dobbins S. S., Lappi S. E., Slotkin T. A. Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in developing brain. J Pharmacol Exp Ther. 1989 Dec;251(3):894–900. [PubMed] [Google Scholar]
- Navarro H. A., Seidler F. J., Schwartz R. D., Baker F. E., Dobbins S. S., Slotkin T. A. Prenatal exposure to nicotine impairs nervous system development at a dose which does not affect viability or growth. Brain Res Bull. 1989 Sep;23(3):187–192. doi: 10.1016/0361-9230(89)90146-9. [DOI] [PubMed] [Google Scholar]
- Navarro H. A., Seidler F. J., Whitmore W. L., Slotkin T. A. Prenatal exposure to nicotine via maternal infusions: effects on development of catecholamine systems. J Pharmacol Exp Ther. 1988 Mar;244(3):940–944. [PubMed] [Google Scholar]
- Owman C., Fuxe K., Janson A. M., Kåhrström J. Chronic nicotine treatment eliminates asymmetry in striatal glucose utilization following unilateral transection of the mesostriatal dopamine pathway in rats. Neurosci Lett. 1989 Jul 31;102(2-3):279–283. doi: 10.1016/0304-3940(89)90092-x. [DOI] [PubMed] [Google Scholar]
- Pope C. N., Chakraborti T. K., Chapman M. L., Farrar J. D., Arthun D. Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothioate insecticides. Toxicology. 1991;68(1):51–61. doi: 10.1016/0300-483x(91)90061-5. [DOI] [PubMed] [Google Scholar]
- Pope C. N., Chakraborti T. K. Dose-related inhibition of brain and plasma cholinesterase in neonatal and adult rats following sublethal organophosphate exposures. Toxicology. 1992;73(1):35–43. doi: 10.1016/0300-483x(92)90168-e. [DOI] [PubMed] [Google Scholar]
- Pugh P. C., Berg D. K. Neuronal acetylcholine receptors that bind alpha-bungarotoxin mediate neurite retraction in a calcium-dependent manner. J Neurosci. 1994 Feb;14(2):889–896. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribary U., Lichtensteiger W. Effects of acute and chronic prenatal nicotine treatment on central catecholamine systems of male and female rat fetuses and offspring. J Pharmacol Exp Ther. 1989 Feb;248(2):786–792. [PubMed] [Google Scholar]
- Richardson R. J., Moore T. B., Kayyali U. S., Randall J. C. Chlorpyrifos: assessment of potential for delayed neurotoxicity by repeated dosing in adult hens with monitoring of brain acetylcholinesterase, brain and lymphocyte neurotoxic esterase, and plasma butyrylcholinesterase activities. Fundam Appl Toxicol. 1993 Jul;21(1):89–96. doi: 10.1006/faat.1993.1076. [DOI] [PubMed] [Google Scholar]
- Roy T. S., Andrews J. E., Seidler F. J., Slotkin T. A. Chlorpyrifos elicits mitotic abnormalities and apoptosis in neuroepithelium of cultured rat embryos. Teratology. 1998 Aug;58(2):62–68. doi: 10.1002/(SICI)1096-9926(199808)58:2<62::AID-TERA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Scotting P. J., Rex M. Transcription factors in early development of the central nervous system. Neuropathol Appl Neurobiol. 1996 Dec;22(6):469–481. doi: 10.1111/j.1365-2990.1996.tb01118.x. [DOI] [PubMed] [Google Scholar]
- Seidler F. J., Levin E. D., Lappi S. E., Slotkin T. A. Fetal nicotine exposure ablates the ability of postnatal nicotine challenge to release norepinephrine from rat brain regions. Brain Res Dev Brain Res. 1992 Oct 23;69(2):288–291. doi: 10.1016/0165-3806(92)90170-2. [DOI] [PubMed] [Google Scholar]
- Seidler F. J., Slotkin T. A. Effects of acute hypoxia on neonatal rat brain: regionally selective, long-term alterations in catecholamine levels and turnover. Brain Res Bull. 1990 Feb;24(2):157–161. doi: 10.1016/0361-9230(90)90200-j. [DOI] [PubMed] [Google Scholar]
- Shacka J. J., Robinson S. E. Postnatal developmental regulation of neuronal nicotinic receptor subunit alpha 7 and multiple alpha 4 and beta 2 mRNA species in the rat. Brain Res Dev Brain Res. 1998 Jul 1;109(1):67–75. doi: 10.1016/s0165-3806(98)00058-3. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Cho H., Whitmore W. L. Effects of prenatal nicotine exposure on neuronal development: selective actions on central and peripheral catecholaminergic pathways. Brain Res Bull. 1987 May;18(5):601–611. doi: 10.1016/0361-9230(87)90130-4. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Cowdery T. S., Orband L., Pachman S., Whitmore W. L. Effects of neonatal hypoxia on brain development in the rat: immediate and long-term biochemical alterations in discrete regions. Brain Res. 1986 May 21;374(1):63–74. doi: 10.1016/0006-8993(86)90395-1. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998 Jun;285(3):931–945. [PubMed] [Google Scholar]
- Slotkin T. A., Greer N., Faust J., Cho H., Seidler F. J. Effects of maternal nicotine injections on brain development in the rat: ornithine decarboxylase activity, nucleic acids and proteins in discrete brain regions. Brain Res Bull. 1986 Jul;17(1):41–50. doi: 10.1016/0361-9230(86)90159-0. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., McCook E. C., Lappi S. E., Seidler F. J. Altered development of basal and forskolin-stimulated adenylate cyclase activity in brain regions of rats exposed to nicotine prenatally. Brain Res Dev Brain Res. 1992 Aug 21;68(2):233–239. doi: 10.1016/0165-3806(92)90065-5. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., McCook E. C., Seidler F. J. Cryptic brain cell injury caused by fetal nicotine exposure is associated with persistent elevations of c-fos protooncogene expression. Brain Res. 1997 Mar 7;750(1-2):180–188. doi: 10.1016/s0006-8993(96)01345-5. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Navarro H. A., McCook E. C., Seidler F. J. Fetal nicotine exposure produces postnatal up-regulation of adenylate cyclase activity in peripheral tissues. Life Sci. 1990;47(17):1561–1567. doi: 10.1016/0024-3205(90)90185-t. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Orband-Miller L., Queen K. L. Development of [3H]nicotine binding sites in brain regions of rats exposed to nicotine prenatally via maternal injections or infusions. J Pharmacol Exp Ther. 1987 Jul;242(1):232–237. [PubMed] [Google Scholar]
- Slotkin T. A., Orband-Miller L., Queen K. L., Whitmore W. L., Seidler F. J. Effects of prenatal nicotine exposure on biochemical development of rat brain regions: maternal drug infusions via osmotic minipumps. J Pharmacol Exp Ther. 1987 Feb;240(2):602–611. [PubMed] [Google Scholar]
- Smith W. T., 4th, Seidler F. J., Slotkin T. A. Acute stimulation of ornithine decarboxylase in neonatal rat brain regions by nicotine: a central receptor-mediated process? Brain Res Dev Brain Res. 1991 Nov 19;63(1-2):85–93. doi: 10.1016/0165-3806(91)90069-u. [DOI] [PubMed] [Google Scholar]
- Song X., Seidler F. J., Saleh J. L., Zhang J., Padilla S., Slotkin T. A. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. 1997 Jul;145(1):158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Song X., Violin J. D., Seidler F. J., Slotkin T. A. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol Appl Pharmacol. 1998 Jul;151(1):182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- Tischler A. S., Greene L. A. Nerve growth factor-induced process formation by cultured rat pheochromocytoma cells. Nature. 1975 Nov 27;258(5533):341–342. doi: 10.1038/258341a0. [DOI] [PubMed] [Google Scholar]
- Tolson C. M., Seidler F. J., McCook E. C., Slotkin T. A. Does concurrent or prior nicotine exposure interact with neonatal hypoxia to produce cardiac cell damage? Teratology. 1995 Nov;52(5):298–305. doi: 10.1002/tera.1420520508. [DOI] [PubMed] [Google Scholar]
- Ward T. R., Mundy W. R. Organophosphorus compounds preferentially affect second messenger systems coupled to M2/M4 receptors in rat frontal cortex. Brain Res Bull. 1996;39(1):49–55. doi: 10.1016/0361-9230(95)02044-6. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia P. M. Role of serotonin and other neurotransmitter receptors in brain development: basis for developmental pharmacology. Pharmacol Rev. 1991 Dec;43(4):553–561. [PubMed] [Google Scholar]
- Whitney K. D., Seidler F. J., Slotkin T. A. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995 Sep;134(1):53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- Yamashita H., Nakamura S. Nicotine rescues PC12 cells from death induced by nerve growth factor deprivation. Neurosci Lett. 1996 Aug 2;213(2):145–147. doi: 10.1016/0304-3940(96)12829-9. [DOI] [PubMed] [Google Scholar]
- Zahalka E. A., Seidler F. J., Lappi S. E., McCook E. C., Yanai J., Slotkin T. A. Deficits in development of central cholinergic pathways caused by fetal nicotine exposure: differential effects on choline acetyltransferase activity and [3H]hemicholinium-3 binding. Neurotoxicol Teratol. 1992 Nov-Dec;14(6):375–382. doi: 10.1016/0892-0362(92)90047-e. [DOI] [PubMed] [Google Scholar]
- Zahalka E. A., Seidler F. J., Yanai J., Slotkin T. A. Fetal nicotine exposure alters ontogeny of M1-receptors and their link to G-proteins. Neurotoxicol Teratol. 1993 Mar-Apr;15(2):107–115. doi: 10.1016/0892-0362(93)90069-z. [DOI] [PubMed] [Google Scholar]
- de Grauw T. J., Myers R. E., Scott W. J. Fetal growth retardation in rats from different levels of hypoxia. Biol Neonate. 1986;49(2):85–89. doi: 10.1159/000242515. [DOI] [PubMed] [Google Scholar]
- van Wijk R., Wicks W. D., Bevers M. M., van Rijn J. Rapid arrest of DNA synthesis by N 6 ,O 2' -dibutyryl cyclic adenosine 3',5'-monophosphate in cultured hepatoma cells. Cancer Res. 1973 Jun;33(6):1331–1338. [PubMed] [Google Scholar]