Abstract
Prostate cancer (PC) is an escalating health burden in the western world. A large number of patients still present with extraprostatic (i.e., T3/T4, N0, M0/M1 or any T category and M1 disease or involved lymph nodes) and therefore incurable disease. Since the work of Huggins in 1940, there have been no major therapeutic advances and androgen ablation remains the best treatment option for extraprostatic androgen-responsive PC. Eighty to ninety percent of PC patients respond well to this form of treatment initially. After a median time of approximately 2 years, however, relapse to an androgen-independent (AI) state occurs, followed by death after a further median 6 months. Androgen ablation is rarely curative. The major molecular defect in extraprostatic and AI PC is the inability of PC cells to initiate apoptosis in response to a variety of stimuli, including different forms of androgen ablation and cytotoxic agents. The balance between cellular proliferation and cell death is regulated by multiple genes or families of genes through the cell cycle. The exact mechanisms governing this intricate and complex process are as yet not fully understood. One family of genes involved in cell survival/death control is the Bcl-2 gene family, which consists of homologous proteins that function to regulate distal and crucial commitment steps of the apoptotic pathway. The Bcl-2 family constitutes both agonists and antagonists of apoptosis that function at least in part through protein-protein interactions between various members of the family. The final outcome depends on the relative ratio of death agonists and antagonists. Bcl-2 expression has been closely associated with the AI phenotype of PC. Cytotoxic chemotherapy may be used as palliative therapy in AI PC but has not been found effective. Most chemotherapeutic cytotoxic agents induce apoptosis in cancer cells by direct and indirect action on the cell cycle. In vitro and in vivo studies have established that Bcl-2 expression confers an antiapoptotic activity against androgen withdrawal and cytotoxic chemotherapy. It thus offers a tempting potential target for therapeutic manipulations of PC.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Livingston D. J., Nicholson D. W., Salvesen G., Thornberry N. A., Wong W. W., Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996 Oct 18;87(2):171–171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- Antonsson B., Conti F., Ciavatta A., Montessuit S., Lewis S., Martinou I., Bernasconi L., Bernard A., Mermod J. J., Mazzei G. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997 Jul 18;277(5324):370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- Apakama I., Robinson M. C., Walter N. M., Charlton R. G., Royds J. A., Fuller C. E., Neal D. E., Hamdy F. C. bcl-2 overexpression combined with p53 protein accumulation correlates with hormone-refractory prostate cancer. Br J Cancer. 1996 Oct;74(8):1258–1262. doi: 10.1038/bjc.1996.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffy G., Miyashita T., Williamson J. R., Reed J. C. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993 Mar 25;268(9):6511–6519. [PubMed] [Google Scholar]
- Bargou R. C., Bommert K., Weinmann P., Daniel P. T., Wagener C., Mapara M. Y., Dörken B. Induction of Bax-alpha precedes apoptosis in a human B lymphoma cell line: potential role of the bcl-2 gene family in surface IgM-mediated apoptosis. Eur J Immunol. 1995 Mar;25(3):770–775. doi: 10.1002/eji.1830250322. [DOI] [PubMed] [Google Scholar]
- Bargou R. C., Wagener C., Bommert K., Mapara M. Y., Daniel P. T., Arnold W., Dietel M., Guski H., Feller A., Royer H. D. Overexpression of the death-promoting gene bax-alpha which is downregulated in breast cancer restores sensitivity to different apoptotic stimuli and reduces tumor growth in SCID mice. J Clin Invest. 1996 Jun 1;97(11):2651–2659. doi: 10.1172/JCI118715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr P. J., Tomei L. D. Apoptosis and its role in human disease. Biotechnology (N Y) 1994 May;12(5):487–493. doi: 10.1038/nbt0594-487. [DOI] [PubMed] [Google Scholar]
- Ben-Ezra J. M., Kornstein M. J., Grimes M. M., Krystal G. Small cell carcinomas of the lung express the Bcl-2 protein. Am J Pathol. 1994 Nov;145(5):1036–1040. [PMC free article] [PubMed] [Google Scholar]
- Berchem G. J., Bosseler M., Sugars L. Y., Voeller H. J., Zeitlin S., Gelmann E. P. Androgens induce resistance to bcl-2-mediated apoptosis in LNCaP prostate cancer cells. Cancer Res. 1995 Feb 15;55(4):735–738. [PubMed] [Google Scholar]
- Berges R. R., Vukanovic J., Epstein J. I., CarMichel M., Cisek L., Johnson D. E., Veltri R. W., Walsh P. C., Isaacs J. T. Implication of cell kinetic changes during the progression of human prostatic cancer. Clin Cancer Res. 1995 May;1(5):473–480. [PMC free article] [PubMed] [Google Scholar]
- Bhargava V., Kell D. L., van de Rijn M., Warnke R. A. Bcl-2 immunoreactivity in breast carcinoma correlates with hormone receptor positivity. Am J Pathol. 1994 Sep;145(3):535–540. [PMC free article] [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Boulakia C. A., Chen G., Ng F. W., Teodoro J. G., Branton P. E., Nicholson D. W., Poirier G. G., Shore G. C. Bcl-2 and adenovirus E1B 19 kDA protein prevent E1A-induced processing of CPP32 and cleavage of poly(ADP-ribose) polymerase. Oncogene. 1996 Feb 1;12(3):529–535. [PubMed] [Google Scholar]
- Boyd J. M., Gallo G. J., Elangovan B., Houghton A. B., Malstrom S., Avery B. J., Ebb R. G., Subramanian T., Chittenden T., Lutz R. J. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995 Nov 2;11(9):1921–1928. [PubMed] [Google Scholar]
- Brinkmann A. O., Jenster G., Ris-Stalpers C., van der Korput J. A., Brüggenwirth H. T., Boehmer A. L., Trapman J. Androgen receptor mutations. J Steroid Biochem Mol Biol. 1995 Jun;53(1-6):443–448. doi: 10.1016/0960-0760(95)00090-m. [DOI] [PubMed] [Google Scholar]
- Bronner M. P., Culin C., Reed J. C., Furth E. E. The bcl-2 proto-oncogene and the gastrointestinal epithelial tumor progression model. Am J Pathol. 1995 Jan;146(1):20–26. [PMC free article] [PubMed] [Google Scholar]
- Bubendorf L., Sauter G., Moch H., Jordan P., Blöchlinger A., Gasser T. C., Mihatsch M. J. Prognostic significance of Bcl-2 in clinically localized prostate cancer. Am J Pathol. 1996 May;148(5):1557–1565. [PMC free article] [PubMed] [Google Scholar]
- Byrne R. L., Horne C. H., Robinson M. C., Autzen P., Apakama I., Bishop R. I., Neal D. E., Hamdy F. C. The expression of waf-1, p53 and bcl-2 in prostatic adenocarcinoma. Br J Urol. 1997 Feb;79(2):190–195. doi: 10.1046/j.1464-410x.1997.03399.x. [DOI] [PubMed] [Google Scholar]
- Campos L., Sabido O., Rouault J. P., Guyotat D. Effects of BCL-2 antisense oligodeoxynucleotides on in vitro proliferation and survival of normal marrow progenitors and leukemic cells. Blood. 1994 Jul 15;84(2):595–600. [PubMed] [Google Scholar]
- Carter B. S., Beaty T. H., Steinberg G. D., Childs B., Walsh P. C. Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3367–3371. doi: 10.1073/pnas.89.8.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary K. S., Lu Q. L., Abel P. D., Khandan-Nia N., Shoma A. M., el Baz M., Stamp G. W., Lalani E. N. Expression of bcl-2 and p53 oncoproteins in schistosomiasis-associated transitional and squamous cell carcinoma of urinary bladder. Br J Urol. 1997 Jan;79(1):78–84. doi: 10.1046/j.1464-410x.1997.30717.x. [DOI] [PubMed] [Google Scholar]
- Chittenden T., Harrington E. A., O'Connor R., Flemington C., Lutz R. J., Evan G. I., Guild B. C. Induction of apoptosis by the Bcl-2 homologue Bak. Nature. 1995 Apr 20;374(6524):733–736. doi: 10.1038/374733a0. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Smith S. D., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Colombel M., Symmans F., Gil S., O'Toole K. M., Chopin D., Benson M., Olsson C. A., Korsmeyer S., Buttyan R. Detection of the apoptosis-suppressing oncoprotein bc1-2 in hormone-refractory human prostate cancers. Am J Pathol. 1993 Aug;143(2):390–400. [PMC free article] [PubMed] [Google Scholar]
- Denmeade S. R., Isaacs J. T. Activation of programmed (apoptotic) cell death for the treatment of prostate cancer. Adv Pharmacol. 1996;35:281–306. doi: 10.1016/s1054-3589(08)60278-1. [DOI] [PubMed] [Google Scholar]
- Devesa S. S., Blot W. J., Stone B. J., Miller B. A., Tarone R. E., Fraumeni J. F., Jr Recent cancer trends in the United States. J Natl Cancer Inst. 1995 Feb 1;87(3):175–182. doi: 10.1093/jnci/87.3.175. [DOI] [PubMed] [Google Scholar]
- Distelhorst C. W., Lam M., McCormick T. S. Bcl-2 inhibits hydrogen peroxide-induced ER Ca2+ pool depletion. Oncogene. 1996 May 16;12(10):2051–2055. [PubMed] [Google Scholar]
- Dorai T., Goluboff E. T., Olsson C. A., Buttyan R. Development of a hammerhead ribozyme against BCL-2. II. Ribozyme treatment sensitizes hormone-resistant prostate cancer cells to apoptotic agents. Anticancer Res. 1997 Sep-Oct;17(5A):3307–3312. [PubMed] [Google Scholar]
- Elo J. P., Kvist L., Leinonen K., Isomaa V., Henttu P., Lukkarinen O., Vihko P. Mutated human androgen receptor gene detected in a prostatic cancer patient is also activated by estradiol. J Clin Endocrinol Metab. 1995 Dec;80(12):3494–3500. doi: 10.1210/jcem.80.12.8530589. [DOI] [PubMed] [Google Scholar]
- Fair W. R., Fleshner N. E., Heston W. Cancer of the prostate: a nutritional disease? Urology. 1997 Dec;50(6):840–848. doi: 10.1016/S0090-4295(97)00339-7. [DOI] [PubMed] [Google Scholar]
- Fisher T. C., Milner A. E., Gregory C. D., Jackman A. L., Aherne G. W., Hartley J. A., Dive C., Hickman J. A. bcl-2 modulation of apoptosis induced by anticancer drugs: resistance to thymidylate stress is independent of classical resistance pathways. Cancer Res. 1993 Jul 15;53(14):3321–3326. [PubMed] [Google Scholar]
- Fontanini G., Vignati S., Bigini D., Mussi A., Lucchi M., Angeletti C. A., Basolo F., Bevilacqua G. Bcl-2 protein: a prognostic factor inversely correlated to p53 in non-small-cell lung cancer. Br J Cancer. 1995 May;71(5):1003–1007. doi: 10.1038/bjc.1995.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee J. M., Robertson J. F., Ellis I. O., Willsher P., McClelland R. A., Hoyle H. B., Kyme S. R., Finlay P., Blamey R. W., Nicholson R. I. Immunocytochemical localization of BCL-2 protein in human breast cancers and its relationship to a series of prognostic markers and response to endocrine therapy. Int J Cancer. 1994 Dec 1;59(5):619–628. doi: 10.1002/ijc.2910590508. [DOI] [PubMed] [Google Scholar]
- Gibson L., Holmgreen S. P., Huang D. C., Bernard O., Copeland N. G., Jenkins N. A., Sutherland G. R., Baker E., Adams J. M., Cory S. bcl-w, a novel member of the bcl-2 family, promotes cell survival. Oncogene. 1996 Aug 15;13(4):665–675. [PubMed] [Google Scholar]
- Giovannucci E., Rimm E. B., Colditz G. A., Stampfer M. J., Ascherio A., Chute C. G., Chute C. C., Willett W. C. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993 Oct 6;85(19):1571–1579. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- Gleason D. F. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966 Mar;50(3):125–128. [PubMed] [Google Scholar]
- Gleason D. F., Mellinger G. T. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974 Jan;111(1):58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- Hakimi J. M., Rondinelli R. H., Schoenberg M. P., Barrack E. R. Androgen-receptor gene structure and function in prostate cancer. World J Urol. 1996;14(5):329–337. doi: 10.1007/BF00184606. [DOI] [PubMed] [Google Scholar]
- Haldar S., Basu A., Croce C. M. Bcl2 is the guardian of microtubule integrity. Cancer Res. 1997 Jan 15;57(2):229–233. [PubMed] [Google Scholar]
- Haldar S., Chintapalli J., Croce C. M. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res. 1996 Mar 15;56(6):1253–1255. [PubMed] [Google Scholar]
- Han J., Sabbatini P., Perez D., Rao L., Modha D., White E. The E1B 19K protein blocks apoptosis by interacting with and inhibiting the p53-inducible and death-promoting Bax protein. Genes Dev. 1996 Feb 15;10(4):461–477. doi: 10.1101/gad.10.4.461. [DOI] [PubMed] [Google Scholar]
- Hanada M., Aimé-Sempé C., Sato T., Reed J. C. Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J Biol Chem. 1995 May 19;270(20):11962–11969. doi: 10.1074/jbc.270.20.11962. [DOI] [PubMed] [Google Scholar]
- Hancock S. L., Cox R. S., Bagshaw M. A. Prostate specific antigen after radiotherapy for prostate cancer: a reevaluation of long-term biochemical control and the kinetics of recurrence in patients treated at Stanford University. J Urol. 1995 Oct;154(4):1412–1417. doi: 10.1016/s0022-5347(01)66879-4. [DOI] [PubMed] [Google Scholar]
- Hengartner M. O., Ellis R. E., Horvitz H. R. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992 Apr 9;356(6369):494–499. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- Hengartner M. O., Horvitz H. R. Programmed cell death in Caenorhabditis elegans. Curr Opin Genet Dev. 1994 Aug;4(4):581–586. doi: 10.1016/0959-437x(94)90076-f. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Horm J. W., Sondik E. J. Person-years of life lost due to cancer in the United States, 1970 and 1984. Am J Public Health. 1989 Nov;79(11):1490–1493. doi: 10.2105/ajph.79.11.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrado A. M., Huang Y., Fang G., Liu L., Bhalla K. Overexpression of Bcl-2 or Bcl-xL inhibits Ara-C-induced CPP32/Yama protease activity and apoptosis of human acute myelogenous leukemia HL-60 cells. Cancer Res. 1996 Oct 15;56(20):4743–4748. [PubMed] [Google Scholar]
- Ibrado A. M., Liu L., Bhalla K. Bcl-xL overexpression inhibits progression of molecular events leading to paclitaxel-induced apoptosis of human acute myeloid leukemia HL-60 cells. Cancer Res. 1997 Mar 15;57(6):1109–1115. [PubMed] [Google Scholar]
- Inohara N., Ding L., Chen S., Núez G. harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L). EMBO J. 1997 Apr 1;16(7):1686–1694. doi: 10.1093/emboj/16.7.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs J. T., Lundmo P. I., Berges R., Martikainen P., Kyprianou N., English H. F. Androgen regulation of programmed death of normal and malignant prostatic cells. J Androl. 1992 Nov-Dec;13(6):457–464. [PubMed] [Google Scholar]
- Isaacs J. T. Molecular markers for prostate cancer metastasis. Developing diagnostic methods for predicting the aggressiveness of prostate cancer. Am J Pathol. 1997 May;150(5):1511–1521. [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. D., Raff M. C. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995 Apr 27;374(6525):814–816. doi: 10.1038/374814a0. [DOI] [PubMed] [Google Scholar]
- Kamesaki S., Kamesaki H., Jorgensen T. J., Tanizawa A., Pommier Y., Cossman J. bcl-2 protein inhibits etoposide-induced apoptosis through its effects on events subsequent to topoisomerase II-induced DNA strand breaks and their repair. Cancer Res. 1993 Sep 15;53(18):4251–4256. [PubMed] [Google Scholar]
- Kane D. J., Sarafian T. A., Anton R., Hahn H., Gralla E. B., Valentine J. S., Ord T., Bredesen D. E. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993 Nov 19;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Winterford C. M., Harmon B. V. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994 Apr 15;73(8):2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada S., Takayama S., De Riel K., Tanaka S., Reed J. C. Reversal of chemoresistance of lymphoma cells by antisense-mediated reduction of bcl-2 gene expression. Antisense Res Dev. 1994 Summer;4(2):71–79. doi: 10.1089/ard.1994.4.71. [DOI] [PubMed] [Google Scholar]
- Koivisto P., Visakorpi T., Kallioniemi O. P. Androgen receptor gene amplification: a novel molecular mechanism for endocrine therapy resistance in human prostate cancer. Scand J Clin Lab Invest Suppl. 1996;226:57–63. [PubMed] [Google Scholar]
- Krajewska M., Krajewski S., Epstein J. I., Shabaik A., Sauvageot J., Song K., Kitada S., Reed J. C. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996 May;148(5):1567–1576. [PMC free article] [PubMed] [Google Scholar]
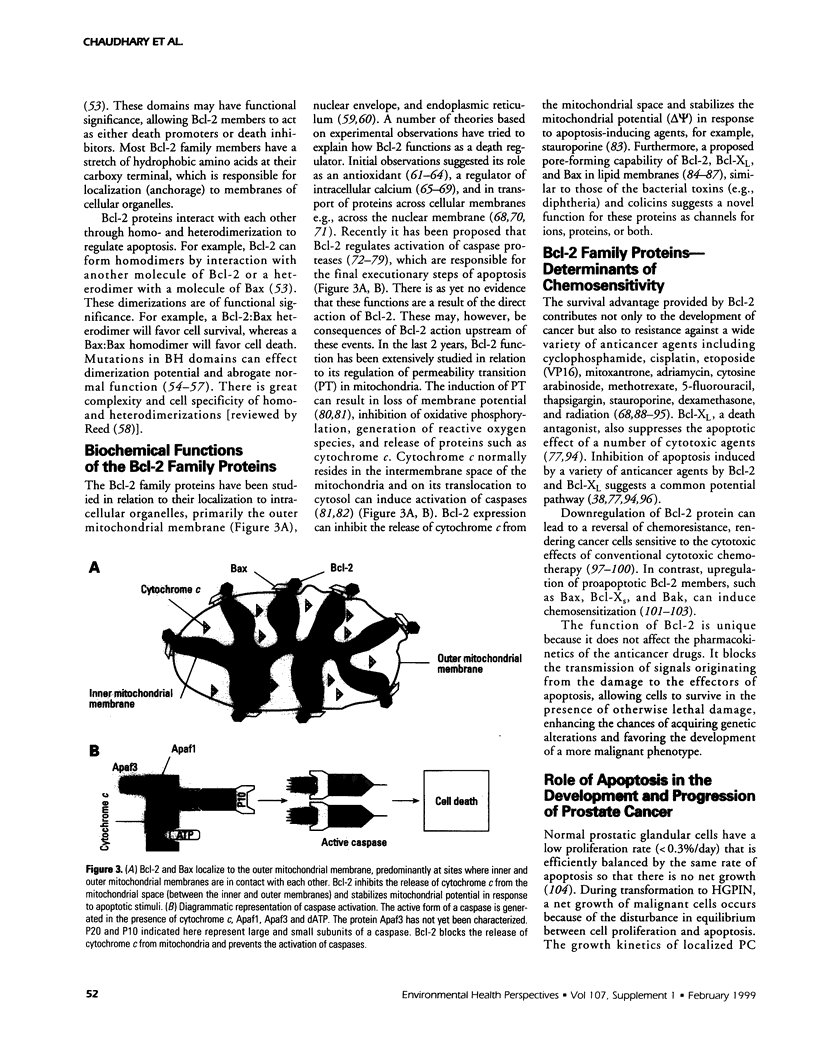
- Krajewski S., Tanaka S., Takayama S., Schibler M. J., Fenton W., Reed J. C. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993 Oct 1;53(19):4701–4714. [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Isaacs J. T. Programmed cell death during regression of PC-82 human prostate cancer following androgen ablation. Cancer Res. 1990 Jun 15;50(12):3748–3753. [PubMed] [Google Scholar]
- Kyprianou N., King E. D., Bradbury D., Rhee J. G. bcl-2 over-expression delays radiation-induced apoptosis without affecting the clonogenic survival of human prostate cancer cells. Int J Cancer. 1997 Jan 27;70(3):341–348. doi: 10.1002/(sici)1097-0215(19970127)70:3<341::aid-ijc16>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Lalani el-N, Laniado M. E., Abel P. D. Molecular and cellular biology of prostate cancer. Cancer Metastasis Rev. 1997 Jun;16(1-2):29–66. doi: 10.1023/a:1005792206377. [DOI] [PubMed] [Google Scholar]
- Lalani E. N., Stubbs A., Stamp G. W. Prostate cancer; the interface between pathology and basic scientific research. Semin Cancer Biol. 1997 Feb;8(1):53–59. doi: 10.1006/scbi.1997.0052. [DOI] [PubMed] [Google Scholar]
- Lam M., Dubyak G., Chen L., Nuñez G., Miesfeld R. L., Distelhorst C. W. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwers G. Y., Scott G. V., Karpeh M. S. Immunohistochemical evaluation of bcl-2 protein expression in gastric adenocarcinomas. Cancer. 1995 May 1;75(9):2209–2213. doi: 10.1002/1097-0142(19950501)75:9<2209::aid-cncr2820750904>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Lin E. Y., Orlofsky A., Wang H. G., Reed J. C., Prystowsky M. B. A1, a Bcl-2 family member, prolongs cell survival and permits myeloid differentiation. Blood. 1996 Feb 1;87(3):983–992. [PubMed] [Google Scholar]
- Lin X. S., Denmeade S. R., Cisek L., Isaacs J. T. Mechanism and role of growth arrest in programmed (apoptotic) death of prostatic cancer cells induced by thapsigargin. Prostate. 1997 Nov 1;33(3):201–207. doi: 10.1002/(sici)1097-0045(19971101)33:3<201::aid-pros9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Lithgow T., van Driel R., Bertram J. F., Strasser A. The protein product of the oncogene bcl-2 is a component of the nuclear envelope, the endoplasmic reticulum, and the outer mitochondrial membrane. Cell Growth Differ. 1994 Apr;5(4):411–417. [PubMed] [Google Scholar]
- Liu X., Kim C. N., Yang J., Jemmerson R., Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996 Jul 12;86(1):147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Lu Q. L., Abel P., Foster C. S., Lalani E. N. bcl-2: role in epithelial differentiation and oncogenesis. Hum Pathol. 1996 Feb;27(2):102–110. doi: 10.1016/s0046-8177(96)90362-7. [DOI] [PubMed] [Google Scholar]
- Lu Q. L., Elia G., Lucas S., Thomas J. A. Bcl-2 proto-oncogene expression in Epstein-Barr-virus-associated nasopharyngeal carcinoma. Int J Cancer. 1993 Jan 2;53(1):29–35. doi: 10.1002/ijc.2910530107. [DOI] [PubMed] [Google Scholar]
- Marin M. C., Fernandez A., Bick R. J., Brisbay S., Buja L. M., Snuggs M., McConkey D. J., von Eschenbach A. C., Keating M. J., McDonnell T. J. Apoptosis suppression by bcl-2 is correlated with the regulation of nuclear and cytosolic Ca2+. Oncogene. 1996 Jun 6;12(11):2259–2266. [PubMed] [Google Scholar]
- McConkey D. J., Greene G., Pettaway C. A. Apoptosis resistance increases with metastatic potential in cells of the human LNCaP prostate carcinoma line. Cancer Res. 1996 Dec 15;56(24):5594–5599. [PubMed] [Google Scholar]
- McDonnell T. J., Troncoso P., Brisbay S. M., Logothetis C., Chung L. W., Hsieh J. T., Tu S. M., Campbell M. L. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992 Dec 15;52(24):6940–6944. [PubMed] [Google Scholar]
- Meikrantz W., Gisselbrecht S., Tam S. W., Schlegel R. Activation of cyclin A-dependent protein kinases during apoptosis. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3754–3758. doi: 10.1073/pnas.91.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer U. K., Reimer D. M., Reed J. C., Brüne B. Nitric oxide induced poly(ADP-ribose) polymerase cleavage in RAW 264.7 macrophage apoptosis is blocked by Bcl-2. FEBS Lett. 1996 Apr 15;384(2):162–166. doi: 10.1016/0014-5793(96)00311-0. [DOI] [PubMed] [Google Scholar]
- Minn A. J., Rudin C. M., Boise L. H., Thompson C. B. Expression of bcl-xL can confer a multidrug resistance phenotype. Blood. 1995 Sep 1;86(5):1903–1910. [PubMed] [Google Scholar]
- Minn A. J., Vélez P., Schendel S. L., Liang H., Muchmore S. W., Fesik S. W., Fill M., Thompson C. B. Bcl-x(L) forms an ion channel in synthetic lipid membranes. Nature. 1997 Jan 23;385(6614):353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993 Jan 1;81(1):151–157. [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res. 1992 Oct 1;52(19):5407–5411. [PubMed] [Google Scholar]
- Monney L., Otter I., Olivier R., Ravn U., Mirzasaleh H., Fellay I., Poirier G. G., Borner C. Bcl-2 overexpression blocks activation of the death protease CPP32/Yama/apopain. Biochem Biophys Res Commun. 1996 Apr 16;221(2):340–345. doi: 10.1006/bbrc.1996.0597. [DOI] [PubMed] [Google Scholar]
- Murphy A. N., Bredesen D. E., Cortopassi G., Wang E., Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Pollina L., Pacini F., Fontanini G., Vignati S., Bevilacqua G., Basolo F. bcl-2, p53 and proliferating cell nuclear antigen expression is related to the degree of differentiation in thyroid carcinomas. Br J Cancer. 1996 Jan;73(2):139–143. doi: 10.1038/bjc.1996.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffo A. J., Perlman H., Chen M. W., Day M. L., Streitman J. S., Buttyan R. Overexpression of bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res. 1995 Oct 1;55(19):4438–4445. [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 family proteins: strategies for overcoming chemoresistance in cancer. Adv Pharmacol. 1997;41:501–532. doi: 10.1016/s1054-3589(08)61070-4. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Cuddy M., Haldar S., Croce C., Nowell P., Makover D., Bradley K. BCL2-mediated tumorigenicity of a human T-lymphoid cell line: synergy with MYC and inhibition by BCL2 antisense. Proc Natl Acad Sci U S A. 1990 May;87(10):3660–3664. doi: 10.1073/pnas.87.10.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Stein C., Subasinghe C., Haldar S., Croce C. M., Yum S., Cohen J. Antisense-mediated inhibition of BCL2 protooncogene expression and leukemic cell growth and survival: comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res. 1990 Oct 15;50(20):6565–6570. [PubMed] [Google Scholar]
- Ruizeveld de Winter J. A., Janssen P. J., Sleddens H. M., Verleun-Mooijman M. C., Trapman J., Brinkmann A. O., Santerse A. B., Schröder F. H., van der Kwast T. H. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol. 1994 Apr;144(4):735–746. [PMC free article] [PubMed] [Google Scholar]
- Ryan J. J., Prochownik E., Gottlieb C. A., Apel I. J., Merino R., Nuñez G., Clarke M. F. c-myc and bcl-2 modulate p53 function by altering p53 subcellular trafficking during the cell cycle. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5878–5882. doi: 10.1073/pnas.91.13.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh H., Hida M., Shimbo T., Nakamura K., Yamagata J., Satoh T. Metastatic patterns of prostatic cancer. Correlation between sites and number of organs involved. Cancer. 1984 Dec 15;54(12):3078–3084. doi: 10.1002/1097-0142(19841215)54:12<3078::aid-cncr2820541245>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Schendel S. L., Xie Z., Montal M. O., Matsuyama S., Montal M., Reed J. C. Channel formation by antiapoptotic protein Bcl-2. Proc Natl Acad Sci U S A. 1997 May 13;94(10):5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger P. H., Gross A., Yin X. M., Yamamoto K., Saito M., Waksman G., Korsmeyer S. J. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc Natl Acad Sci U S A. 1997 Oct 14;94(21):11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder F. H. Detection of prostate cancer. BMJ. 1995 Jan 21;310(6973):140–141. doi: 10.1136/bmj.310.6973.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal N. H., Cohen R. J., Haffejee Z., Savage N. BCL-2 proto-oncogene expression in prostate cancer and its relationship to the prostatic neuroendocrine cell. Arch Pathol Lab Med. 1994 Jun;118(6):616–618. [PubMed] [Google Scholar]
- Shiina H., Igawa M., Urakami S., Honda S., Shirakawa H., Ishibe T. Immunohistochemical analysis of bcl-2 expression in transitional cell carcinoma of the bladder. J Clin Pathol. 1996 May;49(5):395–399. doi: 10.1136/jcp.49.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Eguchi Y., Kosaka H., Kamiike W., Matsuda H., Tsujimoto Y. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature. 1995 Apr 27;374(6525):811–813. doi: 10.1038/374811a0. [DOI] [PubMed] [Google Scholar]
- Shirahama T., Sakakura C., Sweeney E. A., Ozawa M., Takemoto M., Nishiyama K., Ohi Y., Igarashi Y. Sphingosine induces apoptosis in androgen-independent human prostatic carcinoma DU-145 cells by suppression of bcl-X(L) gene expression. FEBS Lett. 1997 Apr 21;407(1):97–100. doi: 10.1016/s0014-5793(97)00304-9. [DOI] [PubMed] [Google Scholar]
- Smyth M. J., Perry D. K., Zhang J., Poirier G. G., Hannun Y. A., Obeid L. M. prICE: a downstream target for ceramide-induced apoptosis and for the inhibitory action of Bcl-2. Biochem J. 1996 May 15;316(Pt 1):25–28. doi: 10.1042/bj3160025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A., Foster L. M., Testa M. P., Ord T., Keane R. W., Bredesen D. E., Kayalar C. Bcl-2 expression in neural cells blocks activation of ICE/CED-3 family proteases during apoptosis. J Neurosci. 1996 Sep 15;16(18):5654–5660. doi: 10.1523/JNEUROSCI.16-18-05654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Huang D. C., Vaux D. L. The role of the bcl-2/ced-9 gene family in cancer and general implications of defects in cell death control for tumourigenesis and resistance to chemotherapy. Biochim Biophys Acta. 1997 Oct 24;1333(2):F151–F178. doi: 10.1016/s0304-419x(97)00019-x. [DOI] [PubMed] [Google Scholar]
- Sumantran V. N., Ealovega M. W., Nuñez G., Clarke M. F., Wicha M. S. Overexpression of Bcl-XS sensitizes MCF-7 cells to chemotherapy-induced apoptosis. Cancer Res. 1995 Jun 15;55(12):2507–2510. [PubMed] [Google Scholar]
- Susin S. A., Zamzami N., Castedo M., Hirsch T., Marchetti P., Macho A., Daugas E., Geuskens M., Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996 Oct 1;184(4):1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Sato N., Watabe Y., Masai M., Seino S., Shimazaki J. Androgen receptor gene mutations in human prostate cancer. J Steroid Biochem Mol Biol. 1993 Dec;46(6):759–765. doi: 10.1016/0960-0760(93)90316-o. [DOI] [PubMed] [Google Scholar]
- Tang C., Willingham M. C., Reed J. C., Miyashita T., Ray S., Ponnathpur V., Huang Y., Mahoney M. E., Bullock G., Bhalla K. High levels of p26BCL-2 oncoprotein retard taxol-induced apoptosis in human pre-B leukemia cells. Leukemia. 1994 Nov;8(11):1960–1969. [PubMed] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990 Jan;29(1-2):8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- Thompson C. B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995 Mar 10;267(5203):1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Trapman J., Brinkmann A. O. Mutations in the androgen receptor. Ann N Y Acad Sci. 1993 Jun 11;684:85–93. doi: 10.1111/j.1749-6632.1993.tb32273.x. [DOI] [PubMed] [Google Scholar]
- Tsuji M., Murakami Y., Kanayama H., Sano T., Kagawa S. Immunohistochemical analysis of Ki-67 antigen and Bcl-2 protein expression in prostate cancer: effect of neoadjuvant hormonal therapy. Br J Urol. 1998 Jan;81(1):116–121. doi: 10.1046/j.1464-410x.1998.00492.x. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Cossman J., Jaffe E., Croce C. M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985 Jun 21;228(4706):1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Finger L. R., Yunis J., Nowell P. C., Croce C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984 Nov 30;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- Tu H., Jacobs S. C., Borkowski A., Kyprianou N. Incidence of apoptosis and cell proliferation in prostate cancer: relationship with TGF-beta1 and bcl-2 expression. Int J Cancer. 1996 Oct 21;69(5):357–363. doi: 10.1002/(SICI)1097-0215(19961021)69:5<357::AID-IJC1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visakorpi T., Hyytinen E., Koivisto P., Tanner M., Keinänen R., Palmberg C., Palotie A., Tammela T., Isola J., Kallioniemi O. P. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995 Apr;9(4):401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- Walsh P. C., Partin A. W., Epstein J. I. Cancer control and quality of life following anatomical radical retropubic prostatectomy: results at 10 years. J Urol. 1994 Nov;152(5 Pt 2):1831–1836. doi: 10.1016/s0022-5347(17)32396-0. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Whysong D., O'Connor P. M., Hockenbery D., Korsmeyer S. J., Kohn K. W. Constitutive expression of human Bcl-2 modulates nitrogen mustard and camptothecin induced apoptosis. Cancer Res. 1993 Apr 15;53(8):1853–1861. [PubMed] [Google Scholar]
- Wang K., Yin X. M., Chao D. T., Milliman C. L., Korsmeyer S. J. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996 Nov 15;10(22):2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- Westin P., Stattin P., Damber J. E., Bergh A. Castration therapy rapidly induces apoptosis in a minority and decreases cell proliferation in a majority of human prostatic tumors. Am J Pathol. 1995 Jun;146(6):1368–1375. [PMC free article] [PubMed] [Google Scholar]
- Whitmore W. F., Jr Natural history of low-stage prostatic cancer and the impact of early detection. Urol Clin North Am. 1990 Nov;17(4):689–697. [PubMed] [Google Scholar]
- Whittemore A. S., Kolonel L. N., Wu A. H., John E. M., Gallagher R. P., Howe G. R., Burch J. D., Hankin J., Dreon D. M., West D. W. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J Natl Cancer Inst. 1995 May 3;87(9):652–661. doi: 10.1093/jnci/87.9.652. [DOI] [PubMed] [Google Scholar]
- Wingo P. A., Landis S., Ries L. A. An adjustment to the 1997 estimate for new prostate cancer cases. Cancer. 1997 Nov 1;80(9):1810–1813. doi: 10.1002/(sici)1097-0142(19971101)80:9<1810::aid-cncr19>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yagoda A., Petrylak D. Cytotoxic chemotherapy for advanced hormone-resistant prostate cancer. Cancer. 1993 Feb 1;71(3 Suppl):1098–1109. doi: 10.1002/1097-0142(19930201)71:3+<1098::aid-cncr2820711432>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Yang E., Zha J., Jockel J., Boise L. H., Thompson C. B., Korsmeyer S. J. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995 Jan 27;80(2):285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yang J., Liu X., Bhalla K., Kim C. N., Ibrado A. M., Cai J., Peng T. I., Jones D. P., Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997 Feb 21;275(5303):1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Yin X. M., Oltvai Z. N., Korsmeyer S. J. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994 May 26;369(6478):321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- Zamzami N., Susin S. A., Marchetti P., Hirsch T., Gómez-Monterrey I., Castedo M., Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996 Apr 1;183(4):1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha H., Aimé-Sempé C., Sato T., Reed J. C. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem. 1996 Mar 29;271(13):7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- Zhou P., Qian L., Kozopas K. M., Craig R. W. Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood. 1997 Jan 15;89(2):630–643. [PubMed] [Google Scholar]
- Zou H., Henzel W. J., Liu X., Lutschg A., Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997 Aug 8;90(3):405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]