Abstract
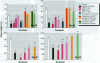
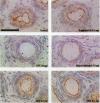
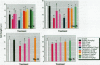
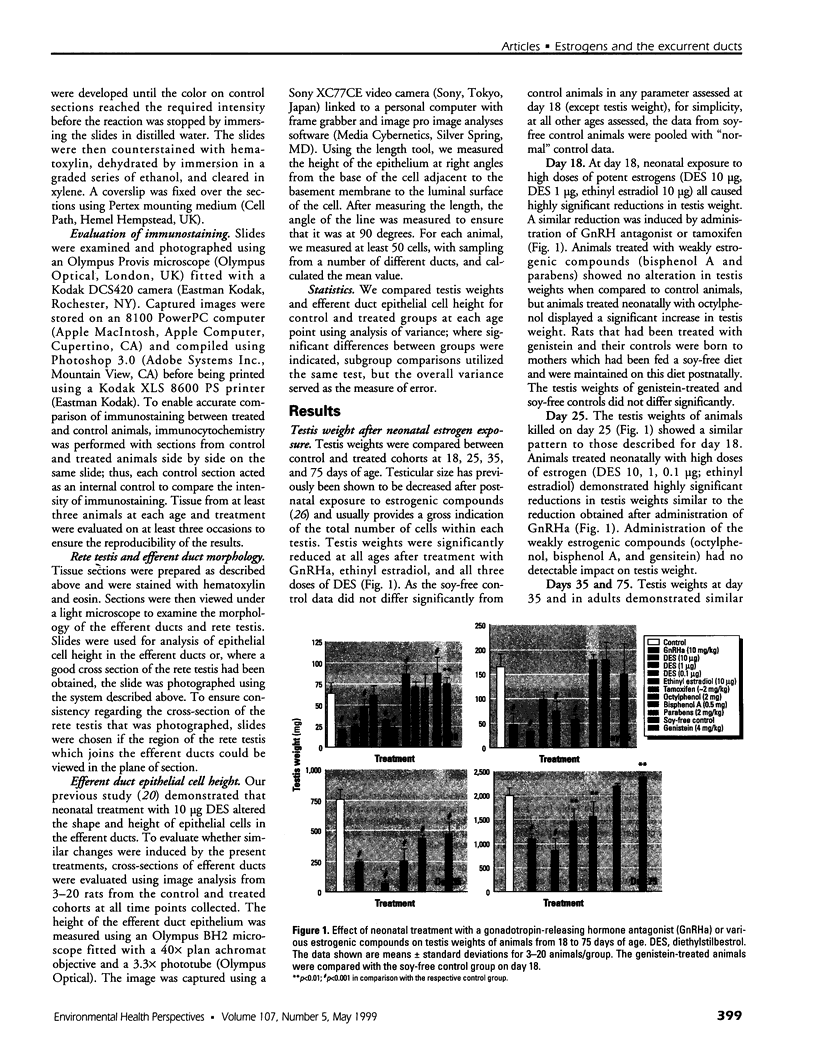
Neonatal exposure to diethylstilbestrol (DES) can alter the structure of the testicular excurrent ducts in rats. We characterized these changes according to dose and time posttreatment and established whether potent estrogens (ethinyl estradiol), environmental estrogens (genistein, octylphenol, bisphenol A, parabens), and tamoxifen induce such changes. Rats were administered these compounds neonatally and assessed at several time points during (day 10, or day 18 for some treatments) and after (days 18, 25, 35, and 75) the treatment period to detect any changes in testis weight, distension of the rete testis and efferent ducts, epithelial cell height in the efferent ducts, and immunoexpression of the water channel aquaporin-1 (AQP-1). Treatment with DES (10, 1, or 0.1 microg/injection; equivalent to 0.37, 0.037, or 0.0037 mg/kg/day, respectively) induced dose-dependent changes in testis weight and all parameters. These effects were most pronounced at days 18 and 25 and appeared to lessen with time, although some persisted into adulthood. Neonatal treatment with ethinyl estradiol (10 microg/injection; equivalent to 0.37 mg/kg/day) caused changes broadly similar to those induced by 10 mg DES. Administration of tamoxifen (2 mg/kg/day) caused changes at 18 days that were similar to those induced by 1 microg DES. Treatment with genistein (4 mg/kg/day), octylphenol (2 mg/injection; equivalent to 150 mg/kg/day), or bisphenol A (0.5 mg/injection; equivalent to 37 mg/kg/day) caused minor but significant (p<0.05) decreases in epithelial cell height of the efferent ducts at days 18 and/or 25. In animals that were followed through to 35 days and/or adulthood, these changes were no longer obvious; other parameters were either unaffected or were affected only marginally and transiently. Administration of parabens (2 mg/kg/day) had no detectable effect on any parameter at day 18. To establish whether these effects of estrogens were direct or indirect (i.e., resulting from reduced follicle-stimulating hormone/luteinizing hormone secretion), the above end points were assessed in animals in which gonadotropin secretion was suppressed neonatally by administration of a gonadotropin-releasing hormone antagonist. This treatment permanently reduced testis weight, but did not affect any of the other end points, apart from a minor transient reduction in efferent duct epithelial cell height at 18 days. This study suggests that structural and functional (expression of AQP-1) development of the excurrent ducts is susceptible to impairment by neonatal estrogen exposure, probably as a consequence of direct effects. The magnitude and duration of adverse changes induced by treatment with a range of estrogenic compounds was broadly comparable to their estrogenic potencies reported from in vitro assays.
Full text
PDF


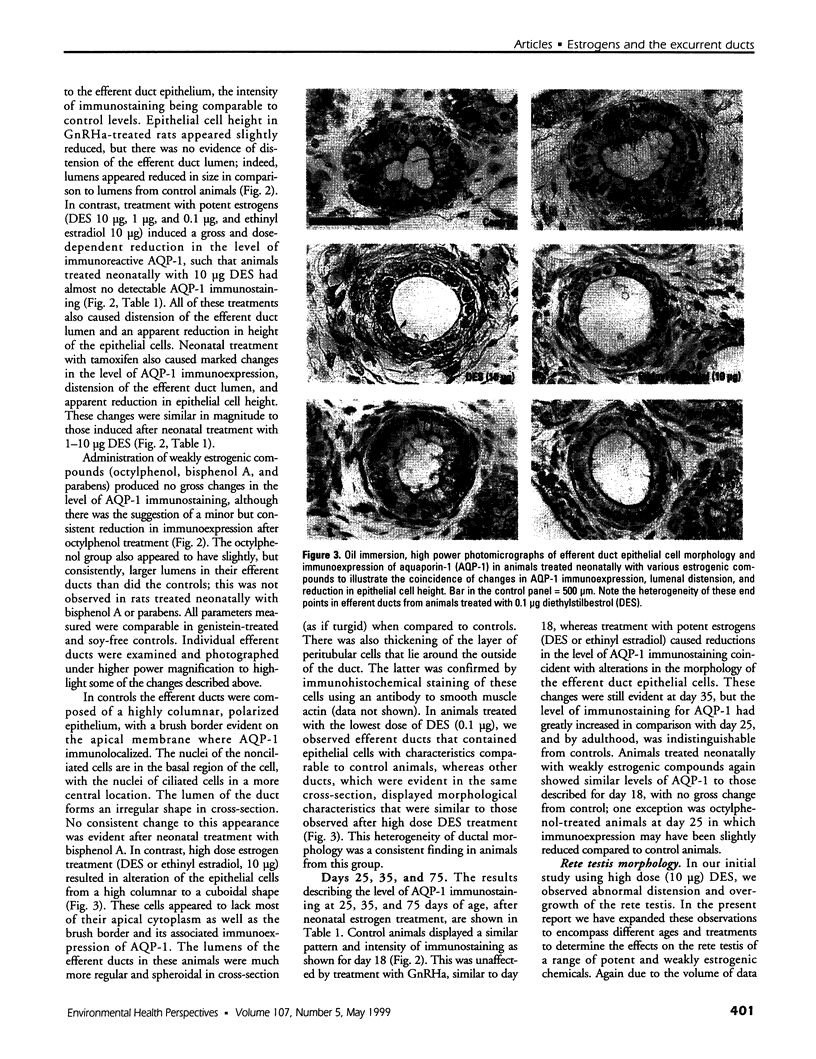
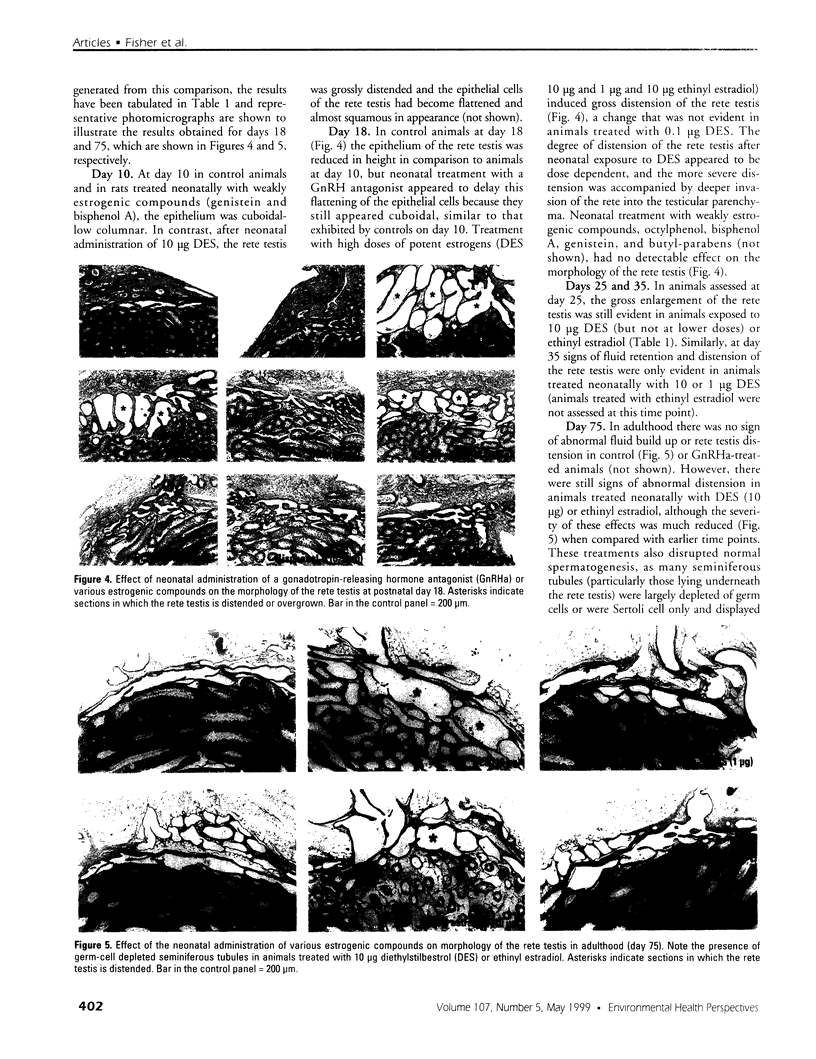
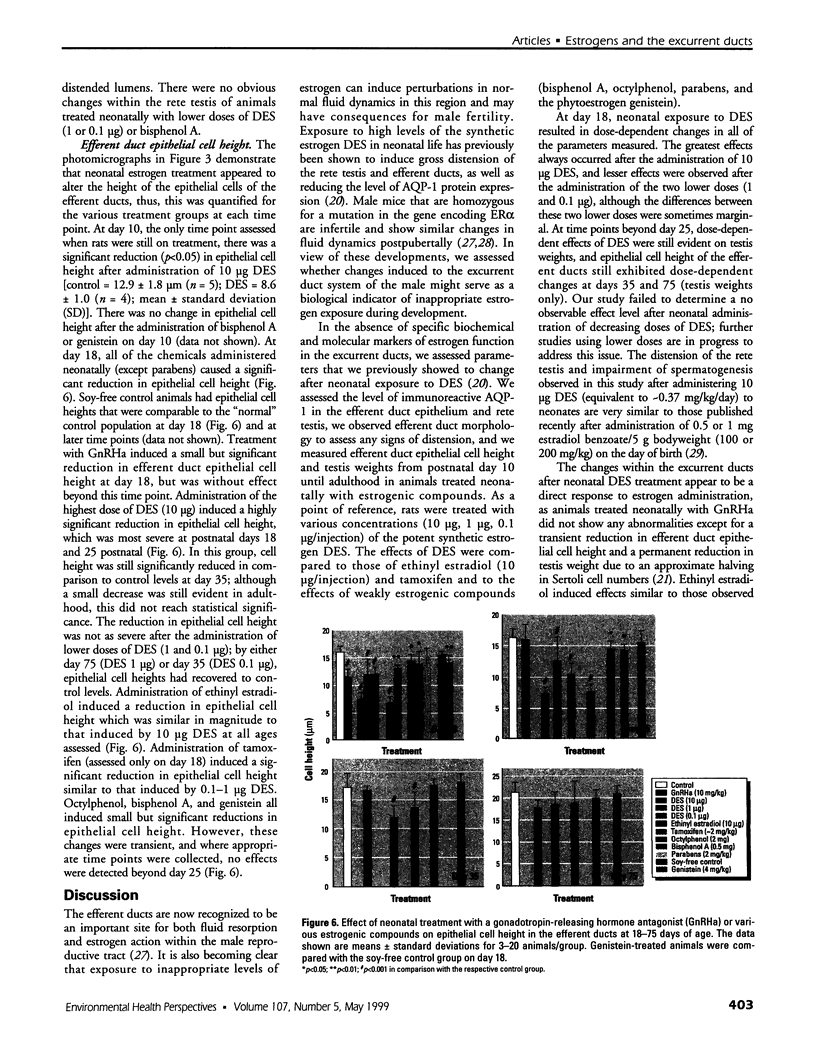


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aceitero J., Llanero M., Parrado R., Peña E., Lopez-Beltran A. Neonatal exposure of male rats to estradiol benzoate causes rete testis dilation and backflow impairment of spermatogenesis. Anat Rec. 1998 Sep;252(1):17–33. doi: 10.1002/(SICI)1097-0185(199809)252:1<17::AID-AR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Adami H. O., Bergström R., Möhner M., Zatoński W., Storm H., Ekbom A., Tretli S., Teppo L., Ziegler H., Rahu M. Testicular cancer in nine northern European countries. Int J Cancer. 1994 Oct 1;59(1):33–38. doi: 10.1002/ijc.2910590108. [DOI] [PubMed] [Google Scholar]
- Auger J., Kunstmann J. M., Czyglik F., Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995 Feb 2;332(5):281–285. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- Bicknell R. J., Herbison A. E., Sumpter J. P. Oestrogenic activity of an environmentally persistent alkylphenol in the reproductive tract but not the brain of rodents. J Steroid Biochem Mol Biol. 1995 Jul;54(1-2):7–9. doi: 10.1016/0960-0760(95)00118-j. [DOI] [PubMed] [Google Scholar]
- Bujan L., Mieusset R., Audran F., Lumbroso S., Sultan C. Increased oestradiol level in seminal plasma in infertile men. Hum Reprod. 1993 Jan;8(1):74–77. doi: 10.1093/oxfordjournals.humrep.a137878. [DOI] [PubMed] [Google Scholar]
- Byers S., Graham R. Distribution of sodium-potassium ATPase in the rat testis and epididymis. Am J Anat. 1990 May;188(1):31–43. doi: 10.1002/aja.1001880105. [DOI] [PubMed] [Google Scholar]
- Carlsen E., Giwercman A., Keiding N., Skakkebaek N. E. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992 Sep 12;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilvers C., Pike M. C., Forman D., Fogelman K., Wadsworth M. E. Apparent doubling of frequency of undescended testis in England and Wales in 1962-81. Lancet. 1984 Aug 11;2(8398):330–332. doi: 10.1016/s0140-6736(84)92697-7. [DOI] [PubMed] [Google Scholar]
- Clulow J., Hansen L. A., Jones R. C. In vivo microperfusion of the ductuli efferentes testis of the rat: flow dependence of fluid reabsorption. Exp Physiol. 1996 Jul;81(4):633–644. doi: 10.1113/expphysiol.1996.sp003964. [DOI] [PubMed] [Google Scholar]
- Clulow J., Jones R. C., Hansen L. A. Micropuncture and cannulation studies of fluid composition and transport in the ductuli efferentes testis of the rat: comparisons with the homologous metanephric proximal tubule. Exp Physiol. 1994 Nov;79(6):915–928. doi: 10.1113/expphysiol.1994.sp003817. [DOI] [PubMed] [Google Scholar]
- Colerangle J. B., Roy D. Profound effects of the weak environmental estrogen-like chemical bisphenol A on the growth of the mammary gland of Noble rats. J Steroid Biochem Mol Biol. 1997 Jan;60(1-2):153–160. doi: 10.1016/s0960-0760(96)00130-6. [DOI] [PubMed] [Google Scholar]
- Eddy E. M., Washburn T. F., Bunch D. O., Goulding E. H., Gladen B. C., Lubahn D. B., Korach K. S. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996 Nov;137(11):4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- Fisher J. S., Turner K. J., Fraser H. M., Saunders P. T., Brown D., Sharpe R. M. Immunoexpression of aquaporin-1 in the efferent ducts of the rat and marmoset monkey during development, its modulation by estrogens, and its possible role in fluid resorption. Endocrinology. 1998 Sep;139(9):3935–3945. doi: 10.1210/endo.139.9.6213. [DOI] [PubMed] [Google Scholar]
- Hess R. A., Bunick D., Lee K. H., Bahr J., Taylor J. A., Korach K. S., Lubahn D. B. A role for oestrogens in the male reproductive system. Nature. 1997 Dec 4;390(6659):509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R. A., Gist D. H., Bunick D., Lubahn D. B., Farrell A., Bahr J., Cooke P. S., Greene G. L. Estrogen receptor (alpha and beta) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl. 1997 Nov-Dec;18(6):602–611. [PubMed] [Google Scholar]
- Huang S. J., Fu W. O., Chung Y. W., Zhou T. S., Wong P. Y. Properties of cAMP-dependent and Ca(2+)-dependent whole cell Cl- conductances in rat epididymal cells. Am J Physiol. 1993 Apr;264(4 Pt 1):C794–C802. doi: 10.1152/ajpcell.1993.264.4.C794. [DOI] [PubMed] [Google Scholar]
- Ilio K. Y., Hess R. A. Localization and activity of Na+,K(+)-ATPase in the ductuli efferentes of the rat. Anat Rec. 1992 Oct;234(2):190–200. doi: 10.1002/ar.1092340206. [DOI] [PubMed] [Google Scholar]
- Jobling S., Reynolds T., White R., Parker M. G., Sumpter J. P. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995 Jun;103(6):582–587. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A. V., Stathis P., Permuth S. F., Tokes L., Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993 Jun;132(6):2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Bullock B. C., McLachlan J. A. Adenocarcinoma of the rete testis. Diethylstilbestrol-induced lesions of the mouse rete testis. Am J Pathol. 1986 Dec;125(3):625–628. [PMC free article] [PubMed] [Google Scholar]
- Newbold R. R., Bullock B. C., McLachlan J. A. Lesions of the rete testis in mice exposed prenatally to diethylstilbestrol. Cancer Res. 1985 Oct;45(10):5145–5150. [PubMed] [Google Scholar]
- Paulozzi L. J., Erickson J. D., Jackson R. J. Hypospadias trends in two US surveillance systems. Pediatrics. 1997 Nov;100(5):831–834. doi: 10.1542/peds.100.5.831. [DOI] [PubMed] [Google Scholar]
- Pollard C. E., Harris A., Coleman L., Argent B. E. Chloride channels on epithelial cells cultured from human fetal epididymis. J Membr Biol. 1991 Dec;124(3):275–284. doi: 10.1007/BF01994360. [DOI] [PubMed] [Google Scholar]
- Rochwerger L., Buchwald M. Stimulation of the cystic fibrosis transmembrane regulator expression by estrogen in vivo. Endocrinology. 1993 Aug;133(2):921–930. doi: 10.1210/endo.133.2.7688293. [DOI] [PubMed] [Google Scholar]
- Rochwerger L., Dho S., Parker L., Foskett J. K., Buchwald M. Estrogen-dependent expression of the cystic fibrosis transmembrane regulator gene in a novel uterine epithelial cell line. J Cell Sci. 1994 Sep;107(Pt 9):2439–2448. doi: 10.1242/jcs.107.9.2439. [DOI] [PubMed] [Google Scholar]
- Rosenfeld C. S., Ganjam V. K., Taylor J. A., Yuan X., Stiehr J. R., Hardy M. P., Lubahn D. B. Transcription and translation of estrogen receptor-beta in the male reproductive tract of estrogen receptor-alpha knock-out and wild-type mice. Endocrinology. 1998 Jun;139(6):2982–2987. doi: 10.1210/endo.139.6.6028. [DOI] [PubMed] [Google Scholar]
- Routledge E. J., Parker J., Odum J., Ashby J., Sumpter J. P. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998 Nov;153(1):12–19. doi: 10.1006/taap.1998.8544. [DOI] [PubMed] [Google Scholar]
- Sabolić I., Valenti G., Verbavatz J. M., Van Hoek A. N., Verkman A. S., Ausiello D. A., Brown D. Localization of the CHIP28 water channel in rat kidney. Am J Physiol. 1992 Dec;263(6 Pt 1):C1225–C1233. doi: 10.1152/ajpcell.1992.263.6.C1225. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Atanassova N., McKinnell C., Parte P., Turner K. J., Fisher J. S., Kerr J. B., Groome N. P., Macpherson S., Millar M. R. Abnormalities in functional development of the Sertoli cells in rats treated neonatally with diethylstilbestrol: a possible role for estrogens in Sertoli cell development. Biol Reprod. 1998 Nov;59(5):1084–1094. doi: 10.1095/biolreprod59.5.1084. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., Fisher J. S., Millar M. M., Jobling S., Sumpter J. P. Gestational and lactational exposure of rats to xenoestrogens results in reduced testicular size and sperm production. Environ Health Perspect. 1995 Dec;103(12):1136–1143. doi: 10.1289/ehp.951031136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe R. M., Turner K. J., McKinnell C., Groome N. P., Atanassova N., Millar M. R., Buchanan D. L., Cooke P. S. Inhibin B levels in plasma of the male rat from birth to adulthood: effect of experimental manipulation of Sertoli cell number. J Androl. 1999 Jan-Feb;20(1):94–101. [PubMed] [Google Scholar]
- Soto A. M., Justicia H., Wray J. W., Sonnenschein C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ Health Perspect. 1991 May;92:167–173. doi: 10.1289/ehp.9192167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizzano E. F., Silver M. M., Chitayat D., Benichou J. C., Buchwald M. Differential cellular expression of cystic fibrosis transmembrane regulator in human reproductive tissues. Clues for the infertility in patients with cystic fibrosis. Am J Pathol. 1994 May;144(5):906–914. [PMC free article] [PubMed] [Google Scholar]
- Toppari J., Larsen J. C., Christiansen P., Giwercman A., Grandjean P., Guillette L. J., Jr, Jégou B., Jensen T. K., Jouannet P., Keiding N. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996 Aug;104 (Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994 Jul;135(1):175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- de Mouzon J., Thonneau P., Spira A., Multigner L. Declining sperm count. Semen quality has declined among men born in France since 1950. BMJ. 1996 Jul 6;313(7048):43–45. doi: 10.1136/bmj.313.7048.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Sant'Agnese P. A. Guest editorial--fertility and the young adult with cystic fibrosis. N Engl J Med. 1968 Jul 11;279(2):103–105. doi: 10.1056/NEJM196807112790213. [DOI] [PubMed] [Google Scholar]
- van der Ven K., Messer L., van der Ven H., Jeyendran R. S., Ober C. Cystic fibrosis mutation screening in healthy men with reduced sperm quality. Hum Reprod. 1996 Mar;11(3):513–517. doi: 10.1093/humrep/11.3.513. [DOI] [PubMed] [Google Scholar]