Abstract
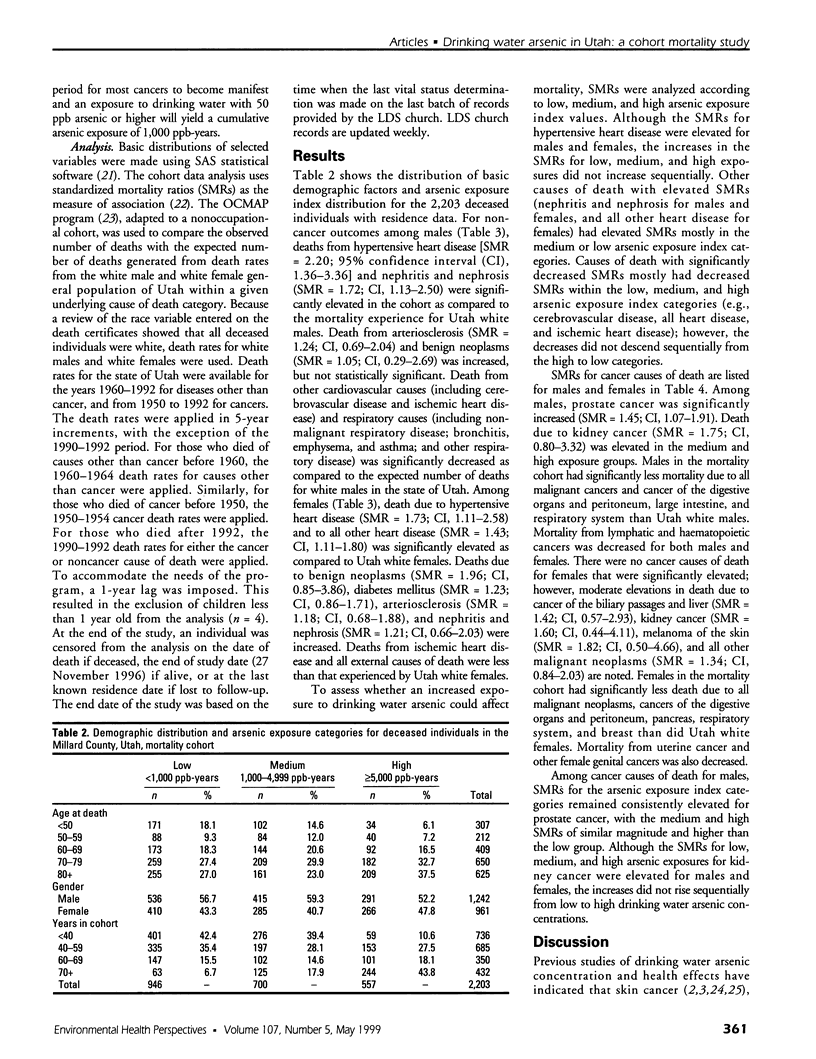
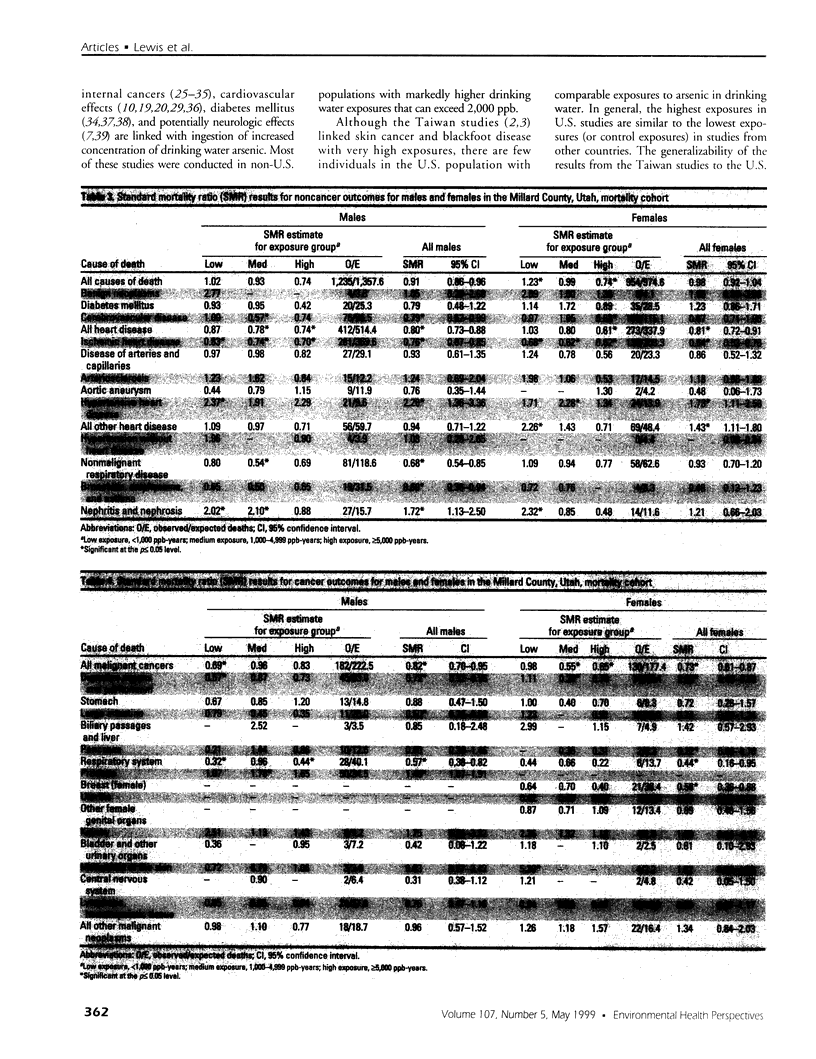
The association of drinking water arsenic and mortality outcome was investigated in a cohort of residents from Millard County, Utah. Median drinking water arsenic concentrations for selected study towns ranged from 14 to 166 ppb and were from public and private samples collected and analyzed under the auspices of the State of Utah Department of Environmental Quality, Division of Drinking Water. Cohort members were assembled using historical documents of the Church of Jesus Christ of Latter-day Saints. Standard mortality ratios (SMRs) were calculated. Using residence history and median drinking water arsenic concentration, a matrix for cumulative arsenic exposure was created. Without regard to specific exposure levels, statistically significant findings include increased mortality from hypertensive heart disease [SMR = 2.20; 95% confidence interval (CI), 1.36-3.36], nephritis and nephrosis (SMR = 1.72; CI, 1.13-2.50), and prostate cancer (SMR = 1.45; CI, 1.07-1. 91) among cohort males. Among cohort females, statistically significant increased mortality was found for hypertensive heart disease (SMR = 1.73; CI, 1.11-2.58) and for the category of all other heart disease, which includes pulmonary heart disease, pericarditis, and other diseases of the pericardium (SMR = 1.43; CI, 1.11-1.80). SMR analysis by low, medium, and high arsenic exposure groups hinted at a dose relationship for prostate cancer. Although the SMRs by exposure category were elevated for hypertensive heart disease for both males and females, the increases were not sequential from low to high groups. Because the relationship between health effects and exposure to drinking water arsenic is not well established in U.S. populations, further evaluation of effects in low-exposure populations is warranted.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aschengrau A., Zierler S., Cohen A. Quality of community drinking water and the occurrence of spontaneous abortion. Arch Environ Health. 1989 Sep-Oct;44(5):283–290. doi: 10.1080/00039896.1989.9935895. [DOI] [PubMed] [Google Scholar]
- Bates M. N., Smith A. H., Cantor K. P. Case-control study of bladder cancer and arsenic in drinking water. Am J Epidemiol. 1995 Mar 15;141(6):523–530. doi: 10.1093/oxfordjournals.aje.a117467. [DOI] [PubMed] [Google Scholar]
- Borgoño J. M., Vicent P., Venturino H., Infante A. Arsenic in the drinking water of the city of Antofagasta: epidemiological and clinical study before and after the installation of a treatment plant. Environ Health Perspect. 1977 Aug;19:103–105. doi: 10.1289/ehp.19-1637404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Chen C. W., Wu M. M., Kuo T. L. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer. 1992 Nov;66(5):888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Chiou H. Y., Chiang M. H., Lin L. J., Tai T. Y. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol. 1996 Apr;16(4):504–510. doi: 10.1161/01.atv.16.4.504. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Chuang Y. C., Lin T. M., Wu H. Y. Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: high-arsenic artesian well water and cancers. Cancer Res. 1985 Nov;45(11 Pt 2):5895–5899. [PubMed] [Google Scholar]
- Chen C. J., Chuang Y. C., You S. L., Lin T. M., Wu H. Y. A retrospective study on malignant neoplasms of bladder, lung and liver in blackfoot disease endemic area in Taiwan. Br J Cancer. 1986 Mar;53(3):399–405. doi: 10.1038/bjc.1986.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Hsueh Y. M., Lai M. S., Shyu M. P., Chen S. Y., Wu M. M., Kuo T. L., Tai T. Y. Increased prevalence of hypertension and long-term arsenic exposure. Hypertension. 1995 Jan;25(1):53–60. [PubMed] [Google Scholar]
- Chen C. J., Wang C. J. Ecological correlation between arsenic level in well water and age-adjusted mortality from malignant neoplasms. Cancer Res. 1990 Sep 1;50(17):5470–5474. [PubMed] [Google Scholar]
- Chen C. J., Wu M. M., Lee S. S., Wang J. D., Cheng S. H., Wu H. Y. Atherogenicity and carcinogenicity of high-arsenic artesian well water. Multiple risk factors and related malignant neoplasms of blackfoot disease. Arteriosclerosis. 1988 Sep-Oct;8(5):452–460. doi: 10.1161/01.atv.8.5.452. [DOI] [PubMed] [Google Scholar]
- Chiang H. S., Guo H. R., Hong C. L., Lin S. M., Lee E. F. The incidence of bladder cancer in the black foot disease endemic area in Taiwan. Br J Urol. 1993 Mar;71(3):274–278. doi: 10.1111/j.1464-410x.1993.tb15942.x. [DOI] [PubMed] [Google Scholar]
- Chiou H. Y., Hsueh Y. M., Liaw K. F., Horng S. F., Chiang M. H., Pu Y. S., Lin J. S., Huang C. H., Chen C. J. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res. 1995 Mar 15;55(6):1296–1300. [PubMed] [Google Scholar]
- Chiou H. Y., Huang W. I., Su C. L., Chang S. F., Hsu Y. H., Chen C. J. Dose-response relationship between prevalence of cerebrovascular disease and ingested inorganic arsenic. Stroke. 1997 Sep;28(9):1717–1723. doi: 10.1161/01.str.28.9.1717. [DOI] [PubMed] [Google Scholar]
- Engel R. R., Smith A. H. Arsenic in drinking water and mortality from vascular disease: an ecologic analysis in 30 counties in the United States. Arch Environ Health. 1994 Sep-Oct;49(5):418–427. doi: 10.1080/00039896.1994.9954996. [DOI] [PubMed] [Google Scholar]
- Enterline P. E., Henderson V. L., Marsh G. M. Exposure to arsenic and respiratory cancer. A reanalysis. Am J Epidemiol. 1987 Jun;125(6):929–938. doi: 10.1093/oxfordjournals.aje.a114631. [DOI] [PubMed] [Google Scholar]
- Feinglass E. J. Arsenic intoxication from well water in the United States. N Engl J Med. 1973 Apr 19;288(16):828–830. doi: 10.1056/NEJM197304192881608. [DOI] [PubMed] [Google Scholar]
- Gerhardsson L., Dahlgren E., Eriksson A., Lagerkvist B. E., Lundström J., Nordberg G. F. Fatal arsenic poisoning--a case report. Scand J Work Environ Health. 1988 Apr;14(2):130–133. doi: 10.5271/sjweh.1944. [DOI] [PubMed] [Google Scholar]
- Hansen E. S. International Commission for Protection Against Environmental Mutagens and Carcinogens. ICPEMC Working Paper 7/1/2. Shared risk factors for cancer and atherosclerosis--a review of the epidemiological evidence. Mutat Res. 1990 Nov;239(3):163–179. doi: 10.1016/0165-1110(90)90004-u. [DOI] [PubMed] [Google Scholar]
- Harrington J. M., Middaugh J. P., Morse D. L., Housworth J. A survey of a population exposed to high concentrations of arsenic in well water in Fairbanks, Alaska. Am J Epidemiol. 1978 Nov;108(5):377–385. doi: 10.1093/oxfordjournals.aje.a112635. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C., Biggs M. L., Fuchs A., Bergoglio R., Tello E. E., Nicolli H., Smith A. H. Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology. 1996 Mar;7(2):117–124. doi: 10.1097/00001648-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Kreiss K., Zack M. M., Landrigan P. J., Feldman R. G., Niles C. A., Chirico-Post J., Sax D. S., Boyd M. H., Cox D. H. Neurologic evaluation of a population exposed to arsenic in Alaskan well water. Arch Environ Health. 1983 Mar-Apr;38(2):116–121. doi: 10.1080/00039896.1983.10543990. [DOI] [PubMed] [Google Scholar]
- Lai M. S., Hsueh Y. M., Chen C. J., Shyu M. P., Chen S. Y., Kuo T. L., Wu M. M., Tai T. Y. Ingested inorganic arsenic and prevalence of diabetes mellitus. Am J Epidemiol. 1994 Mar 1;139(5):484–492. doi: 10.1093/oxfordjournals.aje.a117031. [DOI] [PubMed] [Google Scholar]
- Lerman S., Clarkson T. W. The metabolism of arsenite and arsenate by the rat. Fundam Appl Toxicol. 1983 Jul-Aug;3(4):309–314. doi: 10.1016/s0272-0590(83)80145-6. [DOI] [PubMed] [Google Scholar]
- Lyon J. L., Gardner K., Gress R. E. Cancer incidence among Mormons and non-Mormons in Utah (United States) 1971-85. Cancer Causes Control. 1994 Mar;5(2):149–156. doi: 10.1007/BF01830261. [DOI] [PubMed] [Google Scholar]
- Lyon J. L., Klauber M. R., Gardner J. W., Smart C. R. Cancer incidence in Mormons and non-Mormons in Utah, 1966-1970. N Engl J Med. 1976 Jan 15;294(3):129–133. doi: 10.1056/NEJM197601152940303. [DOI] [PubMed] [Google Scholar]
- Morton W., Starr G., Pohl D., Stoner J., Wagner S., Weswig D. Skin cancer and water arsenic in Lane County, Oregon. Cancer. 1976 May;37(5):2523–2532. doi: 10.1002/1097-0142(197605)37:5<2523::aid-cncr2820370545>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Rahman M., Tondel M., Ahmad S. A., Axelson O. Diabetes mellitus associated with arsenic exposure in Bangladesh. Am J Epidemiol. 1998 Jul 15;148(2):198–203. doi: 10.1093/oxfordjournals.aje.a009624. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rothman K. J. No adjustments are needed for multiple comparisons. Epidemiology. 1990 Jan;1(1):43–46. [PubMed] [Google Scholar]
- Simonato L., Moulin J. J., Javelaud B., Ferro G., Wild P., Winkelmann R., Saracci R. A retrospective mortality study of workers exposed to arsenic in a gold mine and refinery in France. Am J Ind Med. 1994 May;25(5):625–633. doi: 10.1002/ajim.4700250503. [DOI] [PubMed] [Google Scholar]
- Smith A. H., Goycolea M., Haque R., Biggs M. L. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998 Apr 1;147(7):660–669. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- Tseng W. P., Chu H. M., How S. W., Fong J. M., Lin C. S., Yeh S. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J Natl Cancer Inst. 1968 Mar;40(3):453–463. [PubMed] [Google Scholar]
- Tseng W. P. Effects and dose--response relationships of skin cancer and blackfoot disease with arsenic. Environ Health Perspect. 1977 Aug;19:109–119. doi: 10.1289/ehp.7719109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine J. L., He S. Y., Reisbord L. S., Lachenbruch P. A. Health response by questionnaire in arsenic-exposed populations. J Clin Epidemiol. 1992 May;45(5):487–494. doi: 10.1016/0895-4356(92)90097-7. [DOI] [PubMed] [Google Scholar]
- WOOLF C. M. An investigation of the familial aspects of carcinoma of the prostate. Cancer. 1960 Jul-Aug;13:739–744. doi: 10.1002/1097-0142(196007/08)13:4<739::aid-cncr2820130414>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Warner M. L., Moore L. E., Smith M. T., Kalman D. A., Fanning E., Smith A. H. Increased micronuclei in exfoliated bladder cells of individuals who chronically ingest arsenic-contaminated water in Nevada. Cancer Epidemiol Biomarkers Prev. 1994 Oct-Nov;3(7):583–590. [PubMed] [Google Scholar]
- Wong O., Whorton M. D., Foliart D. E., Lowengart R. An ecologic study of skin cancer and environmental arsenic exposure. Int Arch Occup Environ Health. 1992;64(4):235–241. doi: 10.1007/BF00378280. [DOI] [PubMed] [Google Scholar]
- Wu M. M., Kuo T. L., Hwang Y. H., Chen C. J. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989 Dec;130(6):1123–1132. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- Yamauchi H., Yamamura Y. Concentration and chemical species of arsenic in human tissue. Bull Environ Contam Toxicol. 1983 Sep;31(3):267–270. doi: 10.1007/BF01608697. [DOI] [PubMed] [Google Scholar]
- Zierler S., Theodore M., Cohen A., Rothman K. J. Chemical quality of maternal drinking water and congenital heart disease. Int J Epidemiol. 1988 Sep;17(3):589–594. doi: 10.1093/ije/17.3.589. [DOI] [PubMed] [Google Scholar]



