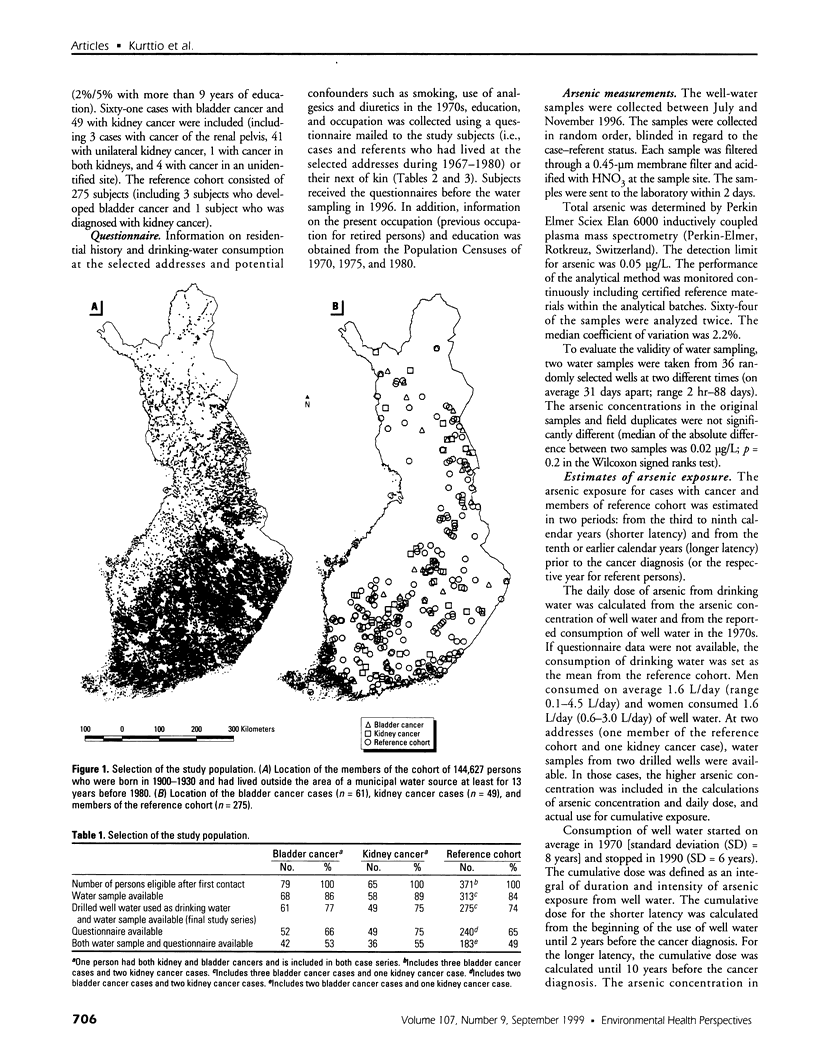
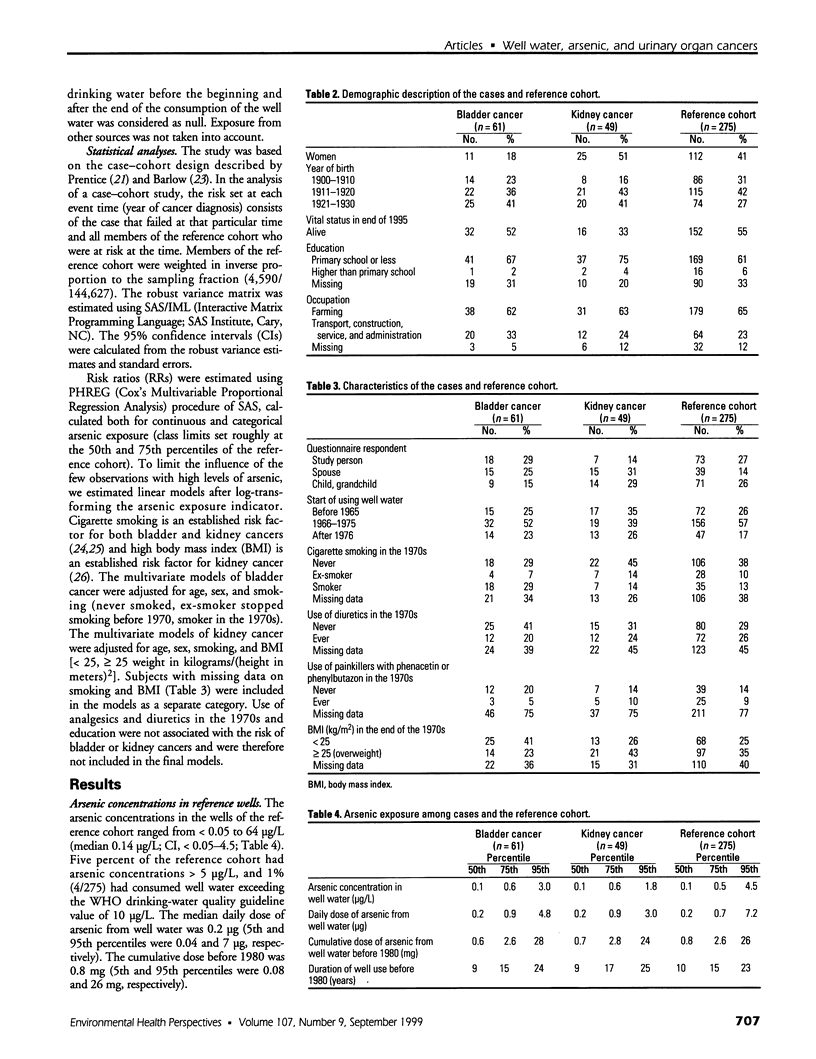
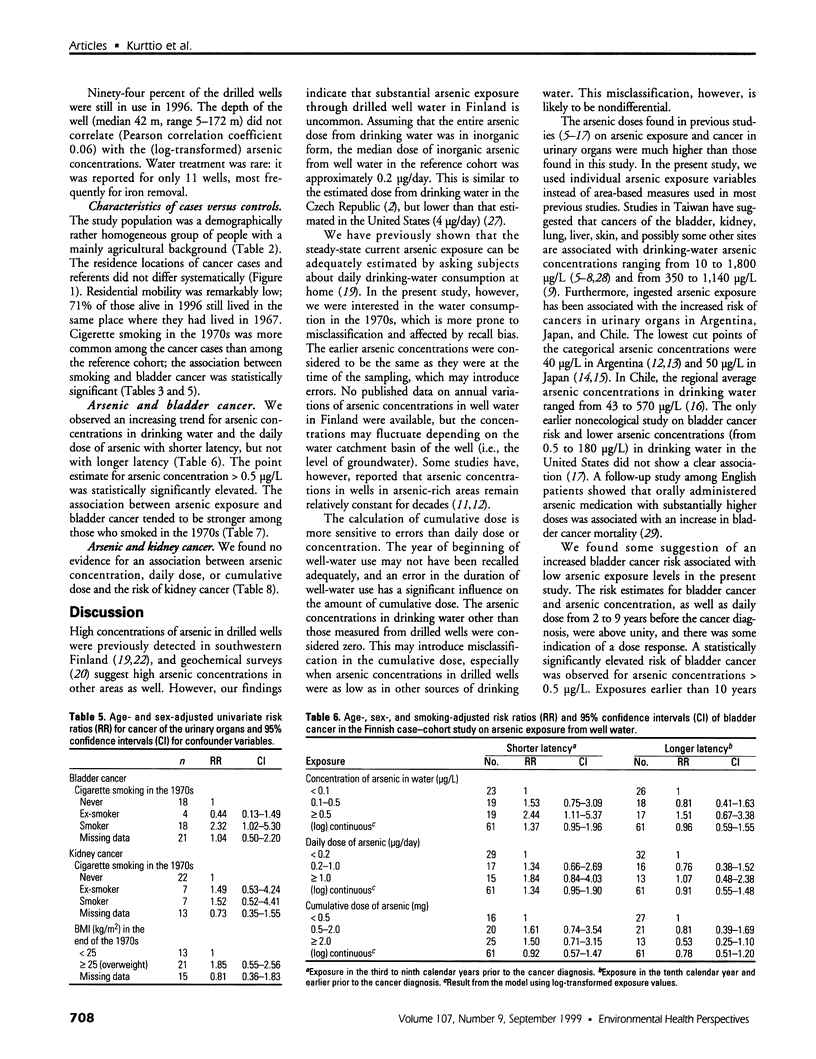
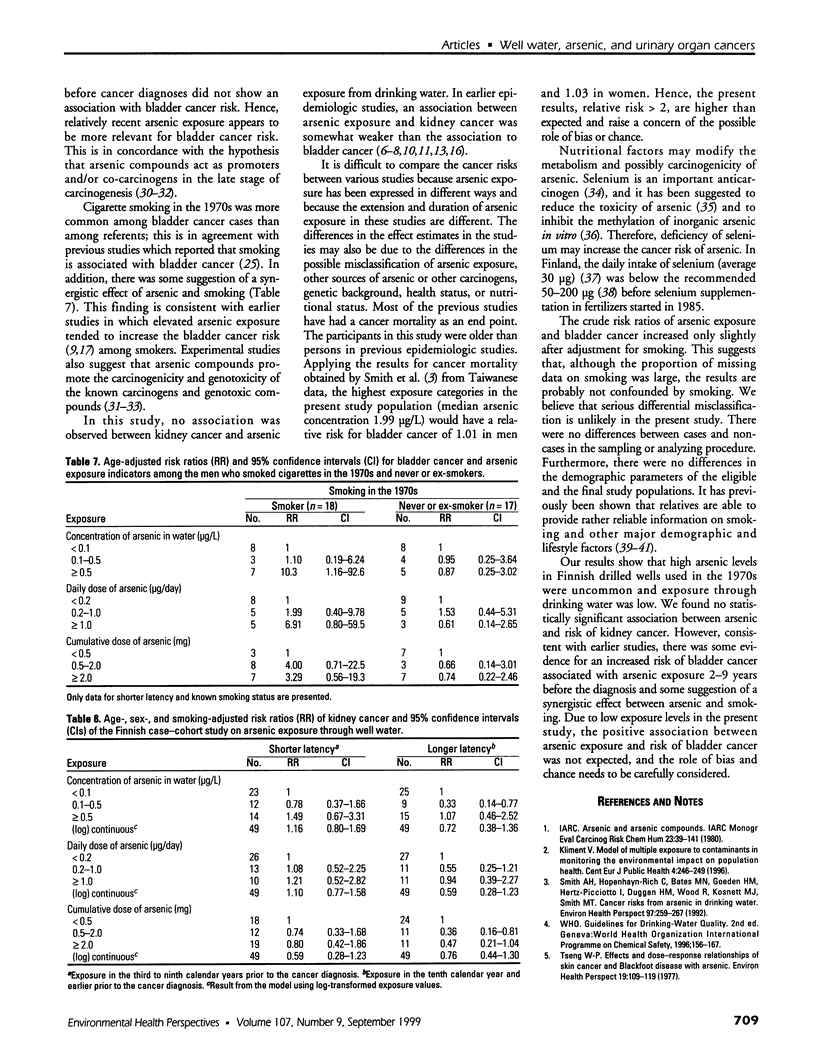
Abstract
We assessed the levels of arsenic in drilled wells in Finland and studied the association of arsenic exposure with the risk of bladder and kidney cancers. The study persons were selected from a register-based cohort of all Finns who had lived at an address outside the municipal drinking-water system during 1967-1980 (n = 144,627). The final study population consisted of 61 bladder cancer cases and 49 kidney cancer cases diagnosed between 1981 and 1995, as well as an age- and sex-balanced random sample of 275 subjects (reference cohort). Water samples were obtained from the wells used by the study population at least during 1967-1980. The total arsenic concentrations in the wells of the reference cohort were low (median = 0.1 microg/L; maximum = 64 microg/L), and 1% exceeded 10 microg/L. Arsenic exposure was estimated as arsenic concentration in the well, daily dose, and cumulative dose of arsenic. None of the exposure indicators was statistically significantly associated with the risk of kidney cancer. Bladder cancer tended to be associated with arsenic concentration and daily dose during the third to ninth years prior to the cancer diagnosis; the risk ratios for arsenic concentration categories 0.1-0.5 and [Greater/equal to] 0.5 microg/L relative to the category with < 0.1 microg/L were 1.53 [95% confidence interval (CI), 0.75-3.09] and 2.44 (CI, 1.11-5.37), respectively. In spite of very low exposure levels, we found some evidence of an association between arsenic and bladder cancer risk. More studies are needed to confirm the possible association between arsenic and bladder cancer risk at such low exposure levels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow W. E. Robust variance estimation for the case-cohort design. Biometrics. 1994 Dec;50(4):1064–1072. [PubMed] [Google Scholar]
- Bates M. N., Smith A. H., Cantor K. P. Case-control study of bladder cancer and arsenic in drinking water. Am J Epidemiol. 1995 Mar 15;141(6):523–530. doi: 10.1093/oxfordjournals.aje.a117467. [DOI] [PubMed] [Google Scholar]
- Berry J. P., Galle P. Selenium-arsenic interaction in renal cells: role of lysosomes. Electron microprobe study. J Submicrosc Cytol Pathol. 1994 Apr;26(2):203–210. [PubMed] [Google Scholar]
- Boyle C. A., Brann E. A. Proxy respondents and the validity of occupational and other exposure data. The Selected Cancers Cooperative Study Group. Am J Epidemiol. 1992 Sep 15;136(6):712–721. doi: 10.1093/oxfordjournals.aje.a116550. [DOI] [PubMed] [Google Scholar]
- Brown J. L., Kitchin K. T. Arsenite, but not cadmium, induces ornithine decarboxylase and heme oxygenase activity in rat liver: relevance to arsenic carcinogenesis. Cancer Lett. 1996 Jan 2;98(2):227–231. [PubMed] [Google Scholar]
- Chen C. J., Chen C. W., Wu M. M., Kuo T. L. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer. 1992 Nov;66(5):888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Kuo T. L., Wu M. M. Arsenic and cancers. Lancet. 1988 Feb 20;1(8582):414–415. doi: 10.1016/s0140-6736(88)91207-x. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Wang C. J. Ecological correlation between arsenic level in well water and age-adjusted mortality from malignant neoplasms. Cancer Res. 1990 Sep 1;50(17):5470–5474. [PubMed] [Google Scholar]
- Chiou H. Y., Hsueh Y. M., Liaw K. F., Horng S. F., Chiang M. H., Pu Y. S., Lin J. S., Huang C. H., Chen C. J. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res. 1995 Mar 15;55(6):1296–1300. [PubMed] [Google Scholar]
- Cuzick J., Sasieni P., Evans S. Ingested arsenic, keratoses, and bladder cancer. Am J Epidemiol. 1992 Aug 15;136(4):417–421. doi: 10.1093/oxfordjournals.aje.a116514. [DOI] [PubMed] [Google Scholar]
- Dreyer L., Winther J. F., Pukkala E., Andersen A. Avoidable cancers in the Nordic countries. Tobacco smoking. APMIS Suppl. 1997;76:9–47. [PubMed] [Google Scholar]
- Guo H. R., Chiang H. S., Hu H., Lipsitz S. R., Monson R. R. Arsenic in drinking water and incidence of urinary cancers. Epidemiology. 1997 Sep;8(5):545–550. doi: 10.1097/00001648-199709000-00012. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C., Biggs M. L., Fuchs A., Bergoglio R., Tello E. E., Nicolli H., Smith A. H. Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology. 1996 Mar;7(2):117–124. doi: 10.1097/00001648-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C., Biggs M. L., Smith A. H. Lung and kidney cancer mortality associated with arsenic in drinking water in Córdoba, Argentina. Int J Epidemiol. 1998 Aug;27(4):561–569. doi: 10.1093/ije/27.4.561. [DOI] [PubMed] [Google Scholar]
- Kliment V. Model of multiple exposure to contaminants in monitoring the environmental impact on population health. Cent Eur J Public Health. 1996 Dec;4(4):246–249. [PubMed] [Google Scholar]
- Kurttio P., Komulainen H., Hakala E., Kahelin H., Pekkanen J. Urinary excretion of arsenic species after exposure to arsenic present in drinking water. Arch Environ Contam Toxicol. 1998 Apr;34(3):297–305. doi: 10.1007/s002449900321. [DOI] [PubMed] [Google Scholar]
- Lahermo P., Alfthan G., Wang D. Selenium and arsenic in the environment in Finland. J Environ Pathol Toxicol Oncol. 1998;17(3-4):205–216. [PubMed] [Google Scholar]
- Li J. H., Rossman T. G. Mechanism of comutagenesis of sodium arsenite with n-methyl-n-nitrosourea. Biol Trace Elem Res. 1989 Jul-Sep;21:373–381. doi: 10.1007/BF02917278. [DOI] [PubMed] [Google Scholar]
- Lyon J. L., Egger M. J., Robison L. M., French T. K., Gao R. Misclassification of exposure in a case-control study: the effects of different types of exposure and different proxy respondents in a study of pancreatic cancer. Epidemiology. 1992 May;3(3):223–231. doi: 10.1097/00001648-199205000-00007. [DOI] [PubMed] [Google Scholar]
- Mass M. J., Wang L. Arsenic alters cytosine methylation patterns of the promoter of the tumor suppressor gene p53 in human lung cells: a model for a mechanism of carcinogenesis. Mutat Res. 1997 Jun;386(3):263–277. doi: 10.1016/s1383-5742(97)00008-2. [DOI] [PubMed] [Google Scholar]
- Muscat J. E., Hoffmann D., Wynder E. L. The epidemiology of renal cell carcinoma. A second look. Cancer. 1995 May 15;75(10):2552–2557. doi: 10.1002/1097-0142(19950515)75:10<2552::aid-cncr2820751023>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Nelson L. M., Longstreth W. T., Jr, Koepsell T. D., Checkoway H., van Belle G. Completeness and accuracy of interview data from proxy respondents: demographic, medical, and life-style factors. Epidemiology. 1994 Mar;5(2):204–217. doi: 10.1097/00001648-199403000-00011. [DOI] [PubMed] [Google Scholar]
- Schrauzer G. N. Selenium. Mechanistic aspects of anticarcinogenic action. Biol Trace Elem Res. 1992 Apr-Jun;33:51–62. doi: 10.1007/BF02783992. [DOI] [PubMed] [Google Scholar]
- Smith A. H., Goycolea M., Haque R., Biggs M. L. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998 Apr 1;147(7):660–669. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- Smith A. H., Hopenhayn-Rich C., Bates M. N., Goeden H. M., Hertz-Picciotto I., Duggan H. M., Wood R., Kosnett M. J., Smith M. T. Cancer risks from arsenic in drinking water. Environ Health Perspect. 1992 Jul;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styblo M., Delnomdedieu M., Thomas D. J. Mono- and dimethylation of arsenic in rat liver cytosol in vitro. Chem Biol Interact. 1996 Jan 5;99(1-3):147–164. doi: 10.1016/0009-2797(95)03666-0. [DOI] [PubMed] [Google Scholar]
- Tseng W. P., Chu H. M., How S. W., Fong J. M., Lin C. S., Yeh S. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J Natl Cancer Inst. 1968 Mar;40(3):453–463. [PubMed] [Google Scholar]
- Tseng W. P. Effects and dose--response relationships of skin cancer and blackfoot disease with arsenic. Environ Health Perspect. 1977 Aug;19:109–119. doi: 10.1289/ehp.7719109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda T., Babazono A., Yamamoto E., Kurumatani N., Mino Y., Ogawa T., Kishi Y., Aoyama H. Ingested arsenic and internal cancer: a historical cohort study followed for 33 years. Am J Epidemiol. 1995 Feb 1;141(3):198–209. doi: 10.1093/oxfordjournals.aje.a117421. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Nagira T., Yamamoto M., Kume Y. An epidemiological study on cancer in certified arsenic poisoning patients in Toroku. Ind Health. 1990;28(2):53–62. doi: 10.2486/indhealth.28.53. [DOI] [PubMed] [Google Scholar]
- Valberg P. A., Beck B. D., Bowers T. S., Keating J. L., Bergstrom P. D., Boardman P. D. Issues in setting health-based cleanup levels for arsenic in soil. Regul Toxicol Pharmacol. 1997 Oct;26(2):219–229. doi: 10.1006/rtph.1997.1148. [DOI] [PubMed] [Google Scholar]
- Wang T. C., Huang J. S., Yang V. C., Lan H. J., Lin C. J., Jan K. Y. Delay of the excision of UV light-induced DNA adducts is involved in the coclastogenicity of UV light plus arsenite. Int J Radiat Biol. 1994 Oct;66(4):367–372. doi: 10.1080/09553009414551301. [DOI] [PubMed] [Google Scholar]
- Wu M. M., Kuo T. L., Hwang Y. H., Chen C. J. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989 Dec;130(6):1123–1132. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]



