Abstract
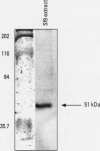
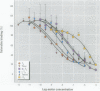
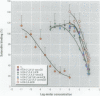
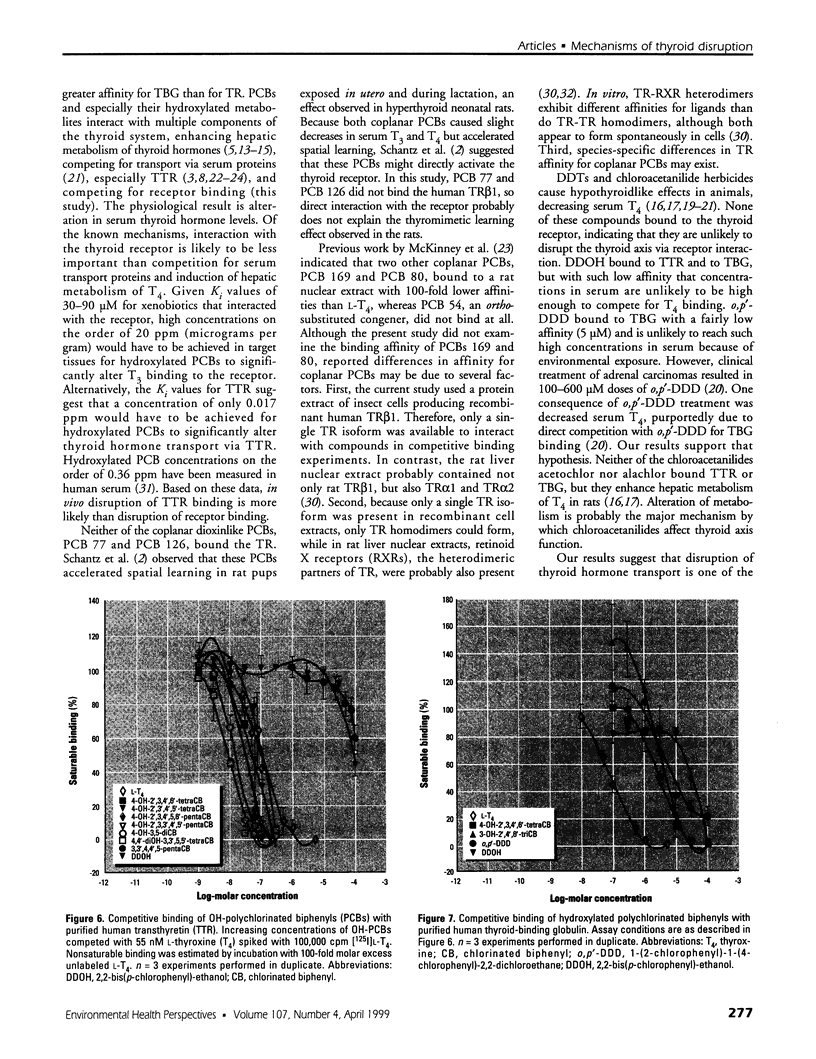
Organochlorine compounds, particularly polychlorinated biphenyls (PCBs), alter serum thyroid hormone levels in humans. Hydroxylated organochlorines have relatively high affinities for the serum transport protein transthyretin, but the ability of these compounds to interact with the human thyroid receptor is unknown. Using a baculovirus expression system in insect cells (Sf9 cells), we produced recombinant human thyroid receptor ss (hTRss). In competitive binding experiments, the recombinant receptor had the expected relative affinity for thyroid hormones and their analogs. In competitive inhibition experiments with PCBs, hydroxylated PCBs (OH-PCBs), DDT and its metabolites, and several organochlorine herbicides, only the OH-PCBs competed for binding. The affinity of hTRss for OH-PCBs was 10,000-fold lower (Ki = 20-50 microM) than its affinity for thyroid hormone (3,3',5-triiodothyronine, T3; Ki = 10 nM). Because their relative affinity for the receptor was low, we tested the ability of OH-PCBs to interact with the serum transport proteins--transthyretin and thyroid-binding globulin (TBG). With the exception of one compound, the OH-PCBs had the same affinity (Ki = 10-80 nM) for transthyretin as thyroid hormone (thyroxine; T4). Only two of the OH-PCBs bound TBG (Ki = 3-7 microM), but with a 100-fold lower affinity than T4. Hydroxylated PCBs have relatively low affinities for the human thyroid receptor in vitro, but they have a thyroid hormonelike affinity for the serum transport protein transthyretin. Based on these results, OH-PCBs in vivo are more likely to compete for binding to serum transport proteins than for binding to the thyroid receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby J., Kier L., Wilson A. G., Green T., Lefevre P. A., Tinwell H., Willis G. A., Heydens W. F., Clapp M. J. Evaluation of the potential carcinogenicity and genetic toxicity to humans of the herbicide acetochlor. Hum Exp Toxicol. 1996 Sep;15(9):702–735. doi: 10.1177/096032719601500902. [DOI] [PubMed] [Google Scholar]
- Barter R. A., Klaassen C. D. Reduction of thyroid hormone levels and alteration of thyroid function by four representative UDP-glucuronosyltransferase inducers in rats. Toxicol Appl Pharmacol. 1994 Sep;128(1):9–17. doi: 10.1006/taap.1994.1174. [DOI] [PubMed] [Google Scholar]
- Barter R. A., Klaassen C. D. UDP-glucuronosyltransferase inducers reduce thyroid hormone levels in rats by an extrathyroidal mechanism. Toxicol Appl Pharmacol. 1992 Mar;113(1):36–42. doi: 10.1016/0041-008x(92)90006-e. [DOI] [PubMed] [Google Scholar]
- Bastomsky C. H. Effects of a polychlorinated biphenyl mixture (aroclor 1254) and DDT on biliary thyroxine excretion in rats. Endocrinology. 1974 Oct;95(4):1150–1155. doi: 10.1210/endo-95-4-1150. [DOI] [PubMed] [Google Scholar]
- Beetstra J. B., van Engelen J. G., Karels P., van der Hoek H. J., de Jong M., Docter R., Krenning E. P., Hennemann G., Brouwer A., Visser T. J. Thyroxine and 3,3',5-triiodothyronine are glucuronidated in rat liver by different uridine diphosphate-glucuronyltransferases. Endocrinology. 1991 Feb;128(2):741–746. doi: 10.1210/endo-128-2-741. [DOI] [PubMed] [Google Scholar]
- Bergman A., Klasson-Wehler E., Kuroki H. Selective retention of hydroxylated PCB metabolites in blood. Environ Health Perspect. 1994 May;102(5):464–469. doi: 10.1289/ehp.94102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bres O., Eales J. G. Thyroid hormone binding to isolated trout (Salmo gairdneri) liver nuclei in vitro: binding affinity, capacity, and chemical specificity. Gen Comp Endocrinol. 1986 Jan;61(1):29–39. doi: 10.1016/0016-6480(86)90246-7. [DOI] [PubMed] [Google Scholar]
- Byrne J. J., Carbone J. P., Hanson E. A. Hypothyroidism and abnormalities in the kinetics of thyroid hormone metabolism in rats treated chronically with polychlorinated biphenyl and polybrominated biphenyl. Endocrinology. 1987 Aug;121(2):520–527. doi: 10.1210/endo-121-2-520. [DOI] [PubMed] [Google Scholar]
- Darnerud P. O., Morse D., Klasson-Wehler E., Brouwer A. Binding of a 3,3', 4,4'-tetrachlorobiphenyl (CB-77) metabolite to fetal transthyretin and effects on fetal thyroid hormone levels in mice. Toxicology. 1996 Jan 8;106(1-3):105–114. doi: 10.1016/0300-483x(95)03169-g. [DOI] [PubMed] [Google Scholar]
- Falcone M., Miyamoto T., Fierro-Renoy F., Macchia E., DeGroot L. J. Antipeptide polyclonal antibodies specifically recognize each human thyroid hormone receptor isoform. Endocrinology. 1992 Nov;131(5):2419–2429. doi: 10.1210/endo.131.5.1425440. [DOI] [PubMed] [Google Scholar]
- Glass C. K. Some new twists in the regulation of gene expression by thyroid hormone and retinoic acid receptors. J Endocrinol. 1996 Sep;150(3):349–357. doi: 10.1677/joe.0.1500349. [DOI] [PubMed] [Google Scholar]
- Goldey E. S., Kehn L. S., Lau C., Rehnberg G. L., Crofton K. M. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol. 1995 Nov;135(1):77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr, Ostby J., Marshall R., Andrews J. Reproductive and thyroid effects of low-level polychlorinated biphenyl (Aroclor 1254) exposure. Fundam Appl Toxicol. 1993 Apr;20(3):288–294. doi: 10.1006/faat.1993.1038. [DOI] [PubMed] [Google Scholar]
- Grässle B., Biessmann A. Effects of DDT, polychlorinated biphenyls and thiouracil on circulating thyroid hormones, thyroid histology and eggshell quality in Japanese quail (Coturnix coturnix japonica). Chem Biol Interact. 1982 Dec;42(3):371–377. doi: 10.1016/0009-2797(82)90080-1. [DOI] [PubMed] [Google Scholar]
- Koopman-Esseboom C., Huisman M., Touwen B. C., Boersma E. R., Brouwer A., Sauer P. J., Weisglas-Kuperus N. Newborn infants diagnosed as neurologically abnormal with relation to PCB and dioxin exposure and their thyroid-hormone status. Dev Med Child Neurol. 1997 Nov;39(11):785–785. [PubMed] [Google Scholar]
- Koopman-Esseboom C., Morse D. C., Weisglas-Kuperus N., Lutkeschipholt I. J., Van der Paauw C. G., Tuinstra L. G., Brouwer A., Sauer P. J. Effects of dioxins and polychlorinated biphenyls on thyroid hormone status of pregnant women and their infants. Pediatr Res. 1994 Oct;36(4):468–473. doi: 10.1203/00006450-199410000-00009. [DOI] [PubMed] [Google Scholar]
- Lans M. C., Klasson-Wehler E., Willemsen M., Meussen E., Safe S., Brouwer A. Structure-dependent, competitive interaction of hydroxy-polychlorobiphenyls, -dibenzo-p-dioxins and -dibenzofurans with human transthyretin. Chem Biol Interact. 1993 Jul;88(1):7–21. doi: 10.1016/0009-2797(93)90081-9. [DOI] [PubMed] [Google Scholar]
- Lans M. C., Spiertz C., Brouwer A., Koeman J. H. Different competition of thyroxine binding to transthyretin and thyroxine-binding globulin by hydroxy-PCBs, PCDDs and PCDFs. Eur J Pharmacol. 1994 Apr 4;270(2-3):129–136. doi: 10.1016/0926-6917(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Larsson M., Pettersson T., Carlström A. Thyroid hormone binding in serum of 15 vertebrate species: isolation of thyroxine-binding globulin and prealbumin analogs. Gen Comp Endocrinol. 1985 Jun;58(3):360–375. doi: 10.1016/0016-6480(85)90108-x. [DOI] [PubMed] [Google Scholar]
- Lazar M. A. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993 Apr;14(2):184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- Marshall J. S., Tompkins L. S. Effect of o,p'-DDD and similar compounds on thyroxine binding globulin. J Clin Endocrinol Metab. 1968 Mar;28(3):386–392. doi: 10.1210/jcem-28-3-386. [DOI] [PubMed] [Google Scholar]
- McKinney J., Fannin R., Jordan S., Chae K., Rickenbacher U., Pedersen L. Polychlorinated biphenyls and related compound interactions with specific binding sites for thyroxine in rat liver nuclear extracts. J Med Chem. 1987 Jan;30(1):79–86. doi: 10.1021/jm00384a014. [DOI] [PubMed] [Google Scholar]
- Morse D. C., Wehler E. K., Wesseling W., Koeman J. H., Brouwer A. Alterations in rat brain thyroid hormone status following pre- and postnatal exposure to polychlorinated biphenyls (Aroclor 1254). Toxicol Appl Pharmacol. 1996 Feb;136(2):269–279. doi: 10.1006/taap.1996.0034. [DOI] [PubMed] [Google Scholar]
- Park J. B., Ashizawa K., Parkison C., Cheng S. Y. One-step immunoaffinity purification of human beta 1 thyroid hormone receptor with DNA and hormone binding activity. J Biochem Biophys Methods. 1993 Sep;27(2):95–103. doi: 10.1016/0165-022x(93)90053-q. [DOI] [PubMed] [Google Scholar]
- Rickenbacher U., McKinney J. D., Oatley S. J., Blake C. C. Structurally specific binding of halogenated biphenyls to thyroxine transport protein. J Med Chem. 1986 May;29(5):641–648. doi: 10.1021/jm00155a010. [DOI] [PubMed] [Google Scholar]
- Sauer P. J., Huisman M., Koopman-Esseboom C., Morse D. C., Smits-van Prooije A. E., van de Berg K. J., Tuinstra L. G., van der Paauw C. G., Boersma E. R., Weisglas-Kuperus N. Effects of polychlorinated biphenyls (PCBs) and dioxins on growth and development. Hum Exp Toxicol. 1994 Dec;13(12):900–906. doi: 10.1177/096032719401301213. [DOI] [PubMed] [Google Scholar]
- Sullivan C. V., Darling D. S., Dickhoff W. W. Nuclear receptors for L-triiodothyronine in trout erythrocytes. Gen Comp Endocrinol. 1987 Jan;65(1):149–160. doi: 10.1016/0016-6480(87)90234-6. [DOI] [PubMed] [Google Scholar]
- Van Birgelen A. P., Smit E. A., Kampen I. M., Groeneveld C. N., Fase K. M., Van der Kolk J., Poiger H., Van den Berg M., Koeman J. H., Brouwer A. Subchronic effects of 2,3,7,8-TCDD or PCBs on thyroid hormone metabolism: use in risk assessment. Eur J Pharmacol. 1995 May 26;293(1):77–85. doi: 10.1016/0926-6917(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Wilson A. G., Thake D. C., Heydens W. E., Brewster D. W., Hotz K. J. Mode of action of thyroid tumor formation in the male Long-Evans rat administered high doses of alachlor. Fundam Appl Toxicol. 1996 Sep;33(1):16–23. doi: 10.1006/faat.1996.0138. [DOI] [PubMed] [Google Scholar]