Abstract
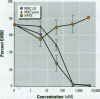
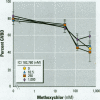
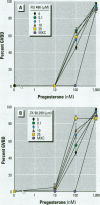
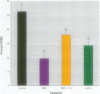
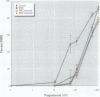
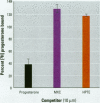
There is currently little evidence of pollution-induced endocrine dysfunction in amphibia, in spite of widespread concern over global declines in this ecologically diverse group. Data regarding the potential effects of endocrine-disrupting contaminants (EDCs) on reproductive function in amphibia are particularly lacking. We hypothesized that estrogenic EDCs may disrupt progesterone-induced oocyte maturation in the adult amphibian ovary, and tested this with an in vitro germinal vesicle breakdown assay using defolliculated oocytes from the African clawed frog, Xenopus laevis. While a variety of natural and synthetic estrogens and xenoestrogens were inactive in this system, the proestrogenic pesticide methoxychlor was a surprisingly potent inhibitor of progesterone-induced oocyte maturation (median inhibitive concentration, 72 nM). This inhibitory activity was specific to methoxychlor, rather than to its estrogenic contaminants or metabolites, and was not antagonized by the estrogen receptor antagonist ICI 182,780, suggesting that this activity is not estrogenic per se. The inhibitory activity of methoxychlor was dose dependent, reversible, and early acting. However, washout was unable to reverse the effect of short methoxychlor exposure, and methoxychlor did not competitively displace [3H]progesterone from a specific binding site in the oocyte plasma membrane. Therefore, methoxychlor may exert its action not directly at the site of progesterone action, but downstream on early events in maturational signaling, although the precise mechanism of action is unclear. The activity of methoxychlor in this system indicates that xenobiotics may exert endocrine-disrupting effects through interference with progestin-regulated processes and through mechanisms other than receptor antagonism.
Full text
PDF
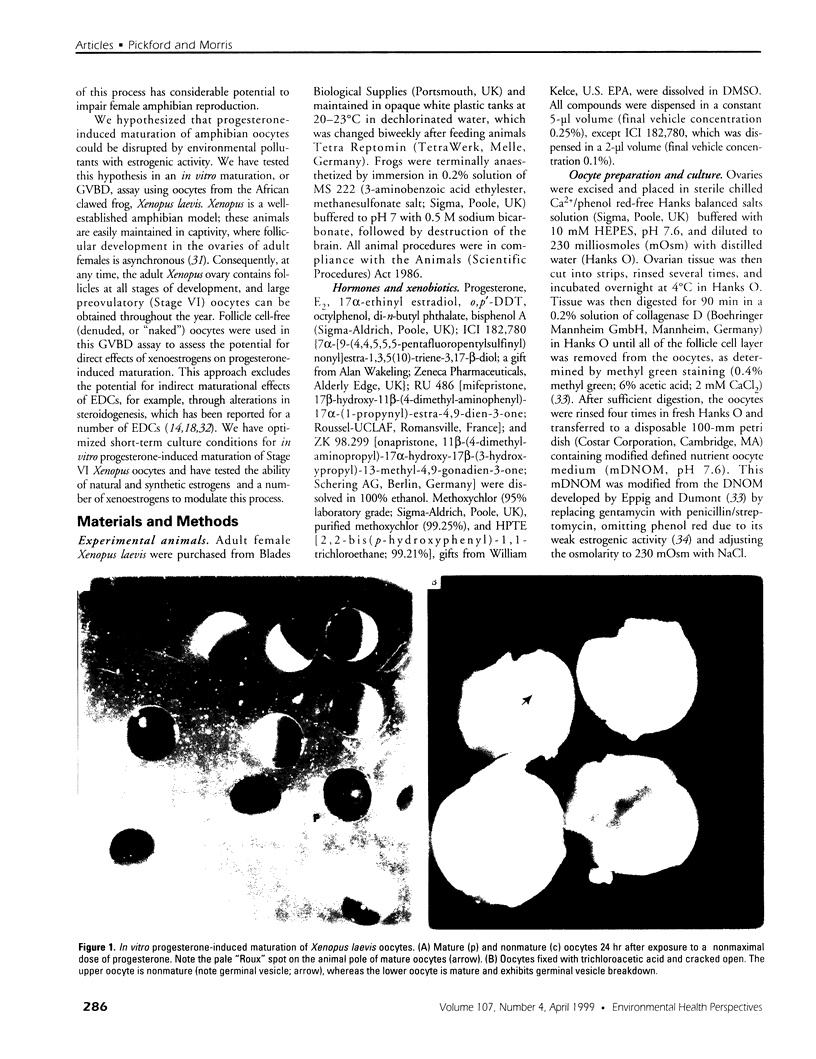
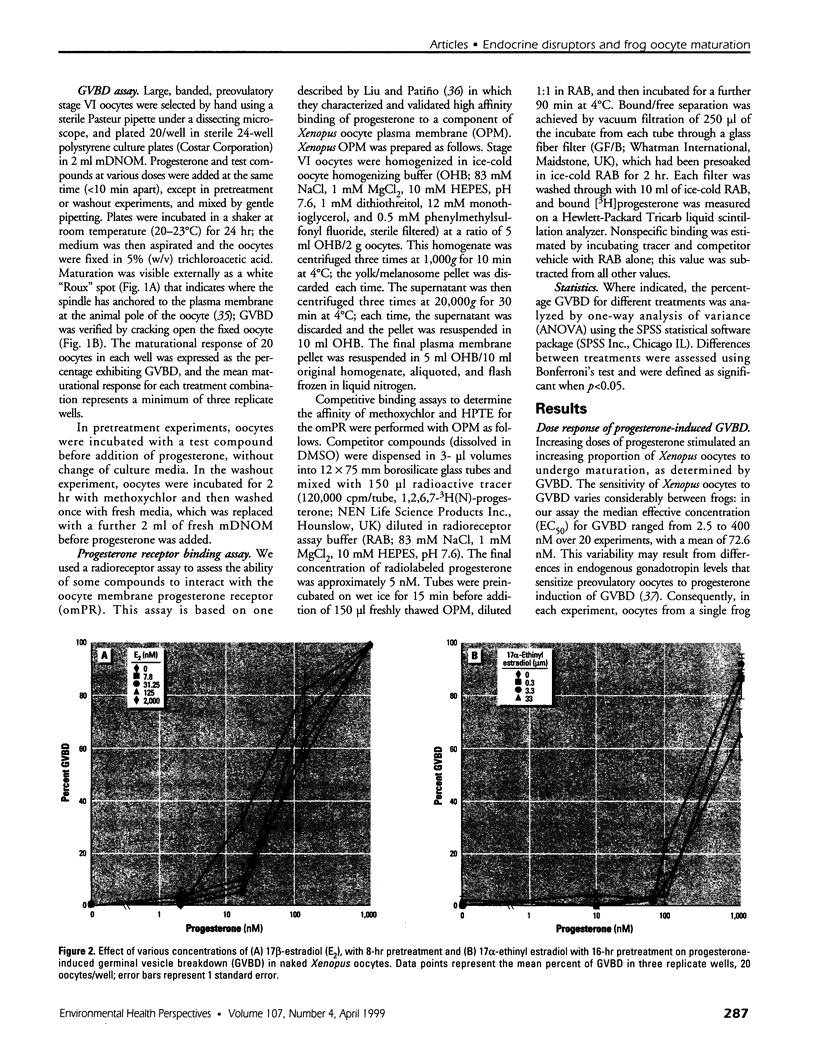
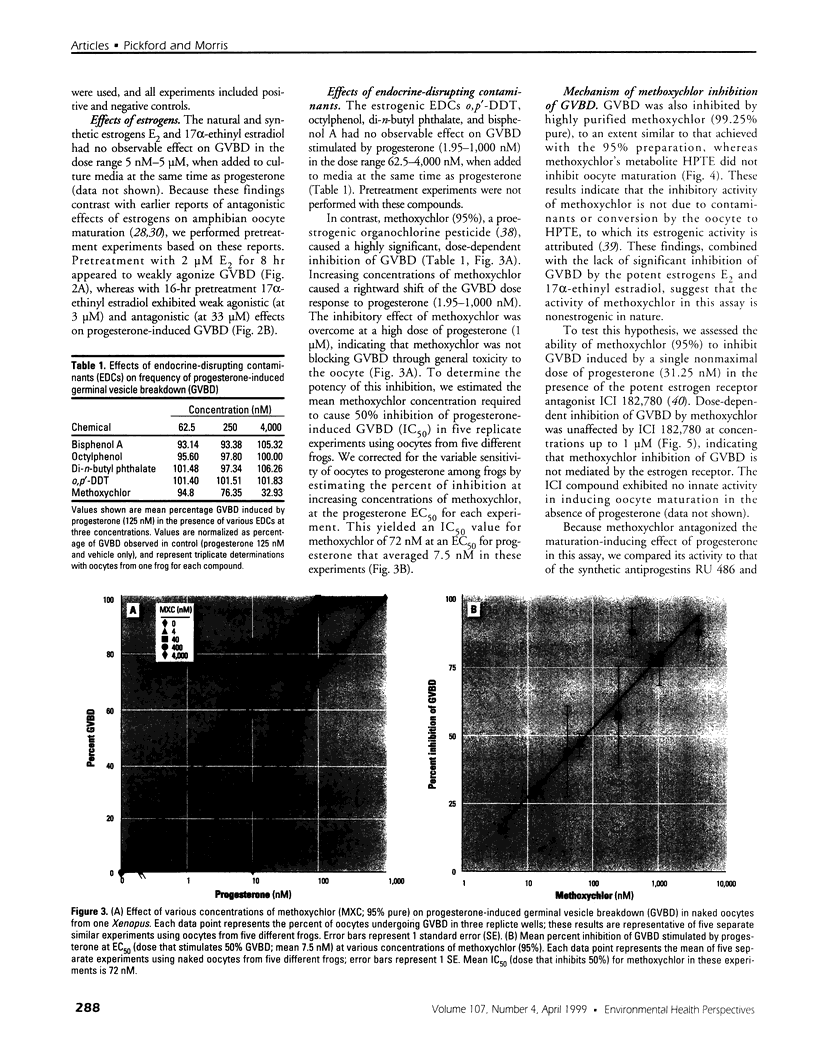
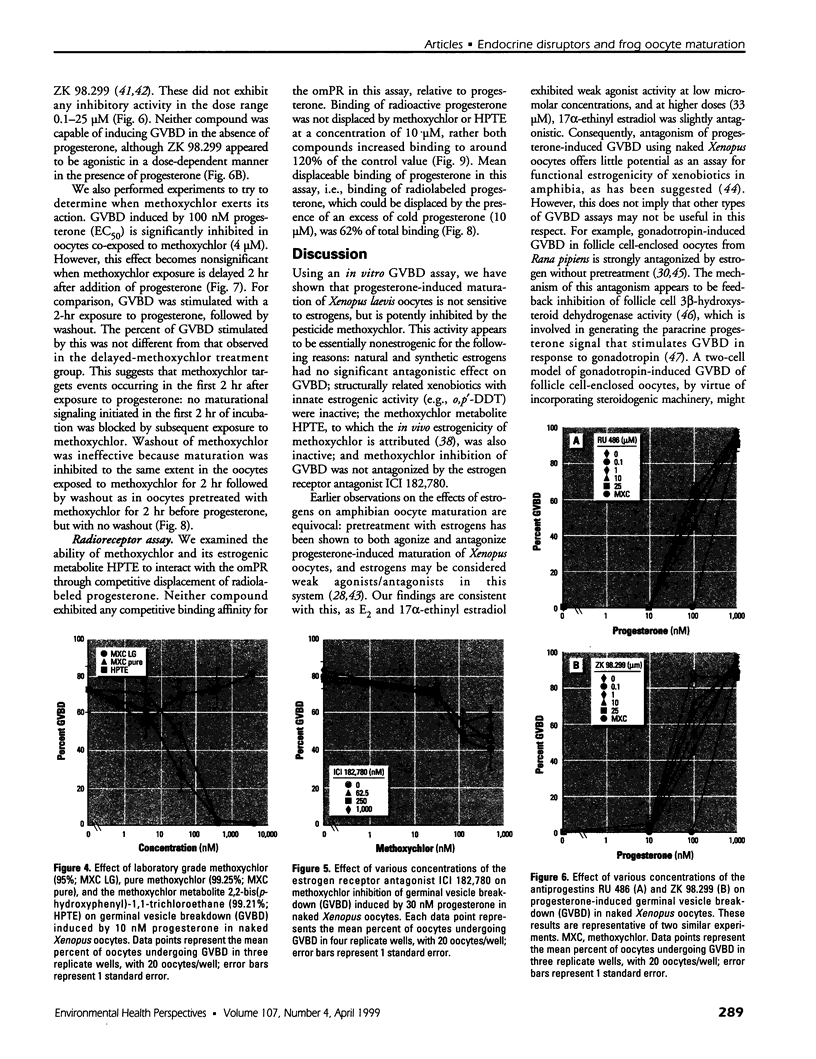
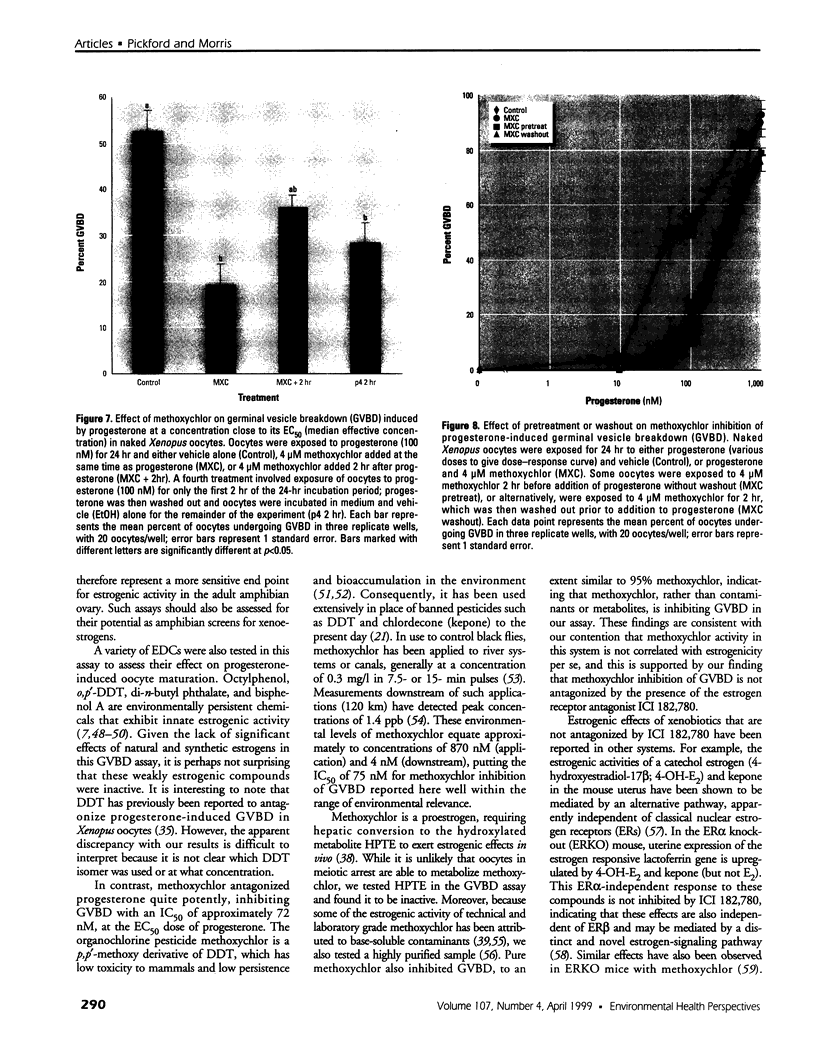

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulieu E. E., Godeau F., Schorderet M., Schorderet-Slatkine S. Steroid-induced meiotic division in Xenopus laevis oocytes: surface and calcium. Nature. 1978 Oct 19;275(5681):593–598. doi: 10.1038/275593a0. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E., Schorderet-Slatkine S. Steroidal and peptidic control mechanisms in membrane of Xenopus laevis oocytes resuming meiotic division. J Steroid Biochem. 1983 Jul;19(1A):139–145. [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein A. R., Hoffman P. D., Hokit D. G., Kiesecker J. M., Walls S. C., Hays J. B. UV repair and resistance to solar UV-B in amphibian eggs: a link to population declines? Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1791–1795. doi: 10.1073/pnas.91.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau J. P., Baulieu E. E. Progesterone receptor characterized by photoaffinity labelling in the plasma membrane of Xenopus laevis oocytes. Biochem J. 1984 May 1;219(3):785–792. doi: 10.1042/bj2190785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer R., Grue C. E. The need for water quality criteria for frogs. Environ Health Perspect. 1995 Apr;103(4):352–357. doi: 10.1289/ehp.95103352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D. W., Hendry L. B., Mahesh V. B. Emerging diversities in the mechanism of action of steroid hormones. J Steroid Biochem Mol Biol. 1995 Feb;52(2):113–133. doi: 10.1016/0960-0760(94)00160-n. [DOI] [PubMed] [Google Scholar]
- Bulger W. H., Feil V. J., Kupfer D. Role of hepatic monooxygenases in generating estrogenic metabolites from methoxychlor and from its identified contaminants. Mol Pharmacol. 1985 Jan;27(1):115–124. [PubMed] [Google Scholar]
- Bulger W. H., Muccitelli R. M., Kupfer D. Interactions of methoxychlor, methoxychlor base-soluble contaminant, and 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane with rat uterine estrogen receptor. J Toxicol Environ Health. 1978 Sep-Nov;4(5-6):881–893. doi: 10.1080/15287397809529709. [DOI] [PubMed] [Google Scholar]
- Bulger W. H., Muccitelli R. M., Kupfer D. Studies on the in vivo and in vitro estrogenic activities of methoxychlor and its metabolites. Role of hepatic mono-oxygenase in methoxychlor activation. Biochem Pharmacol. 1978;27(20):2417–2423. doi: 10.1016/0006-2952(78)90354-4. [DOI] [PubMed] [Google Scholar]
- Carey C., Bryant C. J. Possible interrelations among environmental toxicants, amphibian development, and decline of amphibian populations. Environ Health Perspect. 1995 May;103 (Suppl 4):13–17. doi: 10.1289/ehp.103-1519280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke A. S. Selective predation by newts on frog tadpoles treated with DDT. Nature. 1971 Jan 22;229(5282):275–276. doi: 10.1038/229275a0. [DOI] [PubMed] [Google Scholar]
- Crain D. A., Guillette L. J., Jr, Rooney A. A., Pickford D. B. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Perspect. 1997 May;105(5):528–533. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S. K., Tan J., Johnson D. C., Dey S. K. Differential spatiotemporal regulation of lactoferrin and progesterone receptor genes in the mouse uterus by primary estrogen, catechol estrogen, and xenoestrogen. Endocrinology. 1998 Jun;139(6):2905–2915. doi: 10.1210/endo.139.6.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S. K., Taylor J. A., Korach K. S., Paria B. C., Dey S. K., Lubahn D. B. Estrogenic responses in estrogen receptor-alpha deficient mice reveal a distinct estrogen signaling pathway. Proc Natl Acad Sci U S A. 1997 Nov 25;94(24):12786–12791. doi: 10.1073/pnas.94.24.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosdall L. M., Lehmkuhl D. M. The impact of methoxychlor treatment of the saskatchewan river system on artificial substrate populations of aquatic insects. Environ Pollut. 1989;60(3-4):209–222. doi: 10.1016/0269-7491(89)90105-x. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Ema M., Kurosaka R., Amano H., Ogawa Y. Embryolethality of butyl benzyl phthalate during early pregnancy in rats. Reprod Toxicol. 1994 May-Jun;8(3):231–236. doi: 10.1016/0890-6238(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Eppig J. J., Dumont J. N. Defined nutrient medium for the in vitro maintenance of Xenopus laevis oocytes. In Vitro. 1976 Jun;12(6):418–427. doi: 10.1007/BF02806021. [DOI] [PubMed] [Google Scholar]
- Folmar L. C., Denslow N. D., Rao V., Chow M., Crain D. A., Enblom J., Marcino J., Guillette L. J., Jr Vitellogenin induction and reduced serum testosterone concentrations in feral male carp (Cyprinus carpio) captured near a major metropolitan sewage treatment plant. Environ Health Perspect. 1996 Oct;104(10):1096–1101. doi: 10.1289/ehp.961041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L. E., Jr, Ostby J. S., Kelce W. R. Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat. Toxicol Appl Pharmacol. 1994 Nov;129(1):46–52. doi: 10.1006/taap.1994.1227. [DOI] [PubMed] [Google Scholar]
- Grazzini E., Guillon G., Mouillac B., Zingg H. H. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998 Apr 2;392(6675):509–512. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Crain D. A., Rooney A. A., Pickford D. B. Organization versus activation: the role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environ Health Perspect. 1995 Oct;103 (Suppl 7):157–164. doi: 10.1289/ehp.95103s7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Gross T. S., Gross D. A., Rooney A. A., Percival H. F. Gonadal steroidogenesis in vitro from juvenile alligators obtained from contaminated or control lakes. Environ Health Perspect. 1995 May;103 (Suppl 4):31–36. doi: 10.1289/ehp.95103s431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Gross T. S., Masson G. R., Matter J. M., Percival H. F., Woodward A. R. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994 Aug;102(8):680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette L. J., Jr, Pickford D. B., Crain D. A., Rooney A. A., Percival H. F. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen Comp Endocrinol. 1996 Jan;101(1):32–42. doi: 10.1006/gcen.1996.0005. [DOI] [PubMed] [Google Scholar]
- Harris C. A., Henttu P., Parker M. G., Sumpter J. P. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997 Aug;105(8):802–811. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarrola I., Alejandro A., Marino A., Sancho M. J., Macarulla J. M., Trueba M. Characterization by photoaffinity labeling of a steroid binding protein in rat liver plasma membrane. J Membr Biol. 1992 Jan;125(2):185–191. doi: 10.1007/BF00233357. [DOI] [PubMed] [Google Scholar]
- Kapoor I. P., Metcalf R. L., Nystrom R. F., Sangha G. K. Comparative metabolism of methoxychlor, methiochlor, and DDT in mouse, insects, and in a model ecosystem. J Agric Food Chem. 1970 Nov-Dec;18(6):1145–1152. doi: 10.1021/jf60172a017. [DOI] [PubMed] [Google Scholar]
- Kelce W. R., Stone C. R., Laws S. C., Gray L. E., Kemppainen J. A., Wilson E. M. Persistent DDT metabolite p,p'-DDE is a potent androgen receptor antagonist. Nature. 1995 Jun 15;375(6532):581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- King W., 5th, Ghosh S., Thomas P., Sullivan C. V. A receptor for the oocyte maturation-inducing hormone 17alpha,20beta,21-trihydroxy-4-pregnen-3-one on ovarian membranes of striped bass. Biol Reprod. 1997 Jan;56(1):266–271. doi: 10.1095/biolreprod56.1.266. [DOI] [PubMed] [Google Scholar]
- Krishnan A. V., Stathis P., Permuth S. F., Tokes L., Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993 Jun;132(6):2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- Kuiper G. G., Carlsson B., Grandien K., Enmark E., Häggblad J., Nilsson S., Gustafsson J. A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997 Mar;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- LaMarca M. J., Westphal L. M., Rein D. A. Gonadotropins and the timing of progesterone-induced meiotic maturation of Xenopus laevis oocytes. Dev Biol. 1985 May;109(1):32–40. doi: 10.1016/0012-1606(85)90343-4. [DOI] [PubMed] [Google Scholar]
- Lin Y. W., Schuetz A. W. In vitro estrogen modulation of pituitary and progesterone-induced oocyte maturation in Rana pipiens. J Exp Zool. 1983 May;226(2):281–291. doi: 10.1002/jez.1402260214. [DOI] [PubMed] [Google Scholar]
- Liu Z., Patiño R. High-affinity binding of progesterone to the plasma membrane of Xenopus oocytes: characteristics of binding and hormonal and developmental control. Biol Reprod. 1993 Nov;49(5):980–988. doi: 10.1095/biolreprod49.5.980. [DOI] [PubMed] [Google Scholar]
- Mahesh V. B., Brann D. W., Hendry L. B. Diverse modes of action of progesterone and its metabolites. J Steroid Biochem Mol Biol. 1996 Jan;56(1-6):209–219. doi: 10.1016/0960-0760(95)00238-3. [DOI] [PubMed] [Google Scholar]
- Neef G., Beier S., Elger W., Henderson D., Wiechert R. New steroids with antiprogestational and antiglucocorticoid activities. Steroids. 1984 Oct;44(4):349–372. doi: 10.1016/s0039-128x(84)80027-6. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Mulvihill E. R. Estrogenic activity of the insecticide kepone on the chicken oviduct. Science. 1978 Jul 28;201(4353):356–358. doi: 10.1126/science.78523. [DOI] [PubMed] [Google Scholar]
- Pinter J., Thomas P. Characterization of a progestogen receptor in the ovary of the spotted seatrout, Cynoscion nebulosus. Biol Reprod. 1995 Mar;52(3):667–675. doi: 10.1095/biolreprod52.3.667. [DOI] [PubMed] [Google Scholar]
- Sabeur K., Edwards D. P., Meizel S. Human sperm plasma membrane progesterone receptor(s) and the acrosome reaction. Biol Reprod. 1996 May;54(5):993–1001. doi: 10.1095/biolreprod54.5.993. [DOI] [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. Identification of a steroid receptor on the surface of Xenopus oocytes by photoaffinity labeling. J Biol Chem. 1982 Jan 10;257(1):355–361. [PubMed] [Google Scholar]
- Schuetz A. W. Effect of steroids on germinal vesicle of oocytes of the frog (Rana pipiens) in vitro. Proc Soc Exp Biol Med. 1967 Apr;124(4):1307–1310. doi: 10.3181/00379727-124-31993. [DOI] [PubMed] [Google Scholar]
- Schuetz A. W. Estrogens and ovarian follicular functions in Rana pipiens. Gen Comp Endocrinol. 1972 Feb;18(1):32–36. doi: 10.1016/0016-6480(72)90076-7. [DOI] [PubMed] [Google Scholar]
- Snyder B. W., Schuetz A. W. In vitro evidence of steroidogenesis in the amphibian (Rana pipiens) ovarian follicle and its relationship to meiotic maturation and ovulation. J Exp Zool. 1973 Mar;183(3):333–342. doi: 10.1002/jez.1401830307. [DOI] [PubMed] [Google Scholar]
- Spiegel J., Jones E., Snyder B. W. Estradiol-17 beta interference with meiotic maturation in Rana pipiens ovarian follicles: evidence for inhibition of 3 beta-hydroxysteroid dehydrogenase. J Exp Zool. 1978 May;204(2):187–191. doi: 10.1002/jez.1402040207. [DOI] [PubMed] [Google Scholar]
- Stoltz R. L., Pollock G. A. Methoxychlor residues in treated irrigation canal water in southcentral Idaho. Bull Environ Contam Toxicol. 1982 Apr;28(4):473–476. doi: 10.1007/BF01607713. [DOI] [PubMed] [Google Scholar]
- TULLNER W. W. Uterotrophic action of the insecticide methoxychlor. Science. 1961 Mar 3;133(3453):647–647. doi: 10.1126/science.133.3453.647. [DOI] [PubMed] [Google Scholar]
- Van der Kraak G. J., Munkittrick K. R., McMaster M. E., Portt C. B., Chang J. P. Exposure to bleached kraft pulp mill effluent disrupts the pituitary-gonadal axis of white sucker at multiple sites. Toxicol Appl Pharmacol. 1992 Aug;115(2):224–233. doi: 10.1016/0041-008x(92)90327-o. [DOI] [PubMed] [Google Scholar]
- Vonier P. M., Crain D. A., McLachlan J. A., Guillette L. J., Jr, Arnold S. F. Interaction of environmental chemicals with the estrogen and progesterone receptors from the oviduct of the American alligator. Environ Health Perspect. 1996 Dec;104(12):1318–1322. doi: 10.1289/ehp.961041318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake D. B. Declining amphibian populations. Science. 1991 Aug 23;253(5022):860–860. doi: 10.1126/science.253.5022.860. [DOI] [PubMed] [Google Scholar]
- Wakeling A. E., Dukes M., Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991 Aug 1;51(15):3867–3873. [PubMed] [Google Scholar]
- Watson C. S., Pappas T. C., Gametchu B. The other estrogen receptor in the plasma membrane: implications for the actions of environmental estrogens. Environ Health Perspect. 1995 Oct;103 (Suppl 7):41–50. doi: 10.1289/ehp.95103s741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. M., Levin W., Conney A. H. Estrogenic action of DDT and its analogs. Toxicol Appl Pharmacol. 1969 Mar;14(2):358–367. doi: 10.1016/0041-008x(69)90117-3. [DOI] [PubMed] [Google Scholar]
- White R., Jobling S., Hoare S. A., Sumpter J. P., Parker M. G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994 Jul;135(1):175–182. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]