Abstract
In vivo carcinogenicity testing is an expensive and time-consuming process, and as a result, only a relatively small fraction of new and existing chemicals has been tested in this manner. Therefore, the development and validation of alternative approaches is desirable. We previously developed a mammalian in vitro assay for genotoxicity based on the ability of cells to increase their level of the tumor-suppressor protein p53 in response to DNA damage. Cultured cells are treated with various amounts of the test substances, and at defined times following treatment, they are harvested and lysed. The lysates are analyzed for p53 by Western blot and/or enzyme-linked immunosorbent assay analysis. An increase in cellular p53 following treatment is interpreted as evidence for DNA damage. To determine the ability of this p53-induction assay to predict carcinogenicity in rodents and to compare such results with those obtained using alternate approaches, we subjected 25 chemicals from the predictive toxicology evaluation 2 list to analysis with this method. Five substances (citral, cobalt sulfate heptahydrate, D&C Yellow No. 11, oxymetholone, and t-butylhydroquinone) tested positive in this assay, and three substances (emodin, phenolphthalein, and sodium xylenesulfonate) tested as possibly positive. Comparisons between the results obtained with this assay and those obtained with the in vivo protocol, the Salmonella assay, and the Syrian hamster embryo (SHE) cell assay indicate that the p53-induction assay is an excellent predictor of the limited number of genotoxic carcinogens in this set, and that its accuracy is roughly equivalent to or better than the Salmonella and SHE assays for the complete set of chemicals.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An W. G., Chuman Y., Fojo T., Blagosklonny M. V. Inhibitors of transcription, proteasome inhibitors, and DNA-damaging drugs differentially affect feedback of p53 degradation. Exp Cell Res. 1998 Oct 10;244(1):54–60. doi: 10.1006/excr.1998.4193. [DOI] [PubMed] [Google Scholar]
- Ashby J., Tennant R. W. Definitive relationships among chemical structure, carcinogenicity and mutagenicity for 301 chemicals tested by the U.S. NTP. Mutat Res. 1991 May;257(3):229–306. doi: 10.1016/0165-1110(91)90003-e. [DOI] [PubMed] [Google Scholar]
- Bristol D. W., Wachsman J. T., Greenwell A. The NIEHS Predictive-Toxicology Evaluation Project. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1001–1010. doi: 10.1289/ehp.96104s51001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Fritsche M., Haessler C., Brandner G. Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agents. Oncogene. 1993 Feb;8(2):307–318. [PubMed] [Google Scholar]
- Ginsberg D., Michael-Michalovitz D., Ginsberg D., Oren M. Induction of growth arrest by a temperature-sensitive p53 mutant is correlated with increased nuclear localization and decreased stability of the protein. Mol Cell Biol. 1991 Jan;11(1):582–585. doi: 10.1128/mcb.11.1.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R., Plaumann B., Lutum A. S., Haessler C., Heinz B., Fritsche M., Brandner G. Nuclear accumulation of p53 in response to treatment with DNA-damaging agents. Toxicol Lett. 1994 Jun;72(1-3):43–52. doi: 10.1016/0378-4274(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Hickman A. W., Jaramillo R. J., Lechner J. F., Johnson N. F. Alpha-particle-induced p53 protein expression in a rat lung epithelial cell strain. Cancer Res. 1994 Nov 15;54(22):5797–5800. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
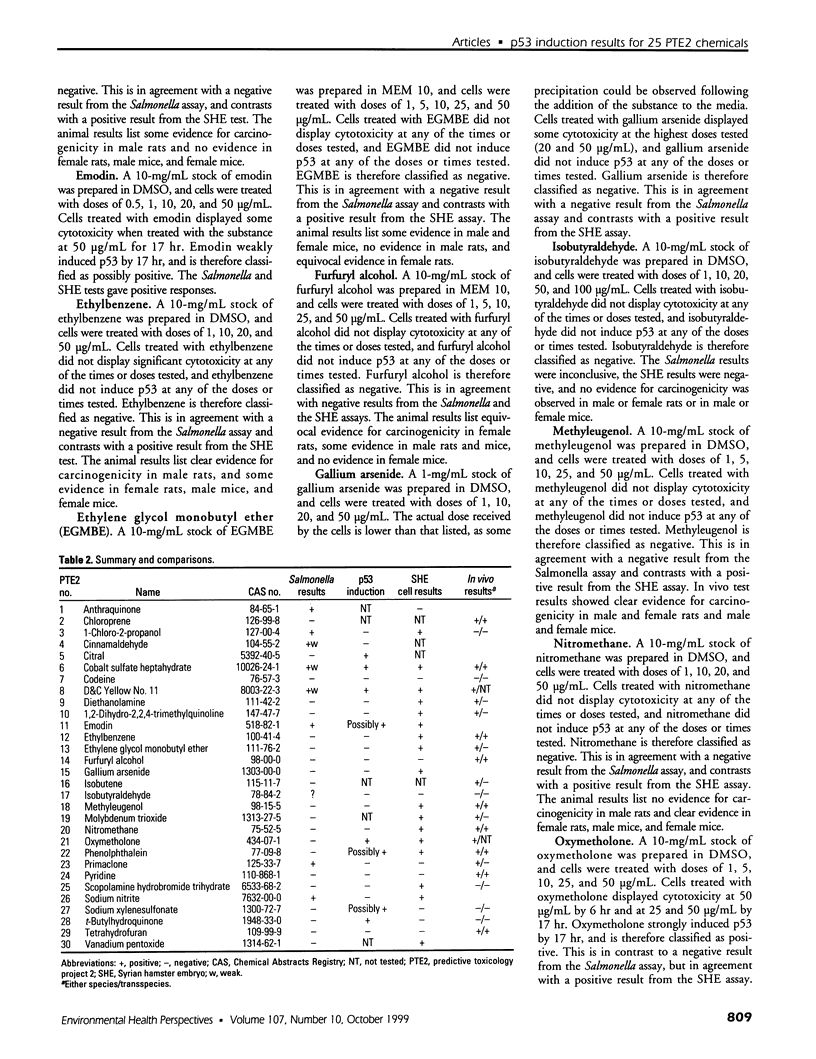
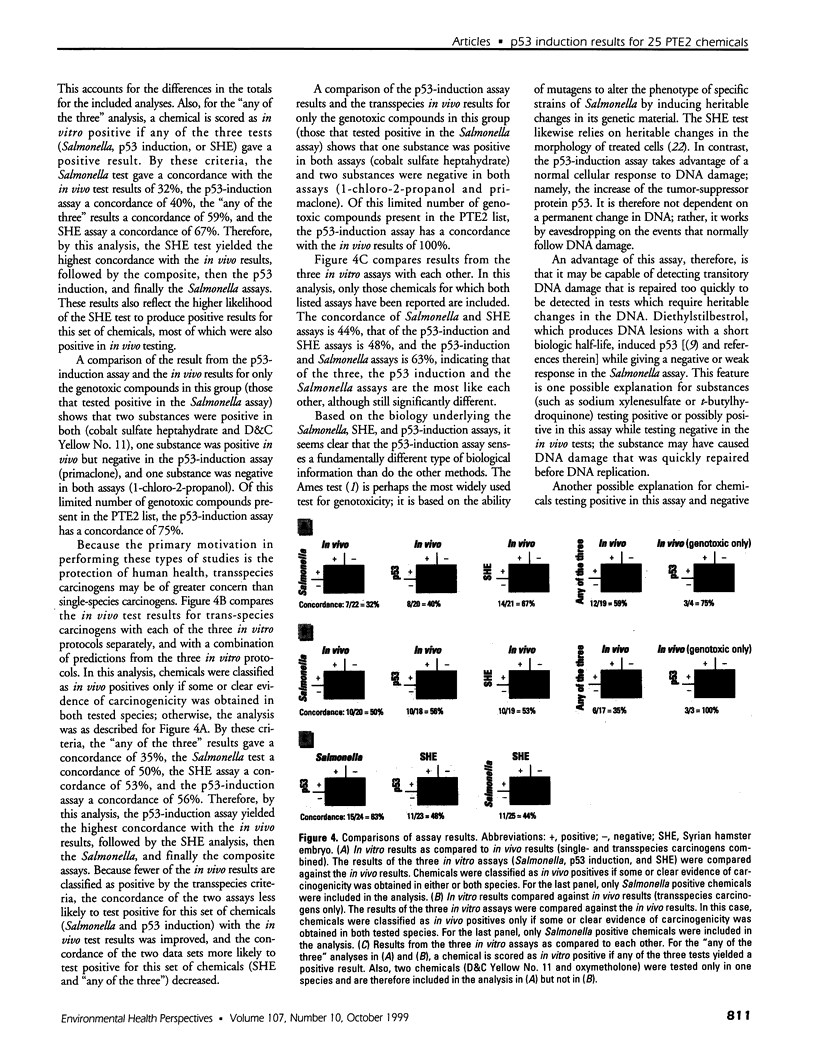
- Kerckaert G. A., Brauninger R., LeBoeuf R. A., Isfort R. J. Use of the Syrian hamster embryo cell transformation assay for carcinogenicity prediction of chemicals currently being tested by the National Toxicology Program in rodent bioassays. Environ Health Perspect. 1996 Oct;104 (Suppl 5):1075–1084. doi: 10.1289/ehp.96104s51075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes U. G., Erhardt P., Yao R., Cooper G. M. p53-dependent induction of apoptosis by proteasome inhibitors. J Biol Chem. 1997 May 16;272(20):12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- Maron D. M., Ames B. N. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983 May;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Nelson W. G., Kastan M. B. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol. 1994 Mar;14(3):1815–1823. doi: 10.1128/mcb.14.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. J., Danish R., Gottlieb C. A., Clarke M. F. Cell cycle analysis of p53-induced cell death in murine erythroleukemia cells. Mol Cell Biol. 1993 Jan;13(1):711–719. doi: 10.1128/mcb.13.1.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K., Tomioka M., Nakano H., Toné S., Ito H., Kawashima S. Apoptosis induction resulting from proteasome inhibition. Biochem J. 1996 Jul 15;317(Pt 2):385–388. doi: 10.1042/bj3170385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Margolin B. H., Shelby M. D., Zeiger E., Haseman J. K., Spalding J., Caspary W., Resnick M., Stasiewicz S., Anderson B. Prediction of chemical carcinogenicity in rodents from in vitro genetic toxicity assays. Science. 1987 May 22;236(4804):933–941. doi: 10.1126/science.3554512. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Spalding J. W., Stasiewicz S., Caspary W. D., Mason J. M., Resnick M. A. Comparative evaluation of genetic toxicity patterns of carcinogens and noncarcinogens: strategies for predictive use of short-term assays. Environ Health Perspect. 1987 Nov;75:87–95. doi: 10.1289/ehp.877587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W. The genetic toxicity database of the National Toxicology Program: evaluation of the relationships between genetic toxicity and carcinogenicity. Environ Health Perspect. 1991 Dec;96:47–51. doi: 10.1289/ehp.919647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsman J. T., Bristol D. W., Spalding J., Shelby M., Tennant R. W. Predicting chemical carcinogenesis in rodents. Environ Health Perspect. 1993 Oct;101(5):444–445. doi: 10.1289/ehp.93101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Duerksen-Hughes P. A new approach to identifying genotoxic carcinogens: p53 induction as an indicator of genotoxic damage. Carcinogenesis. 1998 Jun;19(6):1117–1125. doi: 10.1093/carcin/19.6.1117. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Grunwald D., Wilder S., Kimchi A., May E., Lawrence J. J., May P., Oren M. p53-mediated cell death: relationship to cell cycle control. Mol Cell Biol. 1993 Mar;13(3):1415–1423. doi: 10.1128/mcb.13.3.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- Zeiger E., Haseman J. K., Shelby M. D., Margolin B. H., Tennant R. W. Evaluation of four in vitro genetic toxicity tests for predicting rodent carcinogenicity: confirmation of earlier results with 41 additional chemicals. Environ Mol Mutagen. 1990;16 (Suppl 18):1–14. doi: 10.1002/em.2850160502. [DOI] [PubMed] [Google Scholar]
- Zhan Q., Carrier F., Fornace A. J., Jr Induction of cellular p53 activity by DNA-damaging agents and growth arrest. Mol Cell Biol. 1993 Jul;13(7):4242–4250. doi: 10.1128/mcb.13.7.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]