Abstract
This study investigated whether residence in Aberdeen, North Carolina, the location of the Aberdeen pesticides dumps site (a national priority list Superfund site containing organochlorine pesticides, volatile organic compounds, and metals), is associated with immune suppression as indicated by a higher incidence of herpes zoster and recent occurrences of other common infectious diseases. Study participants included 1,642 residents, 18-64 years of age, who responded to a telephone survey concerning potential occupational and recreational exposures to pesticides and other chemicals, lifetime history of herpes zoster (shingles), and the recent occurrence of other common infectious diseases. Stratified and logistic regression analyses were used to compare the cumulative incidence of herpes zoster among Aberdeen residents and residents of nearby communities. There was little evidence of an overall increased risk of herpes zoster among Aberdeen residents during the period 1951-1994 [relative risk (RR), 1.3; 95% confidence interval (CI), 0.8-2.1]. However, an elevated risk of herpes zoster was noted consistently among Aberdeen residents of younger ages as compared to residents of the nearby communities. The RR was 2.0 (CI, 1.0-4.0) among those 18-40 years of age and was not affected by controlling for potential confounders. The RR of herpes zoster was also consistently elevated in all age groups for the period before 1985. No differences were noted between residents of Aberdeen and those of the nearby communities with respect to the recent occurrence of other common infectious diseases. These results support the plausibility of an association between exposure to the Aberdeen pesticides dumps site and immune suppression and the potential use of herpes zoster as a marker of immune suppression in studies of environmental chemical exposures.
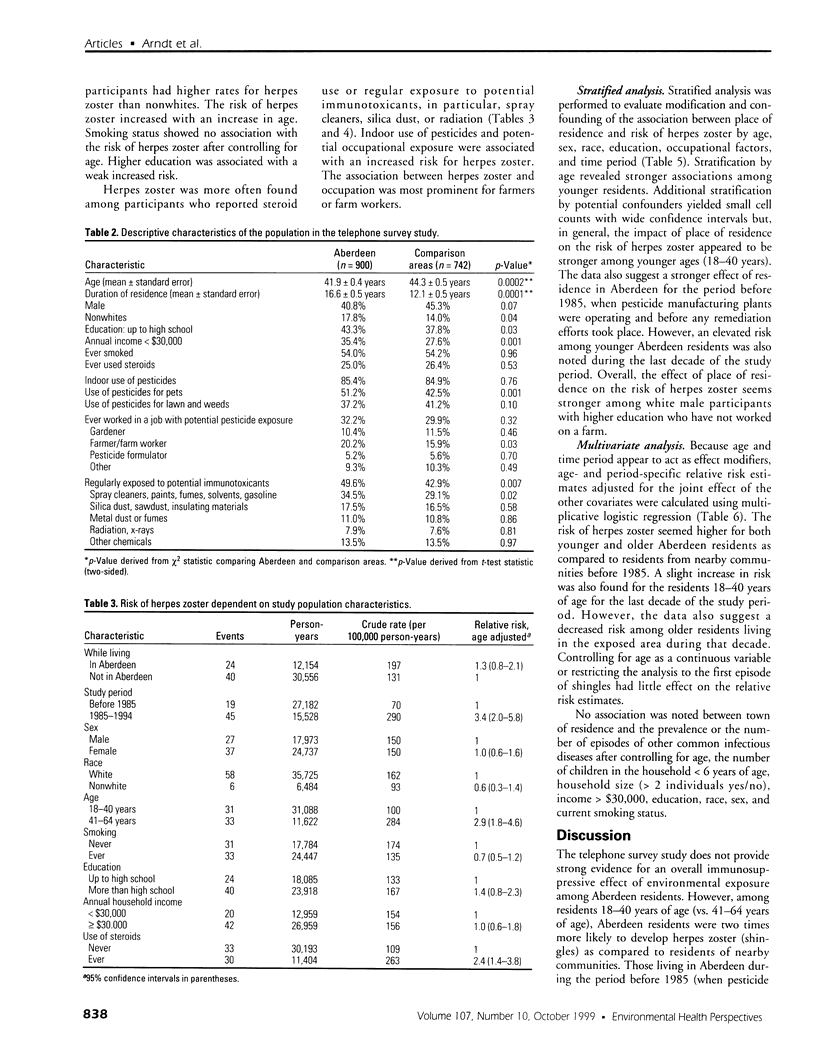
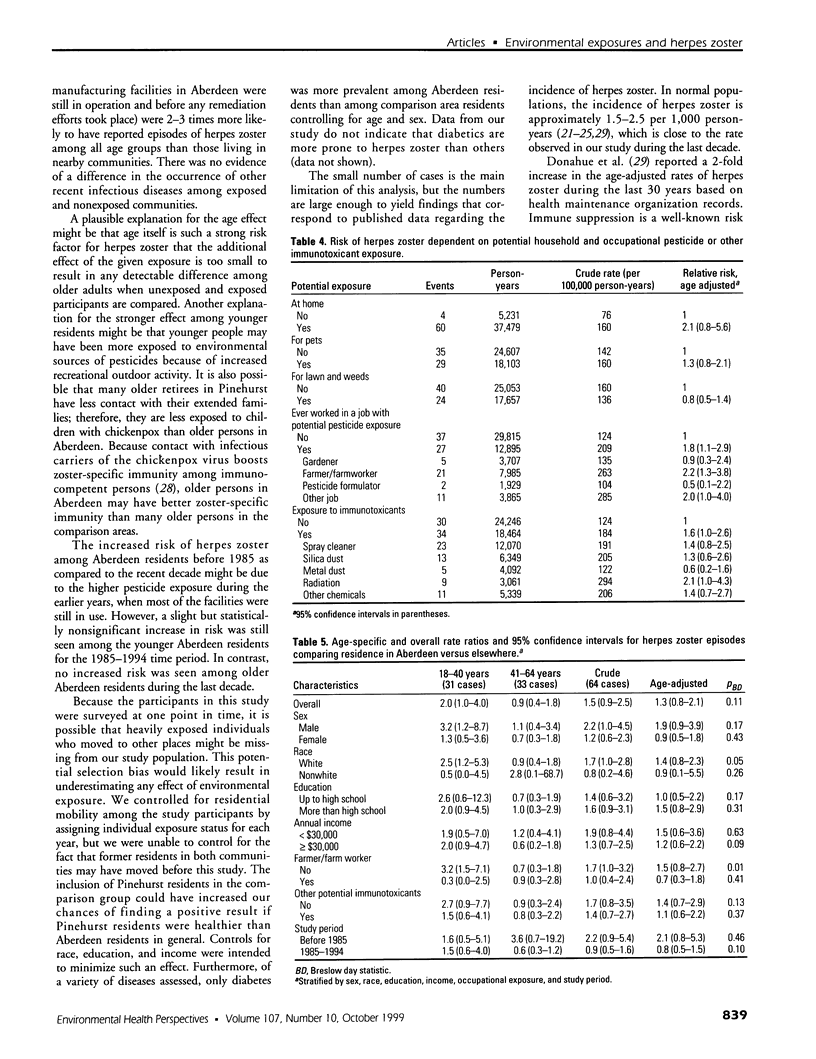
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchbinder S. P., Katz M. H., Hessol N. A., Liu J. Y., O'Malley P. M., Underwood R., Holmberg S. D. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992 Nov;166(5):1153–1156. doi: 10.1093/infdis/166.5.1153. [DOI] [PubMed] [Google Scholar]
- Burrell R. Human immune toxicity. Mol Aspects Med. 1993;14(1):1–81. doi: 10.1016/0098-2997(93)90019-a. [DOI] [PubMed] [Google Scholar]
- Donahue J. G., Choo P. W., Manson J. E., Platt R. The incidence of herpes zoster. Arch Intern Med. 1995 Aug 7;155(15):1605–1609. [PubMed] [Google Scholar]
- Druet P. Metal-induced autoimmunity. Hum Exp Toxicol. 1995 Jan;14(1):120–121. doi: 10.1177/096032719501400129. [DOI] [PubMed] [Google Scholar]
- Finger R., Hughes J. P., Meade B. J., Pelletier A. R., Palmer C. T. Age-specific incidence of chickenpox. Public Health Rep. 1994 Nov-Dec;109(6):750–755. [PMC free article] [PubMed] [Google Scholar]
- Garnett G. P., Grenfell B. T. The epidemiology of varicella-zoster virus infections: a mathematical model. Epidemiol Infect. 1992 Jun;108(3):495–511. doi: 10.1017/s0950268800050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleichmann E., Kimber I., Purchase I. F. Immunotoxicology: suppressive and stimulatory effects of drugs and environmental chemicals on the immune system. A discussion. Arch Toxicol. 1989;63(4):257–273. doi: 10.1007/BF00278639. [DOI] [PubMed] [Google Scholar]
- Glynn C., Crockford G., Gavaghan D., Cardno P., Price D., Miller J. Epidemiology of shingles. J R Soc Med. 1990 Oct;83(10):617–619. doi: 10.1177/014107689008301007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D. A. Posited mechanisms of metal immunotoxicity. Hum Exp Toxicol. 1995 Jan;14(1):114–116. doi: 10.1177/096032719501400127. [DOI] [PubMed] [Google Scholar]
- May D. G., Black C. M., Olsen N. J., Csuka M. E., Tanner S. B., Bellino L., Porter J. A., Wilkinson G. R., Branch R. A. Scleroderma is associated with differences in individual routes of drug metabolism: a study with dapsone, debrisoquin, and mephenytoin. Clin Pharmacol Ther. 1990 Sep;48(3):286–295. doi: 10.1038/clpt.1990.151. [DOI] [PubMed] [Google Scholar]
- Muench R., Nassim C., Niku S., Sullivan-Bolyai J. Z. Seroepidemiology of varicella. J Infect Dis. 1986 Jan;153(1):153–155. doi: 10.1093/infdis/153.1.153. [DOI] [PubMed] [Google Scholar]
- Murphy R., Harvey C. Residues and metabolites of selected persistent halogenated hydrocarbons in blood specimens from a general population survey. Environ Health Perspect. 1985 May;60:115–120. doi: 10.1289/ehp.8560115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino M. W., Melton L. J., 3rd, Kurland L. T., Chu C. P., Perry H. O. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982 Sep;61(5):310–316. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- Schmader K., George L. K., Burchett B. M., Pieper C. F., Hamilton J. D. Racial differences in the occurrence of herpes zoster. J Infect Dis. 1995 Mar;171(3):701–704. doi: 10.1093/infdis/171.3.701. [DOI] [PubMed] [Google Scholar]
- Weigle K. A., Grose C. Molecular dissection of the humoral immune response to individual varicella-zoster viral proteins during chickenpox, quiescence, reinfection, and reactivation. J Infect Dis. 1984 May;149(5):741–749. doi: 10.1093/infdis/149.5.741. [DOI] [PubMed] [Google Scholar]
- Weisenburger D. D. Human health effects of agrichemical use. Hum Pathol. 1993 Jun;24(6):571–576. doi: 10.1016/0046-8177(93)90234-8. [DOI] [PubMed] [Google Scholar]