Abstract
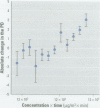
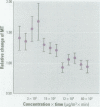
This work was designed to evaluate the toxicity of inhalable particles [less than/equal to] 10 microm in aerodynamic diameter (PM(10)) collected from the urban air in São Paulo, Brazil, to the mucociliary apparatus using the frog palate preparation. Seven groups of frog palates were immersed in different concentrations of PM(10) diluted in Ringer's solution during 120 min: 0 (control, n = 31); 50 (n = 10); 100 (n = 9); 500 (n = 28); 1,000 (n = 10); 5,000 (n = 11); and 10,000 microg/m(3) (n = 10). Mucociliary transport and transepithelial potential difference were determined at 0, 30, 60, and 120 min exposure. Additional groups (control and 500 microg/m(3)) were studied by means of morphometric analyses (quantification of the amount of intraepithelial and surface mucins), measurement of cilia beat frequency, and quantification of total glutathione. Mucociliary transport and transepithelial potential difference were significantly decreased at higher concentrations of PM(10) (p = 0.03 and p = 0.02, respectively). Exposure to PM(10) also elicited a significant decrease of total glutathione (p = 0. 003) and depletion of neutral intraepithelial mucins (p = 0.0461). These results show that PM(10) can promote significant alterations in ciliated epithelium in vitro.
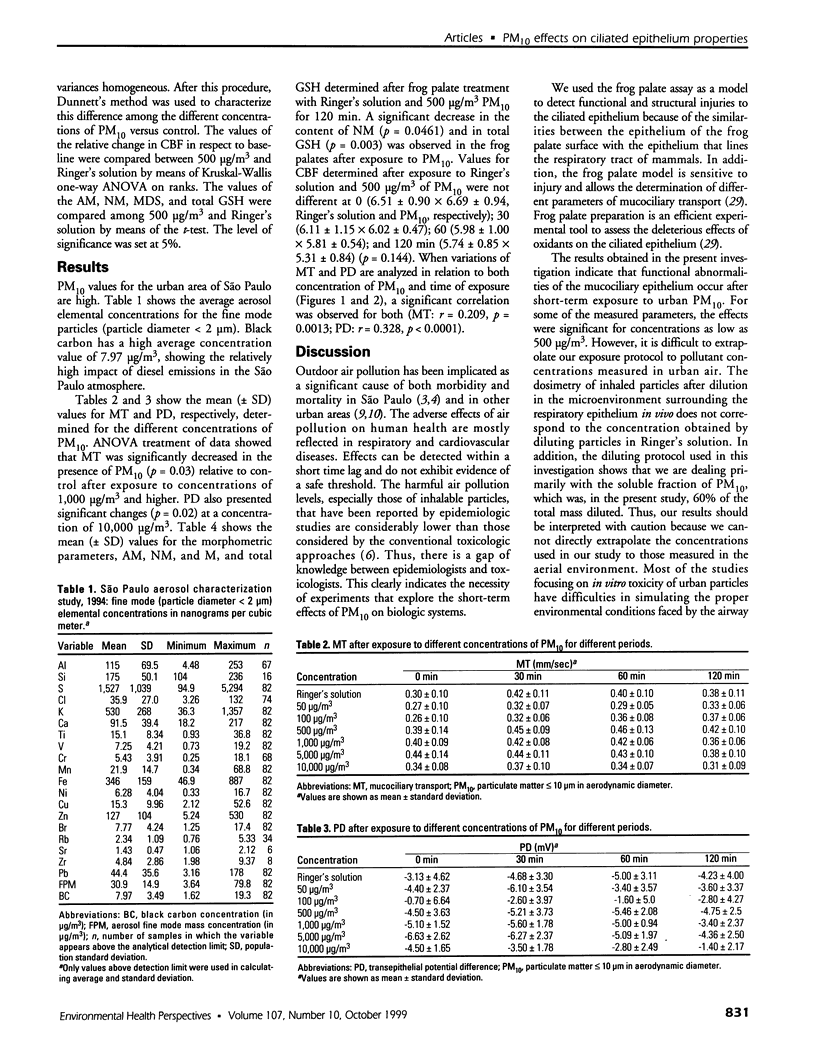
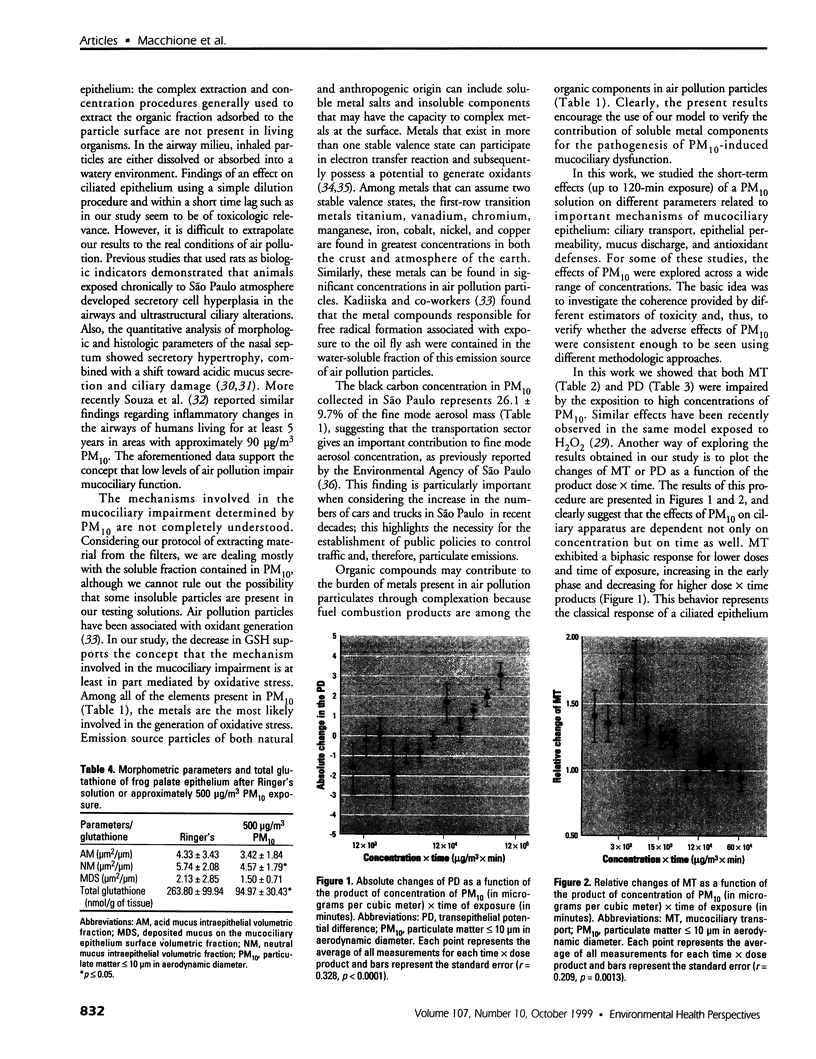
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- CIOCCO A., THOMPSON D. J. A follow-up of Donora ten years after: methodology and findings. Am J Public Health Nations Health. 1961 Feb;51:155–164. doi: 10.2105/ajph.51.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camner P., Helström P. A., Philipson K. Carbon dust and mucociliary transport. Arch Environ Health. 1973 Jun;26(6):294–296. doi: 10.1080/00039896.1973.10666284. [DOI] [PubMed] [Google Scholar]
- Creeth J. M., Cooper B., Donald A. S., Clamp J. R. Studies of the limited degradation of mucus glycoproteins. The effect of dilute hydrogen peroxide. Biochem J. 1983 May 1;211(2):323–332. doi: 10.1042/bj2110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Allen A. Antioxidant protection: a function of tracheobronchial and gastrointestinal mucus. Lancet. 1984 Jun 16;1(8390):1328–1330. doi: 10.1016/s0140-6736(84)91822-1. [DOI] [PubMed] [Google Scholar]
- De Sanctis G. T., App E. M., Trask J. K., De Sanctis B. I., Remmers J. E., Green F. H., Man S. F., King M. Resorptive clearance and transepithelial potential difference in capsaicin-treated F344 rats. J Appl Physiol (1985) 1990 May;68(5):1826–1832. doi: 10.1152/jappl.1990.68.5.1826. [DOI] [PubMed] [Google Scholar]
- Dockery D. W., Pope C. A., 3rd Acute respiratory effects of particulate air pollution. Annu Rev Public Health. 1994;15:107–132. doi: 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- Dockery D. W., Ware J. H., Ferris B. G., Jr, Speizer F. E., Cook N. R., Herman S. M. Change in pulmonary function in children associated with air pollution episodes. J Air Pollut Control Assoc. 1982 Sep;32(9):937–942. doi: 10.1080/00022470.1982.10465494. [DOI] [PubMed] [Google Scholar]
- Donaldson K., Brown D. M., Mitchell C., Dineva M., Beswick P. H., Gilmour P., MacNee W. Free radical activity of PM10: iron-mediated generation of hydroxyl radicals. Environ Health Perspect. 1997 Sep;105 (Suppl 5):1285–1289. doi: 10.1289/ehp.97105s51285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliezer N., Sadé J., Silberberg A., Nevo A. C. The role of mucus in transport by cilia. Am Rev Respir Dis. 1970 Jul;102(1):48–52. doi: 10.1164/arrd.1970.102.1.48. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Von Ritter C., Smith B. F., Lamont J. T., Granger D. N. Interaction between oxygen radicals and gastric mucin. Am J Physiol. 1987 Jul;253(1 Pt 1):G93–G96. doi: 10.1152/ajpgi.1987.253.1.G93. [DOI] [PubMed] [Google Scholar]
- Jones R., Reid L. Secretory cells and their glycoproteins in health and disease. Br Med Bull. 1978 Jan;34(1):9–16. doi: 10.1093/oxfordjournals.bmb.a071466. [DOI] [PubMed] [Google Scholar]
- Kadiiska M. B., Mason R. P., Dreher K. L., Costa D. L., Ghio A. J. In vivo evidence of free radical formation in the rat lung after exposure to an emission source air pollution particle. Chem Res Toxicol. 1997 Oct;10(10):1104–1108. doi: 10.1021/tx970049r. [DOI] [PubMed] [Google Scholar]
- Lacaz-Vieira F., Procopio J. Comparative roles of voltage and Cl ions upon activation of a Cl conductive pathway in toad skin. Pflugers Arch. 1988 Oct;412(6):634–640. doi: 10.1007/BF00583765. [DOI] [PubMed] [Google Scholar]
- Lemos M., Lichtenfels A. J., Amaro Júnior E., Macchione M., Martins M. A., King M., Böhm G. M., Saldiva P. H. Quantitative pathology of nasal passages in rats exposed to urban levels of air pollution. Environ Res. 1994 Jul;66(1):87–95. doi: 10.1006/enrs.1994.1046. [DOI] [PubMed] [Google Scholar]
- Lorenzi G., Böhm G. M., Guimarães E. T., Vaz M. A., King M., Saldiva P. H. Correlation between rheologic properties and in vitro ciliary transport of rat nasal mucus. Biorheology. 1992 Jul-Aug;29(4):433–440. doi: 10.3233/bir-1992-29406. [DOI] [PubMed] [Google Scholar]
- Macchione M., Guimarães E. T., Saldiva P. H., Lorenzi-Filho G. Methods for studying respiratory mucus and mucus clearance. Braz J Med Biol Res. 1995 Nov-Dec;28(11-12):1347–1355. [PubMed] [Google Scholar]
- Macchione M., Lorenzi-Filho G., Guimarães E. T., Junqueira V. B., Saldiva P. H. The use of the frog palate preparation to assess the effects of oxidants on ciliated epithelium. Free Radic Biol Med. 1998 Mar 15;24(5):714–721. doi: 10.1016/s0891-5849(97)00332-8. [DOI] [PubMed] [Google Scholar]
- Menzel D. B. The toxicity of air pollution in experimental animals and humans: the role of oxidative stress. Toxicol Lett. 1994 Jun;72(1-3):269–277. doi: 10.1016/0378-4274(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Parker R. D., Buzzard G. H., Dzubay T. G., Bell J. P. A two stage respirable aerosol sampler using nuclepore filters in series. Atmos Environ. 1977;11(7):617–621. doi: 10.1016/0004-6981(77)90114-7. [DOI] [PubMed] [Google Scholar]
- Peatfield A. C., Richardson P. S. The action of dust in the airways on secretion into the trachea of the cat. J Physiol. 1983 Sep;342:327–334. doi: 10.1113/jphysiol.1983.sp014853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C. A., 3rd, Dockery D. W. Acute health effects of PM10 pollution on symptomatic and asymptomatic children. Am Rev Respir Dis. 1992 May;145(5):1123–1128. doi: 10.1164/ajrccm/145.5.1123. [DOI] [PubMed] [Google Scholar]
- Pope C. A., 3rd Respiratory disease associated with community air pollution and a steel mill, Utah Valley. Am J Public Health. 1989 May;79(5):623–628. doi: 10.2105/ajph.79.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson W. V., Ropes M. W., Bauer W. The degradation of mucins and polysaccharides by ascorbic acid and hydrogen peroxide. Biochem J. 1941 Sep;35(8-9):903–908. doi: 10.1042/bj0350903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B. K., Ramirez O., King M. Mucus-depleted frog palate as a model for the study of mucociliary clearance. J Appl Physiol (1985) 1990 Aug;69(2):424–429. doi: 10.1152/jappl.1990.69.2.424. [DOI] [PubMed] [Google Scholar]
- Saldiva P. H., King M., Delmonte V. L., Macchione M., Parada M. A., Daliberto M. L., Sakae R. S., Criado P. M., Silveira P. L., Zin W. A. Respiratory alterations due to urban air pollution: an experimental study in rats. Environ Res. 1992 Feb;57(1):19–33. doi: 10.1016/s0013-9351(05)80016-7. [DOI] [PubMed] [Google Scholar]
- Saldiva P. H., Lichtenfels A. J., Paiva P. S., Barone I. A., Martins M. A., Massad E., Pereira J. C., Xavier V. P., Singer J. M., Böhm G. M. Association between air pollution and mortality due to respiratory diseases in children in São Paulo, Brazil: a preliminary report. Environ Res. 1994 May;65(2):218–225. doi: 10.1006/enrs.1994.1033. [DOI] [PubMed] [Google Scholar]
- Saldiva P. H., Pope C. A., 3rd, Schwartz J., Dockery D. W., Lichtenfels A. J., Salge J. M., Barone I., Bohm G. M. Air pollution and mortality in elderly people: a time-series study in Sao Paulo, Brazil. Arch Environ Health. 1995 Mar-Apr;50(2):159–163. doi: 10.1080/00039896.1995.9940893. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Lung function and chronic exposure to air pollution: a cross-sectional analysis of NHANES II. Environ Res. 1989 Dec;50(2):309–321. doi: 10.1016/s0013-9351(89)80012-x. [DOI] [PubMed] [Google Scholar]
- Schwartz J. What are people dying of on high air pollution days? Environ Res. 1994 Jan;64(1):26–35. doi: 10.1006/enrs.1994.1004. [DOI] [PubMed] [Google Scholar]
- Souza M. B., Saldiva P. H., Pope C. A., 3rd, Capelozzi V. L. Respiratory changes due to long-term exposure to urban levels of air pollution: a histopathologic study in humans. Chest. 1998 May;113(5):1312–1318. doi: 10.1378/chest.113.5.1312. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- WEIBEL E. R. Principles and methods for the morphometric study of the lung and other organs. Lab Invest. 1963 Feb;12:131–155. [PubMed] [Google Scholar]
- Wanner A., Salathé M., O'Riordan T. G. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996 Dec;154(6 Pt 1):1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- Yeates D. B., Aspin N., Levison H., Jones M. T., Bryan A. C. Mucociliary tracheal transport rates in man. J Appl Physiol. 1975 Sep;39(3):487–495. doi: 10.1152/jappl.1975.39.3.487. [DOI] [PubMed] [Google Scholar]