Abstract
Boric acid (BA) is a naturally occurring agent used in manufacturing processes and numerous consumer products. Because of the potential for both industrial and consumer exposure to boron-containing compounds, and the lack of developmental toxicity data, the National Toxicology Program evaluated the potential for boric acid to cause developmental toxicity in pregnant Swiss (CD-1) mice, Sprague-Dawley rats (n = 26-28/group), and New Zealand rabbits (n = 18-23/group). BA was provided in the feed to mice and rats at 0, 0.1, 0.2, or 0.4% throughout gestation to attain steady-state exposure as early as possible during development. Average doses (mg/kg/day) were 248, 452, or 1003 for mice, and 78, 163, or 330 in rats. A separate group of rats received 0.8% BA in the feed, or 539 mg/kg/day only on gestation days (gd) 6 to 15. Rabbits were given BA (0, 62.5, 125, or 250 mg/kg) by gavage administration on gd 6 to 19. Maternal body weight, food and/or water consumption and signs of toxicity were monitored at regular intervals. At termination, gd 17 (mice), 20 (rats), or 30 (rabbits), the uterus was examined to determine the number of resorptions, dead, or live fetuses. Fetuses were weighed and live fetuses were examined for external, visceral, and skeletal defects. Mouse dams exhibited mild renal lesions (> or = 248 mg/kg/day BA), increased water intake and relative kidney weight (1003 mg/kg/day BA), and decreased weight gain during treatment.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREDDERMAN P. J., FOOTE R. H., YASSEN A. M. AN IMPROVED ARTIFICIAL VAGINA FOR COLLECTING RABBIT SEMEN. J Reprod Fertil. 1964 Jun;7:401–403. doi: 10.1530/jrf.0.0070401. [DOI] [PubMed] [Google Scholar]
- Culver B. D., Shen P. T., Taylor T. H., Lee-Feldstein A., Anton-Culver H., Strong P. L. The relationship of blood- and urine-boron to boron exposure in borax-workers and usefulness of urine-boron as an exposure marker. Environ Health Perspect. 1994 Nov;102 (Suppl 7):133–137. doi: 10.1289/ehp.94102s7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
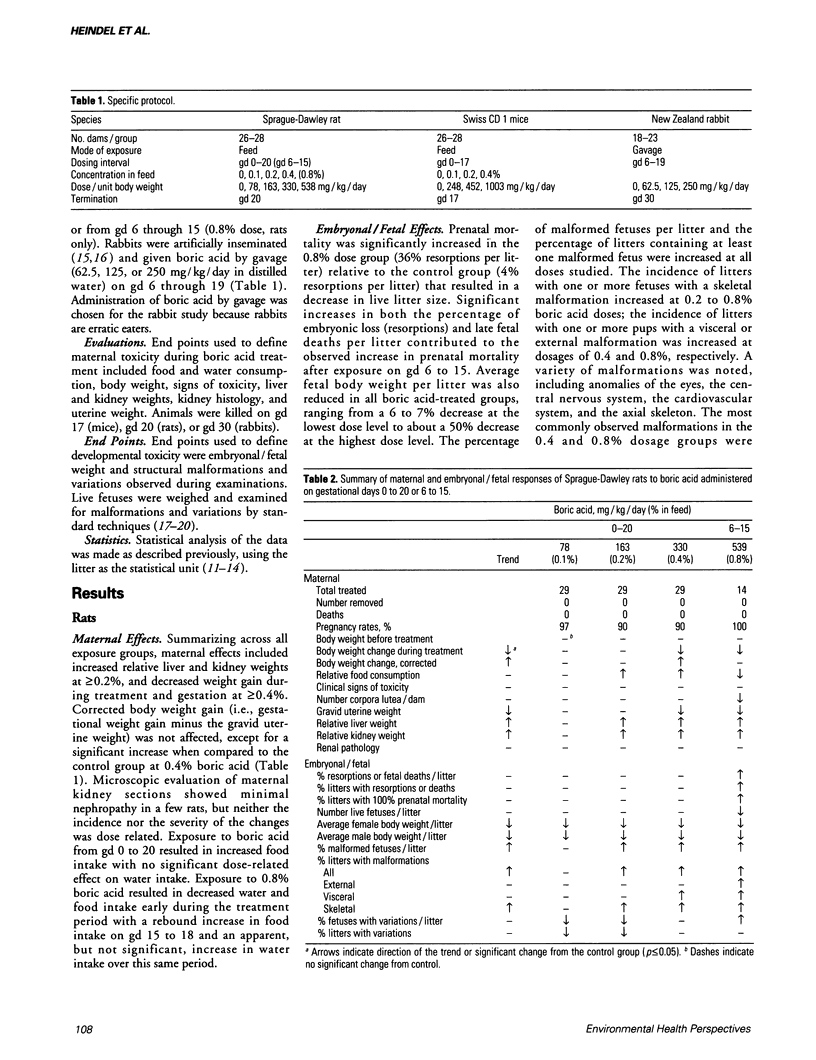
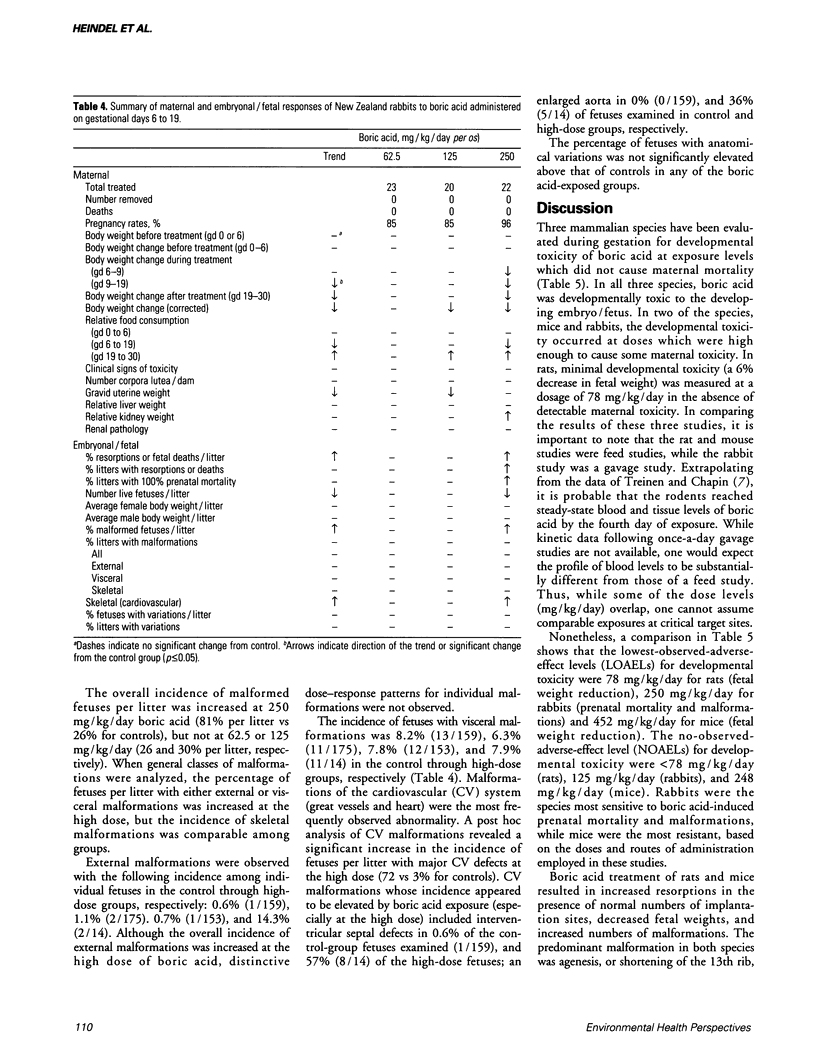
- Heindel J. J., Price C. J., Field E. A., Marr M. C., Myers C. B., Morrissey R. E., Schwetz B. A. Developmental toxicity of boric acid in mice and rats. Fundam Appl Toxicol. 1992 Feb;18(2):266–277. doi: 10.1016/0272-0590(92)90055-m. [DOI] [PubMed] [Google Scholar]
- Lee I. P., Sherins R. J., Dixon R. L. Evidence for induction of germinal aplasia in male rats by environmental exposure to boron. Toxicol Appl Pharmacol. 1978 Aug;45(2):577–590. doi: 10.1016/0041-008x(78)90119-9. [DOI] [PubMed] [Google Scholar]
- Siegel E., Wason S. Boric acid toxicity. Pediatr Clin North Am. 1986 Apr;33(2):363–367. doi: 10.1016/s0031-3955(16)35006-4. [DOI] [PubMed] [Google Scholar]
- Stuckhardt J. L., Poppe S. M. Fresh visceral examination of rat and rabbit fetuses used in teratogenicity testing. Teratog Carcinog Mutagen. 1984;4(2):181–188. doi: 10.1002/tcm.1770040203. [DOI] [PubMed] [Google Scholar]
- Treinen K. A., Chapin R. E. Development of testicular lesions in F344 rats after treatment with boric acid. Toxicol Appl Pharmacol. 1991 Feb;107(2):325–335. doi: 10.1016/0041-008x(91)90212-w. [DOI] [PubMed] [Google Scholar]
- Vorhees C. V., Acuff K. D., Weisenburger W. P., Minck D. R. Teratogenicity of carbamazepine in rats. Teratology. 1990 Mar;41(3):311–317. doi: 10.1002/tera.1420410308. [DOI] [PubMed] [Google Scholar]
- Weir R. J., Jr, Fisher R. S. Toxicologic studies on borax and boric acid. Toxicol Appl Pharmacol. 1972 Nov;23(3):351–364. doi: 10.1016/0041-008x(72)90037-3. [DOI] [PubMed] [Google Scholar]


